Abstract
Dural arteriovenous fistulas (DAVFs) are rare but significant intracranial vascular malformations that are usually idiopathic and can lead to severe complications like venous hypertension and intracranial hemorrhage. We present 2 cases of DAVF occurring after venous sinus stenting (VSS) for presumed idiopathic intracranial hypertension (IIH) in a 51-year-old and a 52-year-old female. In both patients, Cognard type 1 DAVF was detected by Catheter angiography and successfully obliterated with Onyx™ embolization. These cases suggest that VSS may either unmask pre-existing DAVFs by altering intracranial pressure dynamics or induce new fistula formation by angiogenic factors or stent-induced hemodynamic changes. Clinicians should maintain an index of suspicion for DAVF in patients with persistent symptoms post-VSS and catheter angiography might be needed to detect the DAVFs.
Keywords: Dural arteriovenous fistula, Venous sinus stent, Idiopathic intracranial hypertension, Case report
Introduction
Dural arteriovenous fistulas (DAVFs) represent 10-15% of intracranial vascular malformations and are abnormal shunts between the meningeal arteries and the intracranial venous system [1]. Patients with DAVFs often present with tinnitus, headache, and dizziness. DAVFs can potentially cause serious complications such as venous hypertension and intracranial hemorrhage if retrograde cortical venous drainage is present. The exact etiology of DAVFs is unclear; although causes include trauma, inflammation, or sinus thrombosis, most cases are considered idiopathic [2]. We present 2 unusual cases of DAVF following venous sinus stenting (VSS) for presumed idiopathic intracranial hypertension (IIH) and review the English language literature on this rare complication.
Case report
A 52-year-old white female presented with increasing headaches and blurred vision. Her past medical history was notable for migraines and hypertension. She had rheumatoid arthritis and a prior deep vein thrombosis (DVT) with a pulmonary embolism (PE) and nephrolithiasis with secondary hydronephrosis. She was a nonsmoker with a body mass index (BMI) of 31. Her medications included fremanezumab, ubrogepant, hydrochlorothiazide, valsartan, clonidine, rivaroxaban, tizanidine, clonazepam, methotrexate, promethazine, and pramipexole.
A neuro-ophthalmic exam showed visual acuity of 20/25 in both eyes (OU). Pupils were isocoric without relative afferent pupillary defect. Color testing with Ishihara color plates was 3/14 in the right eye (OD) and 1/14 in the left eye (OS). Dilated ophthalmoscopy showed Frisén grade 2 optic disc edema OU. Automated perimetry (Humphrey Visual Field [HVF] 24-2 testing) was normal OU. Optical coherence tomography (OCT) of the peripapillary retinal nerve fiber layer (RNFL) revealed a global thickness of 79 µm OD and 77 µm OS. Magnetic resonance imaging (MRI) of the brain and orbit with and without contrast demonstrated flattening of the globes, partial empty sella, and stenosis but no thrombosis of the distal left transverse sinus. MR venography (MRV) confirmed the narrowing of the left transverse and sigmoid sinuses. Lumbar puncture opening pressure was 34 cm H2O, with unremarkable cerebrospinal fluid (CSF) analysis. Catheter angiogram and venous sinus manometry showed a pressure gradient of 5 mm Hg across the stenosis without evidence of a DAVF or AV malformation.
The patient was diagnosed with IIH by modified Dandy criteria and started on acetazolamide and topiramate. Due to worsening symptoms despite maximal medical therapy, she underwent venous sinus stenting (VSS) on the left transverse sinus and proximal sigmoid sinus (Fig. 1) using both a Nickel-Titanium (Precise™) and a cobalt-chromium alloy (Wallstent™) stent 4 months after her initial presentation. At that time, an external carotid angiogram was negative for DAVF. Four months after VSS, the patient reported new left-sided headaches, memory loss, tinnitus, and hearing loss described as “people talking underwater”. Ophthalmologic examination revealed no disc swelling, normal HVF, and stable OCT. Computed tomography (CT) and MRI of the head were unremarkable. Cerebral Angiogram revealed a Cognard type 1 left DAVF. Liquid (Onyx™) embolization obliterated the DAVF successfully, maintaining patent antegrade flow through bilateral transverse sinuses (Fig. 2).
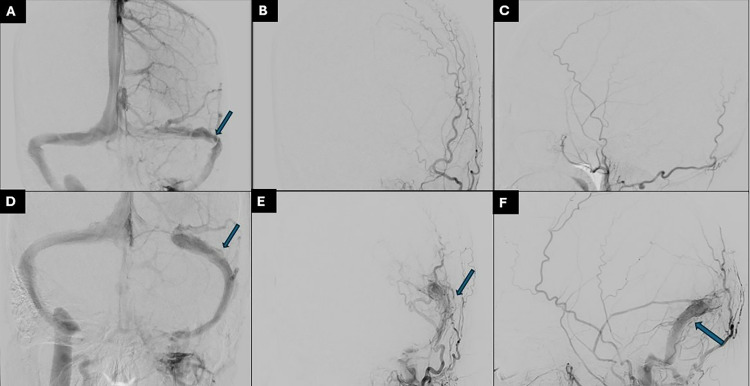
Fig. 1.
(A) Venous phase of the LICA injection showing stenosis of the distal left transverse sinus, (B) and (C) prestenting left ECA show no evidence of DAVF. (D) post stenting of the left transverse-sigmoid sinus. (E) and (F) follow-up External Carotid angiogram shows the interval development of transverse sigmoid sinus dural fistula.
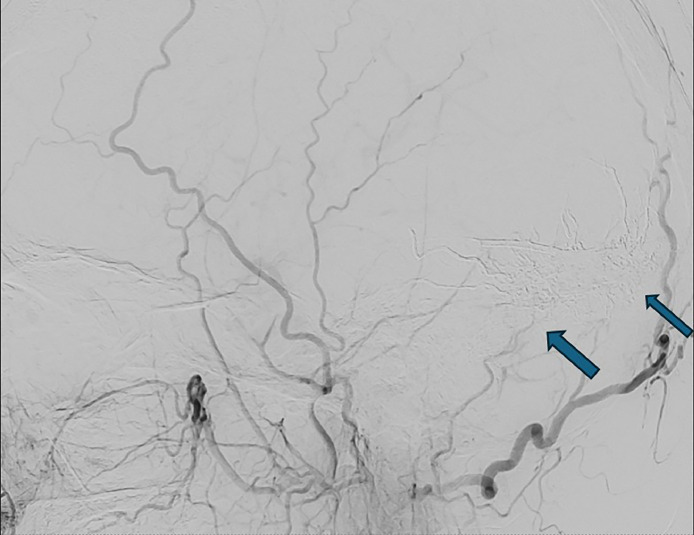
Fig. 2.
Complete occlusion of the fistula using Onyx embolization material.
The second case was a 51-year-old white female, with a medical history significant for migraine, depression, and prediabetes, and no significant family or surgical history. She presented with a prolonged history of right-sided whooshing tinnitus. A cerebral angiogram revealed stenosis at the right transverse-sigmoid sinus junction with an 8 mmHg pressure gradient, alongside a high-riding jugular bulb. Endovascular treatment was planned, leading to the successful placement of 2 wall stents at the right transverse-sigmoid sinus junction, with complete resolution of the stenosis (Fig. 3).
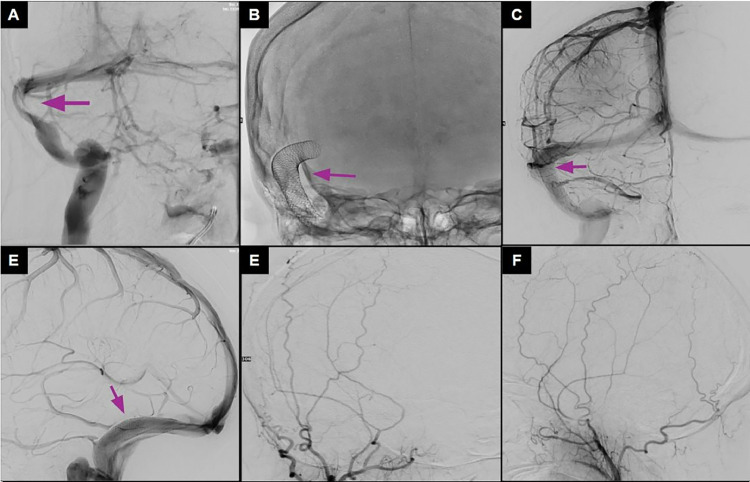
Fig. 3.
Cerebral arteriogram, pre- and poststenting. (A) Stenosis of the right transverse sinus. (B) Wall stent placement in the right transverse-sigmoid sinus. (C, D) Venous phase poststenting, demonstrating recanalization of the stenosis. (E) (F) Anteroposterior and lateral views of the external carotid artery injection showing no Dural fistula on the initial angiogram.
Four months later, she presented to the emergency department with a week-long intermittent right-sided headache, radiating to the back of the head, and not relieved by over-the-counter painkillers. A follow-up cerebral angiogram showed the development of type 1 Cognard classification right transverse-sigmoid sinus dural fistula, primarily supplied by the right middle meningeal artery and right occipital artery, with no reflux into the cortical venous system. Liquid (Onyx™) embolization resulted in full resolution of the fistula (Fig. 4).
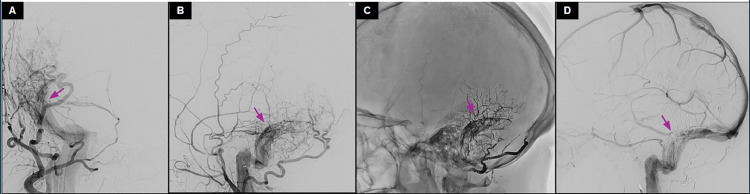
Fig. 4.
Cerebral arteriogram 4 months after venous stenting. (A, B) Right external injection showing Type 1 transverse sinus Dural AVF (DAVF) supplied by right occipital and right middle meningeal artery. (C, D) Lateral view after embolization showing Onyx embolization material with resolution of the Dural AVF (DAVF).
Discussion
DAVFs have been documented with venous congestion, infection, tumors, hormonal imbalance, hypercoagulable states, and arterial dysplasia. These cranial pathologies contribute to chronic hypoperfusion and dural hypoxia which stimulate angiogenesis. The cardiac literature has documented increased vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) after coronary artery stents [3]. Although increased VEGF and PDGF have been documented in the local tissue following VSS [2], the degree of increase is unclear. We propose that a similar angiogenic mechanism could promote DAVF pathogenesis after VSS. Other proposed mechanisms suggest that the stent (foreign body) may have induced transient cortical vein occlusion and subsequent DAVF formation [4], while another case of DAVF after a carotid artery–cavernous sinus stent attributed stent-dependent hemodynamic alterations to have promoted fistula revascularization via small branches of the meningeal supply [5]. In this case, we hypothesize that the increased ICP could have been acting as a tamponade, and paradoxically lowering the ICP may have contributed to the unmasking of the DAVF.
Table 1 summarizes 4 cases of DAVF after stenting. The patients, all females aged 22 to 51 years old, developed DAVFs 3 to 4 months after stenting. Common symptoms included progressive tinnitus, headaches, and visual disturbances. Branches of the external carotid artery typically fed the DAVFs. Management strategies varied, with most cases treated via embolization, although one case was managed conservatively.
Table 1.
Published cases of Dural arteriovenous fistulas (DAVF) after venous sinus stenting (VSS).
| Case report | Year | Patient demographics (age, gender) | Anatomic disruption and VSS location | Time to DAVF diagnosis poststenting | Clinical presentation | Feeding blood supply | DAVF location ± characteristics | Management |
|---|---|---|---|---|---|---|---|---|
| Ellens et al. [6] | 2024 | 30s, female, obese | Right transverse-sigmoid sinus stenosis | 3 months | Progressive tinnitus | Transverse-sigmoid sinus | Borden type 1 DAVF along the stent. | Arterial embolization with venous remodeling using a Copernic RC™ balloon. |
| Dalla et al [7] | 2023 | 50, female | Nondominant left transverse sinus with bilateral transverse sinus stenosis | 3 months | Left progressive bruit | Left external carotid artery | Borden and Cognard type 1 DAVF | Embolization with Onyx™ via arterial and venous approaches |
| Li et al. [8] | 2018 | 51, female, BMI 25 | Right-dominant transverse sinus stenosis | Unknown | Right pulsatile tinnitus | Meningeal branches of right external carotid artery | Borden type 1 DAVF | Partial embolization with Onyx™ via right occipital artery |
| Buell et al. [4] | 2017 | 22, female, BMI 32 | Left transverse sinus stenosis, dominant right transverse sinus, and the right transverse sinus–sigmoid sinus junction | 4 months | Worsening headaches, pulsatile tinnitus, and peripheral vision loss | Branches of the right meningohypophyseal and inferolateral trunks | Borden type 1 DAVF | Managed conservatively |
Our cases raise the question of whether VSS and the resulting altered venous flow could have unmasked the pre-existing DAVF in this patient. In such cases, presumably elevated intracranial pressure acts to tamponade the DAVF. Alternatively, VSS could have triggered the release of angiogenic factors contributing to the development of a de novo DAVF. Clinicians should maintain an index of suspicion for DAVF in patients with persistent or worsening symptoms following VSS. Catheter angiography may be necessary to document the DAVF, confirm retrograde or anterograde flow, and the presence or absence of cortical venous drainage.
The presence of higher-grade DAVF, symptomatic cerebral venous hypertension, or retrograde high-flow cortical venous drainage may be indications for endovascular embolization to prevent potentially life-threatening complications. Radiosurgery is typically used for low-grade (type I Borden) DAVFs with persistent symptoms, such as pulsatile tinnitus in our 2 cases. It can also be considered for DAVFs with patients with significant medical comorbidities, in cases where the anatomy is unsuitable for other interventions, or as a salvage treatment for lesions that remain incompletely treated after endovascular embolization [9,10].
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Patient consent
This report does not contain any personal identifying information and consent was obtained from the patients.
Footnotes
Publication Originality Statement: We confirm this publication is original.
Competing Interests: None.
Acknowledgments: No funding or grant support.
References
- 1.Ahn JY, Kim OJ, Joo YJ, Joo JY. Dural arteriovenous malformation occurring after craniotomy for pial arteriovenous malformation. J Clin Neurosci. 2003;10:134–136. doi: 10.1016/s0967-5868(02)00109-1. [DOI] [PubMed] [Google Scholar]
- 2.S Miyachi E, Izumi T, Matsubara N, Naito T, Haraguchi K, Wakabayashi T. Mechanism of the formation of dural arteriovenous fistula: the role of the emissary vein. Interv Neuroradiol. 2011;17(2):195–202. doi: 10.1177/159101991101700209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bräsen JH, Kivelä A, Röser K, Rissanen TT, Niemi M, Luft FC, et al. vascular endothelial growth factor and platelet-derived growth factor-BB expression, iron deposition, and oxidation-specific epitopes in stented human coronary arteries. Arterioscler Thromb Vasc Biol. 2001;21(11):1720–1726. doi: 10.1161/hq1101.098230. [DOI] [PubMed] [Google Scholar]
- 4.Buell TJ, Raper DM, Ding D, Chen CJ, Liu KC. Development of an intracranial Dural arteriovenous fistula after venous sinus stenting for idiopathic intracranial hypertension. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-013282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lv XL, Li YX, Liu AH, Lv M, Jiang P, Zhang JB, et al. A complex cavernous sinus Dural arteriovenous fistula secondary to covered stent placement for a traumatic carotid artery-cavernous sinus fistula: case report. J Neurosurg. 2008;108(3):588–590. doi: 10.3171/JNS/2008/108/3/0588. [DOI] [PubMed] [Google Scholar]
- 6.Ellens N., Singh A.P., Santangelo G., Bender M.T. Dural arteriovenous fistula embolisation with venous remodelling following venous sinus stenting. BMJ Case Reports CP. 2024;17(1) doi: 10.1136/bcr-2023-256869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalla S, Richards L, Reddy SV. Dural arteriovenous fistula associated with dural sinus stent placement for idiopathic intracranial hypertension. J Vasc Interv Radiol. 2024;35(3):477–479. doi: 10.1016/j.jvir.2023.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Li K., Ren M., Meng R., Wang F., Ji X. Dural arteriovenous fistula formation complicated cerebral venous sinus stenosis after venous sinus stenting. World Neurosurgery. 2018;120:400–402. doi: 10.1016/j.wneu.2018.08.230. [DOI] [PubMed] [Google Scholar]
- 9.Vanlandingham M, Fox B, Hoit D, Elijovich L, Arthur AS. Endovascular treatment of intracranial Dural arteriovenous fistulas. Neurosurgery. 2014;74(Suppl 1):S42–S49. doi: 10.1227/NEU.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 10.Chen CJ, Buell TJ, Diamond J, Ding D, Kumar JS, Taylor DG, et al. Stereotactic radiosurgery for high-grade intracranial Dural arteriovenous fistulas. World Neurosurg. 2018;116:e640–e648. doi: 10.1016/j.wneu.2018.05.062. [DOI] [PubMed] [Google Scholar]