Abstract
Understanding the age and development of a species provides knowledge about its longevity and growth, which are crucial in assessing its life history to maintain the sustainability of its fisheries. Over 3000 samples of Uroteuthis duvaucelii were collected from trawl catches off Concepcion, Iloilo, Western Visayas, Philippines, from April 2018 to September 2019. Daily rings in the statoliths were used to determine their age. The estimated age ranged from 73 to 154 days old, corresponding to sizes of 3.1 to 28.1 cm mantle length. The presence of small individuals (~4 cm) throughout the year indicate continuous spawning of this species. Growth curve patterns of U. duvaucelii were examined using two techniques: growth from sizeat-age data (statolith increments) and growth generated from length-frequency data using the ELEFAN software package. Both methods yielded results that were remarkably different from one another. The growth curve generated from length frequency analysis produced an asymptotic growth. In contrast, sizeat-age data (mantle length-age relationship) revealed that U. duvaucelii does not grow asymptotically. The growth of this tropical squid is best fitted with exponential growth, exhibiting a continuous rapid growth and short lifespan, a prominent characteristic observed in neritic species of squids. This work provides evidence that asymptotic growth is not applicable for the tropical squid species in the Visayan Sea, Philippines.
Keywords: Cephalopods, Growth, Age, Statolith, Length Frequency
BACKGROUND
The Loliginidae family of squids are typically called inshore or neritic squids (Jereb and Roper 2010). This family includes several species that are commercially important worldwide and are found mostly in tropical to temperate environments (Forsythe and Hanlon 1989; Butler et al. 1999; Jackson and Domeier 2003; Sukramungkol et al. 2007; Gonzalez et al. 2010; Jereb and Roper 2010). Thus, the Loliginid squids have been the subject of most squid studies and experimental work. One of the species that belongs to this family is Uroteuthis duvaucelii. It is distributed in the Indo-Pacific region, occurring in depths between 30 and 170 meters (Jereb and Roper 2010). As with all other cephalopods, this species is an active carnivore that feeds mainly on crustaceans (e.g., mysids, euphausiids, ostracods, Acetes spp.) and small fishes and often exhibits cannibalistic behavior (Kore and Joshi 1975; Tehseen et al. 2019). U. duvaucelii is known to spawn all throughout the year (e.g., Meiyappan and Srinath 1989). In the Visayan Sea, Philippines, peak spawning occurs from December until May (Tajolosa 2011), similar to the spawning peak season observed in India (Rao 1988).
The Indian Squid Uroteuthis duvaucelii is among the major commercially important cephalopods in the Philippines due to the increasing demand for local and municipal consumption (PSA 2002–2021). The species is a vital food resource but has also been utilized as fertilizer and as a supplemental food source for highend and expensive cultured animals (del Norte-Campos et al. 2000). The average annual production of squid in the country amounts to about 54, 020.7 metric tons, with a corresponding value of PHP 4,518,456 (PSA 2002–2021). The Western Visayas Region, particularly the Visayan Sea, contributes the most (18.2%) to total landings. In a market survey of commercially important marine invertebrates in Panay Island in the western Visayan Sea, del Norte-Campos et al. (2019) showed that U. duvaucelii was the 2nd most important species due to high catch rates and high price in the local market. While there is an increasing commercial significance of squids, little is known about their growth, which is crucial for stock assessment.
Generally, squids are extensively known for their rapid growth rate, limited lifespan, and complex population structures (e.g., Jin et al. 2019; Sajikumar et al. 2019). Their growth is highly variable (Pecl 2000), and its plasticity is reported to be influenced by temperature (Forsythe and Hanlon 1989; Hatfield 2000; Forsythe 2004), maturation (Moltschaniwskyj 1995), and feeding (Jackson and Moltschaniwskyj 2001b). These distinguishing characteristics, especially in loliginid squids, have made them a good subject for investigating life histories (see Jackson 2004). Squids possess statoliths, a pair of calcareous structures primarily made up of calcium carbonate in aragonite crystal form and a small amount of organic material (Radtke 1983). The statolith contains growth increments which have been observed to form daily, as validated in several species of squids (Jackson 1990; Nakamura and Sakurai 1991). To date, statoliths are considered an effective tool in estimating the growth of squid as they record ontogenetic events chronologically and continue to grow throughout the life span of the squid (Lipinski 2001; Arkhipkin 2005; Arkhipkin and Shcherbich 2012).
Our understanding of growth has improved through the years, yet it is still unclear which method best describes squid growth. Length frequency analysis has been widely applied to determine growth in marine vertebrates and invertebrates. However, various studies have raised questions about its applicability to squid species (Jackson and Choat 1992; Jackson et al. 1997). Although this analysis is still applied in squid growth studies under the assumption that they are long-lived and grow asymptotically (e.g., Meiyappan et al. 1993; Karnik et al. 2003), various researchers have argued that squids have short lifespans and exhibit non-asymptotic growth. The latter has been confirmed by several studies using size-at-age data from statolith analysis, where growth was shown to be exponential (Jackson and Choat 1992; Chang et al. 2014), linear (Butler et al. 1999; Liu et al. 2013) or following a power curve (Jackson and Domeier 2003; Hatfield 2000) pattern. Moreover, studies on their physiology are supportive of non-asymptotic growth (e.g., Moltshaniwsky 1994). It is thus essential to reassess techniques used in determining growth in squid in particular and in cephalopods in general (Jackson and Choat 1992; Semmens et al. 2004).
In this study, we examined and compared two techniques in estimating growth models: lengthfrequency data analyzed through ELEFAN, and agebased data obtained from statolith ring analysis. We also describe which growth model best fits the tropical squid U. duvaucelii. this work provides a more reliable approach to describing the growth of the Indian squid in the Visayan Sea, Philippines, and contributes knowledge to the management for sustainable exploitation of this marine resource.
MATERIALS AND METHODS
Sample Collection
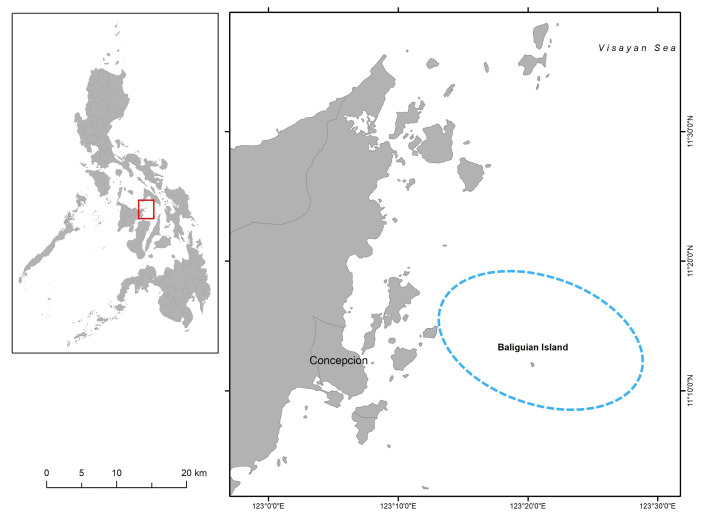
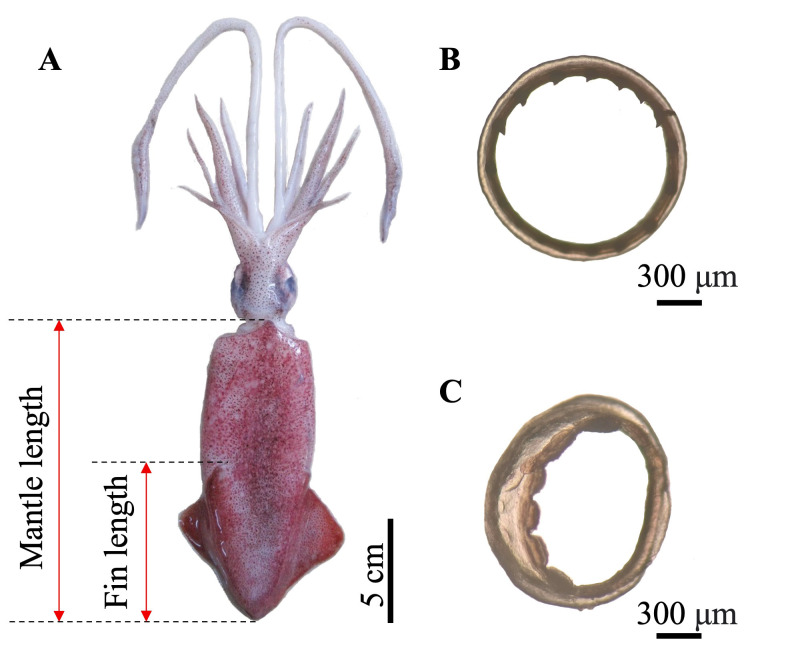
Samples for the study were taken from nighttime catches of trawlers using nets with a mesh size of about 3 cm and operating at depths between 20–25 m in the vicinity of Baliguian Island, Concepcion, Iloilo, Philippines (Fig. 1). Samples were collected monthly from April 2018 to September 2019. Fresh samples were measured (mantle length) (Fig. 2) to the nearest mm using a vernier caliper. A subsample of 20 randomly chosen specimens per month were preserved in 70% ethanol and transported immediately to the laboratory for extraction and further processing of statoliths. Specimens were identified to species using the FAO identification guide (Carpenter and Niem 1998; Jereb and Roper 2010). U. duvaucelii was identified based on the shape of the fin, tentacular club sucker ring, and arm III sucker ring, as illustrated in figure 2.
Fig. 1.
Location of trawl operations encircled in blue around Baliguian Island, Concepcion, Iloilo, Philippines.
Fig. 2.
Diagnostic features of U. duvaucelii based on morphology described in the FAO identification guide for cephalopods (Carpenter and Niem 1998; Jereb and Roper 2010). The species is identified by its short and stout mantle with a rhombic and shortfin shape (A), short and sharp teeth on its tentacular club sucker ring (B), and small, broad, and plate-squared tip teeth of relatively equal size in arm III (C).
Statolith Microstructure Analysis
The statoliths were extracted from the statocysts by making a transverse cut on the lower postero-ventral part of the head, revealing the left and right statoliths located in the anterior part. As with fish otoliths (Green et al. 2009), fine forceps were used to remove any tissue attached to the statolith. Statoliths were then immersed in bleach for three minutes to remove any remaining tissue and debris. The statoliths were then rinsed with distilled water to prevent bleach-crystal formation and stored in a well plate filled with 70–90% ethanol to remove water within the statoliths (Arkhipkin and Shcherbich 2012). After the ethanol evaporated and the wells dried, the statoliths were mounted on a glass slide and polished with diamond lapping film (9 µm) containing alpha aluminum slurry (0.3 µm) until the core was visible under the microscope. A compound light microscope (Leica microsystems) at 400x magnification was used to view the increments and photographed using a Sony high-resolution camera (Sony Cyber-shot DSC WX50 16.2-megapixel camera with 5x optical zoom).
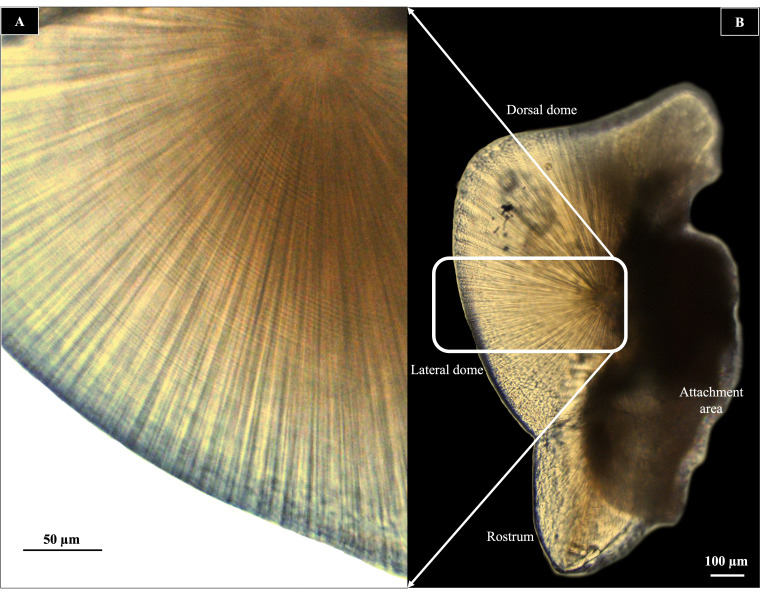
The external morphological features of the statolith follow Clarke’s (1978) identification. Given that the two statoliths in each specimen are known to have identical morphology (Natsukari et al. 1988), one statolith from each pair was randomly selected for further examination (Durholtz et al. 2002). In case one of the statoliths was unavailable or poorly prepared, the remaining statolith was used. Growth increments were counted using image analysis software (Image J). A growth increment is comprised of a ‘dark ring’ and the adjacent ‘light ring’ within the statolith (Bettencourt et al. 1996). Growth increments were counted at the lateral dome since growth rings in loliginid species are more visible in this area (e.g., Lipinski 1986; Jackson 1989), as shown in Fig. 3. Counting of growth increments in loliginid species starts from the natal ring, a distinct first dark ring after the focus, which corresponds to individual hatching until the end of the lateral dome (e.g., Natsukari et al. 1988). Image J was used to mark and count growth increments from the natal ring up to the edge of the lateral dome.
Fig. 3.
Ground statolith of U. duvaucelii (A) and an enlarged view depicted by a rectangle revealing growth rings in the lateral dome area (B).
The same reader conducted three counts successively without knowing the specimen’s mantle length, sex, and age. This was done to assess consistency in counting and avoid potential biases in each reading. The results showed a coefficient of variations ranging from 0.5% to 2.5%.
Length Frequency Analysis
The FiSAT (Fish Stock Assessment Tool) software package uses a von Bertalanffy growth model, which assumes asymptotic growth. This software was used to analyze the length frequency data of the combined sexes of U. duvaucelii, which produced the maximum dorsal mantle length recorded in the study, referred to as the asymptotic length (L∞). The Electronic Length Frequency Analysis (ELEFAN) I built into the FiSAT computer program was used to compute different values of the growth coefficient (K) with fixed L∞, using the von Bertallanfy equation,
where t is the age of the organism at a given time and to is the theoretical age at which the organism would have zero length. The best value of K was determined based on the starting length and sample of the length frequency histogram. By combining L∞ and K parameters, the growth curve was obtained using the best-fitted peaks of the length frequency histogram.
Data Analysis
Age estimates were based on the daily increments obtained. The growth curve was determined in two ways: (1) Following the progression of monthly modal sizes using ELEFAN incorporated in FiSAT employing the von Bertalanffy growth model (VBGM). (2) Determining the best-fitting curve to size at age data using regression analysis based on statolith microstructure.
RESULTS
Length-based Growth
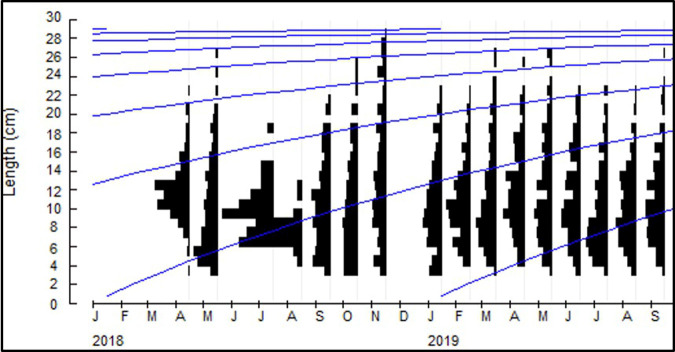
A total of 3,143 fresh specimens were measured during the study. Figure 4 shows the monthly size frequency distributions of U. duvaucelii from April 2018 to September 2019. The recorded sizes ranged from 3.1 to 28.1 cm mantle length (ML), with small individuals (< 4 cm ML) observed in almost all months of the study. In contrast, large individuals (> 25 cm ML) were only recorded just before the northeast monsoon (Oct–Nov) and during the summer (Mar–May). The best-fitting von Bertalanffy growth curve parameters were L∞ = 29.58 cm and K = 0.82 year-1.
Fig. 4.
Von Bertalanffy growth model of U. duvaucelii as derived through ELEFAN I (L∞ = 29.58 cm and K = 0.82 year-1).
These estimates are well within the range of growth parameter values reported in the literature (Table 1) and are very close to the medians of the tabled values: L∞ = 29.80 cm and K = 0.90 year-1. Following this growth model, U. duvaucelii in the Visayan Sea reaches the largest observed size in the study (28.1 cm ML) in over three years and are roughly two months old (~ 4 cm ML) when they are captured by the trawl fishery.
Table 1.
Comparison of VBGF growth parameters (L∞, K) of Indian squid U. duvaucelii from different areas.
Age-based growth
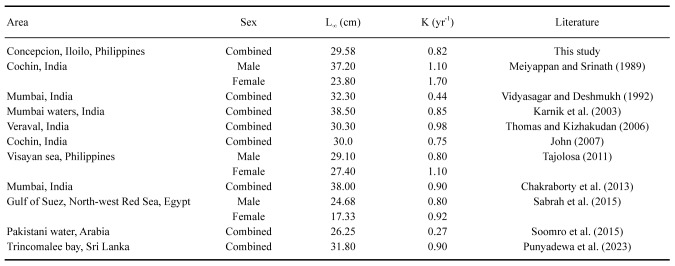
The sizes of specimens (n = 219) whose statoliths were analyzed ranged from 4–21 cm ML, covering the period from May 2018 to April 2019 (Fig. 5a). The corresponding ages (days) estimated from statolith ring counts ranged from 73–154 days old. The agelength plot of all data points shows a reasonably good exponential curve fit (r2 = 0.6032) (Fig. 5b), with increasing scatter beginning at 110–120 days of age or above 9 cm ML.
Fig. 5.
(a) Size frequency distribution of the squids used for statolith analysis, (b) age-length plot of all data points.
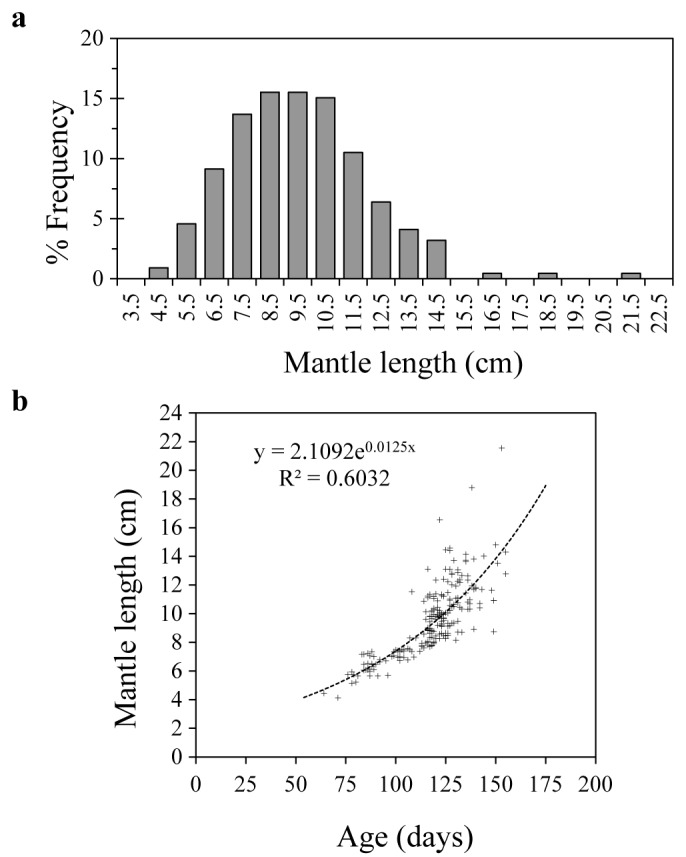
The plot of overall mean growth (mean size at 5-day age intervals) is shown in figure 6. Growth based on age-at-length estimates is clearly not asymptotic and is best represented by an exponential curve (r2 = 0.9446). Based on this model, the largest specimen observed in the study (28.1 cm ML) is just over 1 year old (380 days), whereas those caught by the trawl fishery (4 cm ML) are just below 2 months old (55 days), similar to the projected age using the VBG model above.
Fig. 6.
Overall mean growth with mean size at 5-day age intervals.
DISCUSSION
Using the progression of sizes to obtain a growth model (e.g., ELEFAN) is a common practice in estimating growth because size measurements are more readily available and cost much less to collect than determining ages from scales, otoliths, or statoliths. The premise that typical growth is initially rapid with a gradual slowing down, particularly towards the organism’s maximum age, is based on a balance between metabolic processes that require energy (anabolic) and those that produce energy (catabolic) as the organism ages. This is consistent with asymptotic growth models such as the VBG model. Other similar growth models include the Gompertz, logistic, and the more complex Schnute-Richards model (Flinn and Midway 2021). This VBG model has been used to characterize growth in a wide range of marine invertebrates and vertebrates (Palomares and Pauly 2008), including cephalopods, and has been used to estimate growth of the Indian squid in Arabia (Soomro et al. 2015), the coastal waters of India (Meiyappan and Srinath 1989; Vidyasagar and Deshmukh 1992; Karnik et al. 2003; Thomas and Kizhakudan 2006; John 2007; Chakraborty et al. 2013) and Egypt (Sabrah et al. 2015). However, studies on the same species that determined age-at-size based on statoliths for stocks in Taiwan (Chang et al. 2014) and in the Andaman Sea, Thailand (Sukramungkol et al. 2007) as well as in other loliginid species (Jackson et al. 1997; Gonzalez et al. 2010; Wang et al. 2010) reveal non-asymptotic growth.
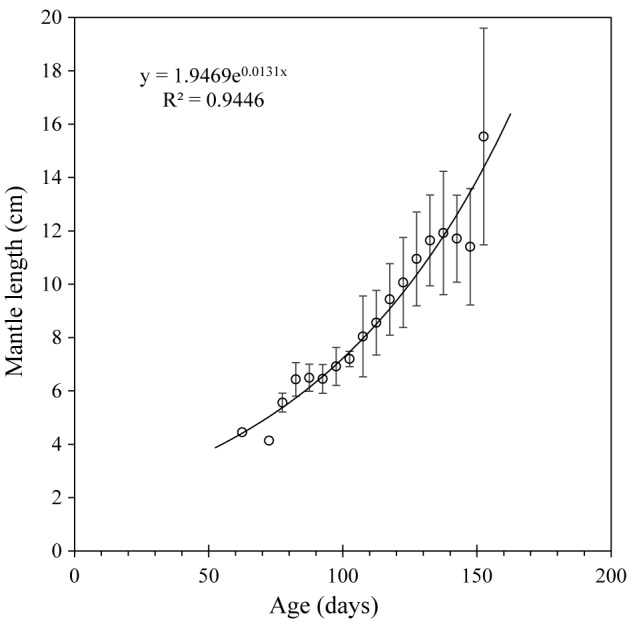
Age-at-size data based on statolith rings show that the Indian squid in the Visayan Sea grows exponentially (Fig. 5b). A comparison of growth curves obtained through the two methods (Fig. 7) shows that age estimates are similar for small individuals as they recruit to the trawl fishery (~ 4 cm DML), but the difference in ages increases with older individuals.
Fig. 7.
Comparison of growth curves obtained between length frequency analysis and age-at-size data.
Legaspi (2020) determined sizes at first maturity to be 9.8 cm ML in females and 12.3 cm ML in males. Using the length-based growth model, this size range corresponds to an age range of 0.5–0.65 years (6–8 months), whereas age-based growth gives a range of 0.3–0.4 years (4–5 months). It is noteworthy that it is also around this age (120–140 days) that growth somewhat stagnates (Fig. 6), consistent with shifting energy towards gonad development rather than somatic growth. The size at first maturity is about 16–20% of the length-based age of the largest recorded specimen in the study while using age data; this is 31–37% of the estimated age of the largest recorded specimen in the study. Since attaining 50% maturity within the 1st 1/3 of the stock’s approximate lifespan is more realistic for a short-lived year-round spawning species, the agebased model seems to represent growth in the Indian squid more accurately. In addition, the year-round occurrence of small squid (~4 cm DML) is consistent with continuous spawning of the stock within the year. Similar results on continuous spawning have also been noted in the same species in the Andaman Sea of Thailand (Sukramungkol et al. 2007) and in other loliginid species (e.g., Guerra and Rocha 1994), possibly to decrease the chances of recruitment failure (O'Dor 1998). Jackson and Molschaniwskyj (2001a) stated that short lifespan and protracted spawning throughout the year result in continuous recruitment for short tropical loliginids.
Length-based growth models of U. duvaucelii (Table 1) demonstrate that this species experiences a slow and asymptotic growth pattern. However, the present study, along with the extensive research on statolith growth increments (e.g., Jackson et al. 1997) as well as culture studies in loliginid squids (Yang et al. 1986), have confirmed that cephalopods typically exhibit rapid exponential growth. For these reasons, the exponential growth curve revealed by age-based growth is a more realistic representation of the growth of Indian squid in the Visayan Sea.
Significant progress has been achieved in the research of squid's life history, particularly the Loliginid species. However, there is currently limited information on the life cycle of U. duvaucelii in the Philippines. Thus, acquiring growth model information on this species is crucial for effective fisheries management and conservation. Here, we present a discussion of the inaccuracy of employing ELEFAN to determine the growth of U. duvaucelii based on its known biology.
U. duvaucelii is a fast-growing species known to have a short lifespan.The study conducted by Srichanngam (2010) demonstrates that the growth rates of male and female squids from the Gulf of Thailand and the Andaman Sea differ significantly. In the Gulf of Thailand, female growth was 1.044 mm/day on average, whereas that for males was 0.959 mm/day, with ages ranging from 61–153 days for sizes ranging from 3.5–16.0 cm ML). In contrast, females from the Andaman Sea grew at 0.706 mm/day, while males grew at 0.730 mm/day, with ages ranging from 76–270 days for sizes ranging from 5.8–23.2 cm ML). These reported growth rates of U. duvaucelii for both sexes are quite rapid, a common characteristic of tropical loliginid species (Jackson and Choat 1992). In the Visayan Sea, the similar range of sizes and ages of the species is consistent with similarly rapid growth, indicating a probable lifespan of 5–6 months.
The short lifespan of U. duvaucelii is consistent with those reported in many tropical loliginid squids, which have estimated lifespans of < 200 days, including Sepioteuthis lessoniana (Jackson 1990), Loligo chinensis (Jackson and Choat 1992; Chang et al. 2014) Lolliguncula brevis (Jackson et al. 1997), and Loliolus hardwickei (Sajikumar et al. 2019). This short longevity has been observed even in the ommastrephid squid, Sthenoteuthis oualaniensis, in the eastern tropical Pacific Ocean, which has a lifespan of < 6 months (Liu et al. 2016).
Figure 7 shows both length and age-based growth models. The length-based model shows that the species will take over two years to reach 28.1 cm ML, the maximum length recorded in this study. However, the statolith age-based model shows that the species has a much shorter lifespan of less than one year, consistent with what is considered typical for loliginid squids in tropical waters (Jackson 2004). From this, it is clear that the length-based model, commonly used to estimate the growth patterns of slow-growing, longlived animals (age in years) (Ricker 1979), is unrealistic in the case of short-lived species such as U. duvaucelii. This highlights the limitations of length-based models in determining growth and deriving lifespan of this species.
U. duvaucelii has been observed to have more than one cohort.This study confirmed that U. duvaucelii has more than one cohort, as demonstrated by the sizefrequency distributions of the specimens (as shown in Fig. 4). Cohorts collected at different times may experience varying environmental conditions, which can undoubtedly impact their growth rates (Forsythe and Hanlon 1989; Hatfield 2000; Forsythe 2004). Laboratory experiments have shown that temperature (Villanueva 2000) and feeding rates (Jackson and Moltschaniwskyj 2001b) are the primary factors that govern cephalopod growth rates. Moreover, squids in the wild exhibit high plasticity in growth (Pecl 2000), displaying greater variability in eproductive investment (Argüelles and Tafur 2010) and recruitment (Jackson et al. 2000)as a direct response to changes in environmental factors. This makes it hard to identify clear modes in length-frequency data (Jackson et al. 2000). As a result, the assessment made by ELEFAN may not be suitable for squids as it may not accurately capture the complexity of populations exhibiting multimodal size structures. Thus, the VBGF growth model is unsuitable for interpreting squids’ growth patterns.
U. duvaucelii might exhibit semelparous reproduction. It has been suggested that reproduction in squids is closely related to their growth pattern (Pecl 2001). According to Bian et al. (2022), the final stage of a cephalopod’s life is characterized by a rapid and severe aging process. In octopuses, this final stage is marked by a shift from the growth pattern observed during the exponential phase (Domain et al. 2000). It is worth noting that some species of squids exhibit a unique reproductive behavior called semelparity, where they reproduce only once and die shortly after (Ikeda et al. 1993; Rocha et al. 2001). Additionally, certain female octopus species tend to die after their eggs hatch, as observed by Anderson et al. (2002). These findings provide valuable insights into the complex life cycle of cephalopods. Rao (1988) studied the biology of U. duvaucelii and reported that this species exhibits semelparous reproduction. In the entire duration of the study, only nine out of 3143 individuals used in the length frequency data were found to have sizes ranging from 27 cm to 28 cm. The scarcity of large specimens in the study area suggests that they probably die shortly after spawning. Thus, this could potentially contribute to the Species’ lack of an asymptotic phase.
In addition, the squid’s growth rate is linked to its unique physiology, which encourages growth by continually producing new muscle fibers (hyperplasia) and increasing the size of the existing ones (hypertrophy) (Jackson and O’Dor 2001). This exceptional physiology has been observed in wild and laboratory-maintained squids (Moltschaniwskyj 1994; Pecl and Moltschaniwskyj 1999). Semmens et al. (2011) also found that hyperplasia plays a role in the growth of Octopus pallidus. Furthermore, the squid’s efficient use of protein-based metabolism and digestion (Jackson and O'Dor 2001), along with the structure of its body that doesn’t restrict oxygen intake, contribute to its continuous growth (Moltschawiskyj 2004). The results from the physiological investigations performed on squids highlight the paramount importance of understanding their biology and the factors that impact their growth when constructing growth models. Moreover, it is recommended that further research be conducted on the physiology of U. duvaucelii to fully understand the mechanism behind the growth of this tropical squid species.
CONCLUSIONS
Studying the growth of U. duvaucelii has been instrumental in expanding our knowledge and improving our understanding of the complex population structure of cephalopods. The use of length frequency to model cephalopod growth is a subject of current debate since there are still studies using this approach. The findings of our study showed that the Indian squid U. duvaucelii grows exponentially, suggesting a rapid growth and very short lifespan (< 200 days) and is consistent with what is known about the ‘live fast, die young” life strategy in squids (Forsythe and Van Heukelem 1987; Jackson and O’Dor 2001). Thus, along with other studies, this work suggests that statolith growth ring analysis is a reliable method to acquire a growth model for tropical squid species. Furthermore, in-depth investigations into individual growth variability are recommended to support the squid’s rapid and continual growth and how environmental factors influence these. The authors are still analysing these findings to obtain a deeper understanding of the life history of U. duvaucelii.
Acknowledgments
The authors would like to thank the following for funding this research: the Mollusk of Panay (MOLLPAN) Project under the DOSTPCAARRD NICER Program on Mollusks, the DOSTASTHRDP scholarship, University of the Philippines Visayas Office of the Vice Chancellor for Research and Extension (OVCRE) writing grant. We wish to thank all the staff and researchers of the OceanBio and Marine Bio labs of the College of Arts and Sciences, University of the Philippines Visayas, especially Karen Villarta-Lane, Alexanra Bagarinao-Regalado, Kris Angeli Sanchez, and Lucas Felix, whose assistance was a milestone in the completion of this study. We are grateful for the support of the Municipal Agricultural Office (MAO) and the Local Government Unit (LGU) of Concepcion, Iloilo, Philippines, our field assistants, and field enumerators.
Footnotes
Authors’ contributions: All authors made significant contributions to the implementation and completion of the study. JML collected and examined the squid samples and prepared the manuscript. WLC and AGC performed conceptualization and interpretation of data. All co-authors shared input regarding the preparation of the manuscript draft. The final manuscript was read and approved by all authors to be published.
Competing interests: The authors declare no competing interests.
Availability of data and materials: The corresponding author may provide the datasets used in this work upon request.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Anderson RC, Wood JB, Byrne RA. 2002. Octopus senescence: the beginning of the end. J Appl Anim Welf Sci 5:275–283. doi:10.1207/S15327604JAWS0504_02. [DOI] [PubMed]
- Argüelles J, Tafur R. 2010. New insights on the biology of the jumbo squid Dosidicus gigas in the Northern Humboldt Current System: Size at maturity, somatic and reproductive investment. Fish Res 106:185–192. doi:10.1016/j.fishres.2010.06.005.
- Arkhipkin AI. 2005. Statoliths as ‘black boxes’ (life recorders) in squids. Mar Freshw Res 56:576–583. doi:10.1071/MF04158.
- Arkhipkin AI, Shcherbich Z. 2012. Thirty years progress in age determination of squid using statoliths. J Mar Biol Assoc UK 92:1389–1398. doi:10.1017/S0025315411001585.
- Bian L, Li F, Chang Q, Liu C, Tan J, Zhang S, Li X, Li M, Sun Y, Xu R, Chen S. 2022. Comparison of behavior, histology and ImpL2 gene expression of Octopus sinensis under starvation and senescence conditions. Front Mar Sci 9: 874777. doi:10.3389/fmars.2022.874777.
- Bettencourt V, Coelho L, Andrade JP, Guerra A. 1996. Age and growth of the squid Loligo vulgaris off the south coast of Portugal, using statolith analysis. J Moll Stud 62: 359–366. doi:10.1093/mollus/62.3.359.
- Butler J, Fuller D, Yaremko M. 1999. Age and growth of market squid (Loligo opalescens) off California during 1998. CalCOFI Rep 40:191–195.
- Carpenter KE, Niem VH (eds). 1998. FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. Volume 2. Cephalopods, crustaceans, holothurians and sharks. Rome, FAO.
- Chakraborty SK, Biradar RS, Jaiswar AK, Palaniswamy R, Kumar P. 2013. Growth, mortality, and population parameters of three cephalopod species, Loligo duvaucelii (Orbigny), Sepia aculeata (Orbigny) and Sepiella inermis (Orbigny) from north-west coast of India. Indian J Fish 60:1–7.
- Chang K, Liao C, Huang H, Wu C, Wang K. 2014. Age and growth of Uroteuthis (Photololigo) chinensis, U. (P.) duvaucelii, and U. (P.) edulis from the waters around Taiwan. J Taiwan Fish Res 22:1–13.
- Clark MR. 1978. The cephalopod statolith –an introduction to its form. J Mar Biol Assoc UK 58: 701–712. doi:10.1017/S0025315400041345.
- del Norte-Campos AG, Beldia RA, Villarta KA, Tajolosa MAO. 2000. A market survey of commercially important invertebrates around Panay Island and use of data to prioritize research. UPV J Nat Sci 5:9–11.
- del Norte-Campos AG, Burgos LA, Villarta KA. 2019. A Ranked Inventory of Commercially-important Mollusks of Panay, West Central Philippines as a Guide to Prioritize Research. Philipp J Fish 26:114–131. doi:10.31398/tpjf/26.2.2019-0004.
- Domain F, Jouffre D, Caverivière A. 2000. Growth of Octopus vulgaris from tagging in Senegalese waters. J Mar Biol Assoc UK 80:699–706. doi:10.1017/S0025315400002526.
- Durholtz MD, Lipinski MR, Field JG. 2002. Laboratory validation of periodicity of incrementation in statoliths of the South African chokka squid Loligo vulgaris reynaudii (d'Orbigny, 1845): a reevaluation. J Exp Mar Biol Ecol 279: 41–59. doi:10.1016/S0022-0981(02)00364-7.
- Flinn SA, Midway SR. 2021. Trends in Growth Modeling in Fisheries Science. Fishes 6:1. doi:10.3390/fishes6010001.
- Forsythe JW. 2004. Accounting for the effect of temperature on squid growth in nature: from hypothesis to practice. Mar Freshw Res 55:331–339. doi:10.1071/MF03146.
- Forsythe JW, Hanlon RT. 1989. Growth of the Eastern Atlantic Squid, Loligo forbesi Steenstrup (Mollusca: Cephalopoda). Aquacul Fish Manag 20:1–14. doi:10.1111/j.1365-2109.1989.tb00437.x.
- Forsythe JW, Van Heukelum WF. 1987. Growth. In: Boyle PR (ed) Cephalopod life cycles, vol II. Comparative reviews. Academic Press London, UK.
- Gonzalez AF, Otero J, Pierce G, Guerra A. 2010. Age, growth, and mortality of Loligo vulgaris wild paralarvae: implications for understanding of the life cycle and longevity. ICES J Mar Sci 67:1119–1127. doi:10.1093/icesjms/fsq014.
- Green BS, Mapstone BD, Carlos GC, Begg GA (eds). 2009. Tropical Fish Otoliths: Information for Assessment, Management and Ecology. Springer Dordrecht Heidelberg London New York.
- Guerra A, Rocha F. 1994. The life history of Loligo vulgaris and Loligo forbesi (Cephalopoda: Loliginidae) in Galician waters (NW Spain). Fish Res 21:43–69. doi:10.1016/0165-7836(94)90095-7.
- Hatfield EMC. 2000. Do some like it hot? Temperature as a possible determinant of variability in growth of the Patagonian squid, Loligo gahi (Cephalopoda: Loliginidae) Fish Res 47:27–40. doi:10.1016/S0165-7836(99)00127-7.
- Ikeda Y, Sakurai Y, Shimazaki K. 1993. Maturation process of the Japanese common squid Todarodes pacificus in captivity. In: Okutani T, O'Dor RK, Kubodera T (eds). Recent advances in cephalopod fisheries biology : contributed papers to 1991 CIAC International Symposium and proceedings of the Workshop on Age, Growth and Population Structure.
- Jackson GD. 1989. The use of statolith microstructures to analyze life history events in the small tropical cephalopod Idiosepius pygmaeus. Fish Bull 87:265–272.
- Jackson GD. 1990. Age and growth of the tropical nearshore loliginid squid Sepioteuthis lessoniana determined from statolith growthring analysis. Fish Bull 88:113–118.
- Jackson GD. 2004. Advances in defining the life histories of myopsid squid. Mar Freshw Res 55:357–365. doi:10.1071/MF03152.
- Jackson GD, Alford R, Choat JH. 2000. Can length frequency analysis be used to determine squid growth? –An assessment of ELEFAN. ICES J Mar Sci 57:948–954. doi:10.1006/jmsc.2000.0582.
- Jackson GD, Choat JH. 1992. Growth in tropical cephalopods:an analysis based on statolith microstructure. Can J Fish Aquat Sci 49:218–228. doi:10.1139/f92-026.
- Jackson GD, Domeier ML. 2003. The effects of an extraordinary El Niño/La Niña event on the size and growth of the squid Loligo opalescens off Southern California. Mar Biol 142:925–935. doi:10.1007/s00227-002-1005-4.
- Jackson GD, Forsythe JW, Hixon RF, Hanlon RT. 1997. Age, growth, and maturation of Lolliguncula brevis (Cephalopoda: Loliginidae) in the northwestern Gulf of Mexico with a comparison of length-requency versus statolith age analysis. Can J Fish Aquat Sci 54:2907–2919. doi:10.1139/f97-192.
- Jackson GD, Moltschaniwskyj NA. 2001a. Temporal variation in growth rates and reproductive parameters in the small near–shore tropical squid, Loliulus noctiluca; is cooler better? Mar Ecol Prog Ser 218:167–177. doi:10.3354/meps218167.
- Jackson GD, Moltschaniwskyj NA. 2001b. The influence of ration level on growth and statolith increment width of the tropical squid Sepioteuthis lessoniana (Cephalopoda: Loliginidae): an experimental approach. Mar Biol 138: 819–825. doi:10.1007/s002270000496.
- Jackson GD, O'Dor RK. 2001. Time, space and the ecophysiology of squid growth, life in the fast lane. Vie et Milieu /Life & Environment 51:205–215. Available at: https://hal.sorbonneuniversite.fr/hal-03192511/document.
- Jereb P, Roper CFE (eds). 2010. Cephalopods of the World. An annotated and illustrated catalogue of cephalopod species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes. Rome, Italy.
- Jin Y, Li N, Chen X, Liu B, Li J. 2019. Comparative age and growth of Uroteuthis chinensis and Uroteuthis edulis from China Seas based on statolith. Aquac Fish 4: 166–172. doi:10.1016/j.aaf.2019.02.002.
- John M. 2007. Studies on some aspects of landings, utilization and export of commercially important cephalopods. PhD dissertation, Cochin University of Science and Technology, Kerala, India.
- Karnik NS, Chakraborty SK, Jaiswar AK, Swamy RP, Rajaprasad R, Boomireddy S, Rizvi AF. 2003. Growth and mortality of Indian squid, Loligo duvaucelii (d'Orbigny) (Mollusca/Cephalopoda/Teuthoidea) from Mumbai waters, India. Indian J Mar Sci 32:67–70.
- Kore BA, Joshi MC. 1975. Food of the squid Loligo duvauceli d'Orbigny. Proc Indian Acad Sci 81: 20–28. doi:10.1007/BF03050743.
- Legaspi JM. 2020. Gonad maturation in the Indian squid Uroteuthis duvaucelii (d'Orbigny, 1835) (Mollusca: Cephalopoda, Loliginidae) in Concepcion, Iloilo correlated with age and growth by statolith observation and length frequency analysis. Master’s thesis, University of the Philippines Visayas, Miagao, Iloilo, Philippines.
- Lipinski MR. 1986. Methods for the validation of squid age from statoliths. J Mar Biol Assoc UK 66: 505–526. doi:10.1017/S0025315400043095.
- Lipinski MR. 2001. Statoliths as archives of cephalopod life cycle: a search for universal rules. Folia Malacol 9:115–123. doi:10.12657/folmal.009.014.
- Liu B, Chen X, Chen Y, Tian S, Li J, Fang Z, Yang M. 2013. Age, maturation, and population structure of the Humboldt squid Dosidicus gigas off the Peruvian Exclusive Economic Zone. Chin J Oceanol Limnol 31:81–91. doi:10.1007/s00343-013-2036-z.
- Liu B, Chen X, Li JH, Chen Y. 2016. Age, growth, and maturation of Stenoteuthis oualaniensis in the eastern tropical Pacific Ocean by statolith analysis. Mar Freshw Res 67: 1973–1981. doi:10.1071/MF14427.
- Meiyappan MM, Srinath M. 1989. Growth and mortality of the Indian squid (Loligo duvaucelii) off Cochin, India. In: Venema SC, van Zalinge NP (eds) Contributions to tropical fish stock assessment in India: FAO/DANIDA/ICAR National Follow-up Training Course on Fish Stock Assessment, Cochin, India, 2–28 November 1987.
- Meiyappan MM, Srinath M, Nair KP, Rao KS, Sarvesan R, Syda Rao G, Mohamed K, Vidyasagar K, Sundaram KS, Lipton AP, Natarajan P, Radhakrishnan G, Narasimham KA, Balan K, Kripa V, Sathianandan TV. 1993. Stock assessment of the Indian squid Loligo duvaucelii Orbigny. Indian J Fish 40:74–84.
- Moltschaniwskyj NA. 1994. Muscle tissue growth and muscle fibre dynamics in the tropical loliginid squid Photololigo sp. (Cephalopoda: Loliginidae). Can J Fish Aquat Sci 51:830–835. doi:10.1139/f94-081.
- Moltschaniwskyj NA. 1995. Multiple spawning in the tropical squid Photololigo sp.: what is the cost in somatic growth? Mar Biol 124:127–135. doi:10.1007/BF00349154.
- Moltschaniwskyj NA. 2004. Understanding the process of growth in cephalopods. Mar Freshw Res 55: 379–386. doi:10.1071/MF03147.
- Nakamura Y, Sakurai Y. 1991. Validation of daily growth increments in statoliths of Japanese common squid Todarodes pacificus. Nippon Suisan Gakkaishi 57:2007–2011. doi:10.2331/suisan.57.2007.
- Natsukari Y, Nakanose T, Oda K. 1988. Age and growth of loliginid squid Photololigo edulis (Hoyle, 1885). J Exp Mar Biol Ecol 116:177–190. doi:10.1016/0022-0981(88)90054-8.
- O'Dor RK. 1998. Can understanding squid life-history strategies and recruitment improve management? S Afr J Mar Sci 20:193–206. doi:10.2989/025776198784126188.
- Palomares ML, Pauly D. 2008. Von Bertalanffy growth parameters of non-fish marine organisms. In: Fisheries Centre Research Reports. Fisheries Centre, University of British Columbia, Canada. Available at: https://oceans.ubc.ca/research/publica tions/research-reports/. Accessed 01 June 2023.
- Pecl GT. 2000. Comparative life history of tropical and temperate Sepioteuthis squids in Australian waters. PhD dissertation, James Cook University, Australia.
- Pecl GT. 2001. Flexible reproductive strategies in tropical and temperate Sepioteuthis squids. Mar Biol 138:93–101. doi:10.1007/s002270000452.
- Pecl GT, Moltschaniwskyj NA. 1999. Somatic growth processes:how are they altered in captivity? Proc R Soc B Biol Sci 266:1133. doi:10.1098/rspb.1999.0754.
- Philippine Statistics Authority (PSA). 2002–2021. Value of squid production in marine fisheries by region and province in Philippines. In: Fisheries Database. Available at: https://psa.gov. ph/content/fisheries-statistics-philippines. Accessed 24 June 2023.
- Punyadewa NBP, Wijayasinghe KLRC, Amarasinghe US. 2023. Population dynamics of two squid species, Uroteuthis duvaucelii (D'Orbigny, 1835) and U. singhalensis (Ortmann, 1891) (Family:Loliginidae) in the Trincomalee bay, Sri Lanka. Sri Lanka J Aquat Sci 28:29–41. doi:10.4038/sljas.v28i1.7605.
- Radtke RL. 1983. Chemical and structural characteristics of statoliths from the short-fined squid Illex illecebrosus. Mar Biol 76:47–54. doi:10.1007/BF00393054.
- Rao SG. 1988. Biology of inshore squid Loligo duvaucelii Orbigny, with a note on its fishery off Mangalore. Ind Journ Fish 35:121–130.
- Ricker WE. 1979. Growth rates and models. In: Hoar WS, Randall DJ, Brett JR (eds) Fish Physiology, vol. VIII. Bioenergetics and growth. Academic Press, New York, pp. 678–738.
- Rocha F, Guerra A, González AF. 2001. A review of the reproductive strategies in cephalopods. Biol Rev 76: 291–304. doi:10.1017/S1464793101005681. [DOI] [PubMed]
- Sabrah M, El-Sayed A, El-Ganiny A. 2015. Fishery and population characteristics of the Indian squids Loligo duvaucelii Orbigny, 1848 from trawl survey along the north-west Red Sea. The Egypt J Aquat Res 41:279–285. doi:10.1016/j.ejar.2015.07.003.
- Sajikumar KK, Sasikumar G, Mohan G, Kripa V, Alloycious PS, Mohamed KS. 2019. Age and growth of the little Indian squid, Loliolus hardwickei (Gray, 1849) in the Arabian Sea. J Mar Biolog Assoc UK 99:1–5. doi:10.1017/S0025315419000560.
- Semmens JM, Doubleday Z, Hoyle K, Pecl GT. 2011. A multilevel approach to examining cephalopod growth using Octopus pallidus as a model. J Exp Biol 214: 2799–2807. doi:10.1242/jeb.051631. [DOI] [PubMed]
- Semmens JM, Pecl GT, Villanueva R, Jouffre D, Sobrino I, Wood JB, Rigby JB. 2004. Understanding octopus growth: patterns, variability, and physiology. Mar Freshw Res 55:367–377. doi:10.1071/MF03155.
- Soomro SH, Liu Q, Kalhoro MA, Memon KH, Zhang K, Liao B. 2015. Growth and mortality parameters of Indian squid Uroteuthis (Photololigo) duvaucelii (d'Orbigny, 1835) from Pakistani waters (Arabian Sea) based on length frequency data. Indian J Geo-Mar Sci 44:1598–1603.
- Srichanngam S. 2010. Age and growth determination and stock identification using statolith microstructure of Indian squid, Loligo duvaucelii. Master’s thesis. University of Bergen.
- Sukramongkol N, Tsuchiya K, Segawa S. 2007. Age and maturation of Loligo duvaucelii and L. chinensis from Andaman Sea of Thailand. Rev Fish Biol Fisheries 17: 237–246. doi:10.1007/s11160-006-9033-7.
- Tajolosa MAO. 2011. Population and reproductive biology of the Indian Squid, Photololigo duvaucelii D’Orbigny 1835 (Cephalopoda: Loliginidae) in the Visayan Sea, Philippines. Master’s thesis. University of the Philippines Visayas.
- Tehseen P, Desai A, Saroj J, Arti J. 2019. Feeding biology and lengthweight relationship of Indian Squid (Uroteuthis duvaucelii) in coastal waters of Gujarat. J Exp Zool India 22:609–661.
- Thomas S, Kizhakudan SJ. 2006. Cephalopod fishery and population dynamics of Loligo duvaucelii (Orbigny) off Saurashtra region, Gujrat. Indian J Fish 53:425–430.
- Vidyasagar K, Deshmukh VD. 1992. Stock assessment of Loligo duvaucelii (d'Orbigny) in Bombay waters. J Mar Biol Assoc India 34:14–17.
- Villanueva R. 2000. Effect of temperature on statolith growth of the European squid Loligo vulgaris during early life. Mar Biol 136:449–460. doi:10.1007/s002270050704.
- Wang KY, Lee KT, Liao CH. 2010. Age, growth, and maturation of swordtip squid (Photololigo edulis) in the Southern East China Sea. J Mar Sci Technol 18: 99–105. doi:10.51400/2709 6998.1870.
- Yang WT, Hixon RF, Turk PE, Krejci ME, Hulet WH, Hanlon RT. 1986. Growth, behavior, and sexual maturation of the market squid, Loligo opalescens, cultured through the life cycle. Fish Bull 84:771–798.