Abstract
Background
Facet synovial cysts (FSCs) are benign, extradural outpouchings arising from the facet joint that can cause radiculopathy. Effectiveness of CT-guided indirect percutaneous cyst rupture (IPCR) alone and direct fenestration (DF) treatment alone have previously been reported in large cohorts. We performed a retrospective review of all FSCs treated under CT-guidance at a single institution where patients underwent IPCR, and IPCR followed by DF if necessary. We hypothesized that CT-guided FSC rupture would demonstrate similar effectiveness to previously reported fluoroscopic-guided methods, with potential improvement due to the opportunity to employ the DF technique in cases of IPCR failure.
Methods
A search was conducted of all CT-guided FSC rupture procedures over 10 years. Data included demographics, needle gauge used for IPCR and DF, rupture success, cyst size and T2 intensity, presence of spinal hardware, and cyst location. Subsequent surgery at the level of the cyst was documented.
Results
90 FSC rupture attempts were performed on 75 patients (28 M/47 F). FSC rupture using IPCR had a 70.0% success rate. In 22 FSC rupture attempts, IPCR failed and was followed by DF, with a success rate of combined IPCR + DF of 90.6 %. Subsequent surgery was required for 36.0% of patients involving the same level as the cyst or cysts.
Conclusion
Rates of successful FSC rupture under CT-guidance increased when the indirect rupture technique could be followed by direct fenestration in cases of failure. Our findings emphasize the benefits of flexibility afforded to the operator with CT-guidance.
1. Introduction
Facet synovial cysts (FSCs) are benign, extradural, fluid-filled outpouchings that arise from synovium in the setting of chronic spinal facet motion and degenerative facet arthropathy, most commonly at the L4/5 level [1,2]. When present, FSCs can impinge upon the intradural neural elements, as well as exiting and transiting nerve roots, which may manifest as intractable back pain, radiculopathies, neurogenic claudication, and, in rare cases, cauda equina syndrome [1].
Treatment options for patients with FSCs generally include non-operative medical management, surgical excision, indirect percutaneous cyst rupture (IPCR) via the facet joint itself (Fig. 1), or direct fenestration (DF) (Fig. 2) [2,3]. IPCR has been found to achieve statistically and clinically significant pain relief in patients [4]. Some imaging markers, specifically inherent T2 signal intensity of FSCs, have been found to directly correlate with success of percutaneous rupture, i.e. high and intermediate signal intensity cysts are significantly easier to rupture than low signal intensity cysts, although the reason for this is not entirely clear [5].
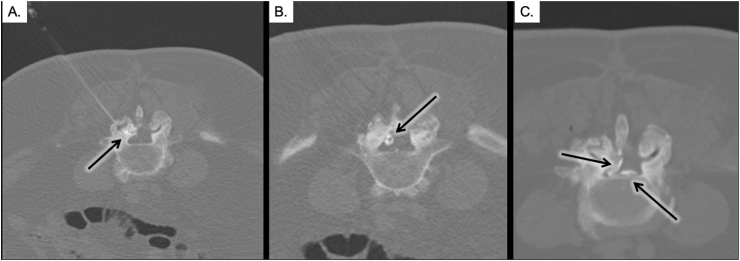
Fig. 1.
The indirect percutaneous cyst rupture technique as shown in three sequential procedural CT axial images. Image A demonstrates placement of a spinal needle into the posterior aspect of the right facet joint, with injection of contrast to fill the joint space (arrow). Image B demonstrates contrast extending from the joint and filling the FSC (arrow). Image C demonstrates extravasation of contrast into the right lateral and ventral aspects of the epidural space after successful FSC rupture (arrows).
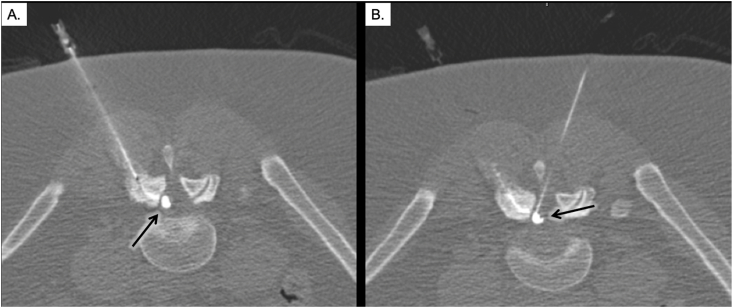
Fig. 2.
Failure of indirect percutaneous cyst rupture followed by successful rupture by direct fenestration. Image A demonstrates indirect filling of the FSC via filling of the right facet joint (arrow). There was failure of rupture after multiple attempts at pressurizing the cyst indirectly. Image B demonstrates direct fenestration of the cyst. This caused subsequent spillage of contrast into the epidural space (early extravasation noted by arrow).
Access of the facet joint under image guidance may be performed using either fluoroscopy or computed tomography (CT). Use of fluoroscopy provides real-time assessment of needle trajectory in the anteroposterior and lateral planes and can also minimize the radiation dose to the patient relative to CT [6]. Fluoroscopically-guided procedures use the IPCR method to treat FSCs, accessing the facet joint from a posterior approach, confirming intra-articular needle placement by contrast injection, and using high pressure to indirectly rupture the cyst [4,7]. FSCs treated with IPCR under fluoroscopic guidance have been reported to be successful in up to 81% of cases [8], with an overall success rate of 55.8% as shown in a meta-analysis of 29 studies [9]. A potential downside of using fluoroscopic technique, however, is that it only shows a two-dimensional evaluation of contrast spread into the epidural space to confirm rupture [10,11], which can be difficult to differentiate from the retrodural space of Okada [12]. On the other hand, intervention with CT, albeit a more limited procedural resource, can be instrumental for accessing joints in patients who may have anatomical variations or large osteophytes which complicate facet joint access [6]. Under CT-guidance, FSCs can be treated using the less invasive IPCR method as in fluoroscopy, as well as the more invasive DF technique via an interlaminar approach to directly aspirate and/or rupture the cyst [2]. The advantages of CT include [1]: accurate visualization of contrast extravasation into the epidural space to confirm successful cyst rupture (Fig. 1) [13], and [2] the ability to use a DF technique in the case of IPCR failure (Fig. 2).
The effectiveness of CT-guided IPCR FSC treatment alone and DF FSC treatment alone have previously been reported in large cohorts. In our study, we performed a retrospective review of all FSCs treated under CT-guidance at a single institution where patients underwent IPCR, DF, or a combination of techniques over the last ten years. We hypothesized that CT-guided IPCR would be similarly effective when compared to previously reported fluoroscopic-guided methods in the literature, with potentially improved effectiveness due to the opportunity to employ the DF technique in cases of IPCR failure. We investigated the patient demographics, pre-procedure FSC imaging features, and procedure techniques in order to determine if any of these were associated with successful cyst rupture or the need for subsequent surgical intervention.
2. Materials and methods
This exploratory retrospective, single-arm cohort/case series was conducted under an institutional review board-approved protocol, and informed consent was waived.
2.1. Subjects
We conducted a search in the Nuance mPower (Montage, Burlington, Massachusetts) database for all CT-guided facet synovial cyst rupture procedures performed within the last ten years, August 2012 - August 2022. Search terms included ‘facet AND synovial AND cyst AND needle AND rupture.’ Results were filtered by Exam description, including ‘CT guidance needle placement’ and ‘CT guided cyst aspiration’ and ‘CT guided needle biopsy’. Data for each case, including accession number, patient sex, patient age, and report text, were exported into Microsoft Excel Spreadsheet (Microsoft Corporation, Redmond, Washington).
Next, a search was conducted in the Picture Archiving and Communication System (PACS) using the accession numbers to record corresponding medical record numbers, demographic information, level of intervention, presence of spinal hardware, needle gauge used, successful indirect rupture (which was determined by contrast extravasating into the ventral or dorsal epidural fat pad or into the foramina), and follow-up direct fenestration and rupture attempt, as applicable. Additionally, pre- and post-procedure pain scores by numeric rating system were recorded as available. For each patient, the most recent pre-intervention lumbar MR images were accessed to record cyst size in greatest biaxial dimension, cyst location, and cyst T2 intensity. Chart review was performed on each patient to assess rates of subsequent surgery at the same level of prior intervention during the study period.
2.2. Procedural technique
All cases were performed by one of three operators with a range of 2–15 years of procedural experience during the 10-year search period and used techniques established by the practice. Pre-procedure MR imaging was reviewed for each patient to determine precise location of each FSC. Potential risks of the procedure were discussed with the patient, including but not limited to bleeding, infection, or injury to surrounding blood vessels, nerves, or the spinal cord. Each patient was positioned prone on the table, and the overlying skin was prepped and draped in the usual sterile fashion and anesthetized locally with 1–2% lidocaine. Conscious sedation was achieved with IV Versed and Fentanyl and titrated to effect under continuous physiologic monitoring.
All FSC ruptures were first attempted using the less invasive IPCR approach prior to consideration of using the more invasive DF approach. Under CT-guidance, either a 17-, 18-, 20-, or 22-gauge needle (as deemed by physician by patient anatomic factors) was placed with its tip in the posterior aspect of the facet joint ipsilateral to the FSC. Contrast was injected into the facet joint in order to visualize the filling of the synovial cyst under CT. Once visualized, a mixture of 80 mg Depo-Medrol, 1 cc of 0.5 % Bupivacaine, and 1 cc of normal saline was forcefully injected into the facet joint to attempt indirect rupture of the synovial cyst and bathe the epidural space at this level. Cyst rupture via this indirect technique was confirmed by visualization of contrast extravasating into the epidural space, after previously being contained within the cyst. Upon procedure completion, the needle was removed, and a sterile dressing applied to the area. If no contrast was seen extravasating into the epidural space, additional indirect attempts were performed using preservative-free normal saline to achieve FSC rupture.
If multiple (i.e. at least 3) subsequent IPCR attempts with normal saline failed, the more invasive DF technique was performed as feasible via an interlaminar approach. Under CT-guidance, a 17-, 18-, 20-, or 22-gauge spinal needle was placed using either [1] an ipsilateral interlaminar approach or [2] a contralateral interlaminar approach in order to access the FSC. Direct fenestration of the cyst was performed, and preservative-free normal saline was injected into the cyst to induce a direct rupture. Subsequent CT images through the intervention site were attained in order to confirm contrast extending from the cyst into the epidural space, demonstrating a successful cyst rupture.
2.3. Statistical analysis
Analyses were performed to evaluate imaging and procedural variables and their effect on the following outcomes: success of IPCR, success of IPCR followed by DF, and necessity for subsequent surgery at the level of FSC. Successful IPCR or rupture via DF was defined by visualization of contrast extravasation through the epidural space. Statistical analysis was performed using the Mann-Whitney test for non-parametric variables, Student t-test for parametric continuous variables, and Fisher Exact test for categorical variables. The variables analyzed were as follows: sex, age, hardware, needle gauge, cyst size in greatest bi-axial dimension, and cyst T2 intensity, all of which were chosen to replicate studies that have preceded ours, in order to determine the relationship between each of these variables and successful cyst rupture at our institution. The change in numeric rating scale between pre- and post-procedure was correlated with procedure success, both in the IPCR and IPCR + DF groups.
The T2 intensity of FSCs was evaluated by a neuroradiology attending with 9 years of experience (BW) and neuroradiology fellow with one year of experience (AH). They assigned categories of T2 signal per the methods originally developed by Cambron et al. [5]. Weighted Cohen's Kappa Coefficient was used to report the reliability of the two radiologists [14].
For analyses of predictor variables and their association with outcomes, a total of 75 patients consisting of 90 FSC rupture attempts/90 cases were analyzed. Three procedures involved bilateral synovial cyst ruptures. Patients' sex and hardware status did not change between procedures (n = 75). Patients’ age, needle gauge, cyst size, and cyst intensity changed between total cases (n = 90). Three procedures used the same MR findings, and as such, T2 cyst intensity and size were excluded from outcome calculations for these procedures (n = 87). Data Analyses were generated using STATA version 17 statistical software [StataCorp. College Station, TX].
3. Results
3.1. Subjects
Ninety cases were yielded from our search. Three cases had no intervention performed due to no cyst filling with contrast during the procedure (one cyst previously seen on MRI not found to communicate with the facet joint, one case found to represent facet hypertrophy on the CT planning scan prior to intervention instead of a cyst, and one case demonstrating a vascular structure rather than a cyst). However, we included these cases as they would have likely undergone intervention in the fluoroscopic setting. The 90 FSC attempted ruptures remaining for review were performed on 75 patients (28 males with an average 62.3 years of age; 47 females with an average 61.1 years of age) under CT-guidance. Three cases involved bilateral FSCs (3.2%), with the remainder being unilateral (96.8%). Eleven patients required repeated FSC interventions [1]: 9 were treated twice [2], 1 was treated 3 times, and [3] 1 treated 4 times in total. This resulted in a total of 90 FSCs being treated in 90 cases involving 75 patients (Supplemental Table). There were no procedural complications.
3.2. Inter-rater reliability
An inter-rater reliability analysis of cyst T2 signal intensity showed Kw = 0.65, which falls in the substantial level of agreement [15]. The agreement values were defined as follows: ≤0 poor, 0.01–0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81–1.00 almost perfect.
3.3. Overall rates of successful cyst rupture
63/90 (70.0%) FSCs were successfully treated using the IPCR method alone. In 27 FSCs the IPCR method did not result in cyst rupture. In 22 of these FSCs the failed rupture was subsequently followed by DF, which was successful in 14/22 FSCs (63.6%). In 5 of these FSCs, DF was not attempted due to anatomy barriers (no interlaminar window). The overall success rate for FSC rupture using IPCR alone was 70.0% (63/90 FSCs), and the success rate of combined IPCR +DF was 90.6% in FSCs where both techniques were attempted (77/85 FSCs, as 5 FSCs never had a DF attempted).
Twenty-seven out of 75 patients required subsequent surgery involving the same level as the cyst or cysts (36.0%). The average amount of time after the attempted percutaneous cyst rupture to subsequent surgery was 10.21 (standard deviation±15.23) months.
3.4. Association of age, sex, and presence of surgical hardware with treatment outcomes
Neither age nor sex were found to be associated with success of IPCR alone, IPCR +DF, or subsequent need for surgery (Table 1, Table 2).
Table 1.
Patient-level characteristics and outcomes. (n = 75).
| Overall Success |
Success IPCR alone |
Subsequent surgery |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | p-value | No | Yes | p-value | No | Yes | p-value | ||
| Age mean (SD), y | 61.52 (10.7) | |||||||||
| Female | 61.06 (10.7) | |||||||||
| Male | 62.28 (10.8) | |||||||||
| Sex no. (%) | ||||||||||
| Female | 47 (62.6) | 10 (71.4) | 37 (60.6) | .45 | 19 (731) | 28 (57.2) | .17 | 32 (66.6) | 15 (55.5) | .34 |
| Male | 28 (37.3) | 4 (28.5) | 24 (39.3) | 7 (27) | 21 (42.8) | 16 (33.3) | 12 (44.4) | |||
| Hardware no. (%) | ||||||||||
| No | 64 (85.3) | 12 (85.7) | 52 (85.2) | .96 | 24 (92.3) | 40 (81.6) | .21 | 41 (85.4) | 23 (85.2) | .97 |
| Yes | 11 (14.7) | 2 (14.3) | 9 (14.8) | 2 (7.7) | 9 (18.4) | 7 (14.6) | 4 (14.8) | |||
-Student t-test for parametric continuous variables and Fisher Exact or chi-squared test for categorical variables where appropriate.
Table 2.
FSC rupture attempt characteristics and outcome.
| Overall Success |
Success IPCR alone |
Subsequent surgery |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | p-value | No | Yes | p-value | No | Yes | p-value | |
| Age mean (SD), y (n = 90) | 66 (9.5) | 61.7 (10.5) | .15 | 62.7 (10.5) | 62.1 (10.5) | .78 | 62 (11.5) | 63 (8.5) | .67 |
| Needle Gauge no. (%) (n = 90) | |||||||||
| Any 18 | 13 (100) | 69 (89.6) | .22 | 26 (96.3) | 56 (88.9) | .25 | 51 (89.572) | 31 (94) | .47 |
| Other | 0 | 8 (10.4) | 1 (3.7) | 7 (11.1) | 6 (10.5) | 2 (6) | |||
| Cyst size median [IQR], mm (n = 87)a | 46 [32.2, 52.5] | 46.7 [35.7, 91.5] | .44 | 47.6 [32.8, 66] | 45.8 [35.7, 80.2] | .92 | 50 [37.2, 100.7] | 44.2 [30, 57.6] | .012 |
| T2 Signal no. (%) (n = 87)a | |||||||||
| Hyper-intense (H) | 3 (23) | 39 (52.7) | .10 | 9 (33.3) | 33 (55) | .15 | 24 (43.5) | 18 (56.3) | .41 |
| Intermediate (I) | 9 (69.3) | 31 (41.9) | 16 (59.3) | 24 (40) | 28 (51) | 12 (37.5) | |||
| Hypo-intense (L) | 1 (7.7) | 4 (5.4) | 2 (7.4) | 3 (5) | 3 (5.5) | 2 (6.1) | |||
-Student t-test used for parametric continuous variables (Age),-Mann-Whitney test for non-parametric variable (Cyst Size), and Fisher Exact or chi-squared test for categorical variableswhere appropriate.
-Cyst size: L x W (mm), calculated by multiplying the two greatest biaxial dimensions in millimeters.
a In three FSC rupture attempts same MR was used and were excluded.
Fourteen patients had surgical hardware, and of these, 13 (92.9%) had the hardware present either directly adjacent to or at the same level as the FSC. The presence of surgical hardware was also not found to be associated with treatment success or subsequent need for surgery (Table 1).
3.5. Association of procedural technique and treatment outcomes
An 18-gauge Quincke needle was most used in the IPCR technique (82/90 FSCs, 91.1 %). Four FSC ruptures were attempted with a 20-gauge Quincke needle, three with a 22-gauge Quincke needle, and one used a 17-gauge needle. Needle gauge was not found to be associated with treatment success of IPCR alone, IPCR +DF, or subsequent need for surgery (Table 2).
3.6. Association of procedural outcomes and pain relief
Pre- and post-procedure pain scores were available in both the IPCR + DF and IPCR alone groups. There were only 46 paired pain scores in the IPCR + DF and 45 paired pain scores in IPCR alone group.
In IPCR +DF group, the mean change in pain scores was the same between successful and unsuccessful procedures (−3.8 vs. −3.3, p = .83). In the IPCR alone group, those with unsuccessful rupture had a slightly greater reduction in pain (−4.3) compared to those with successful rupture (−3.5); however, it was not statistically significant (p = .45), (Table 3).
Table 3.
Absolute pain score change from pre-to post-procedure.
| obs | mean change (SD) | p-value | |
|---|---|---|---|
| IPCR + DF Success (n = 46) | |||
| Yes | 43 | −3.8 (3.1) | 0.83 |
| No | 3 | −3.3 (3.5) | |
| IPCR Alone Success (n = 45) | |||
| Yes | 33 | −3.5 (3.3) | 0.45 |
| No | 12 | −4.3 (2.7) |
- Student t-test.
- Pain scores were documented on a scale of 0–10 (0: no pain, 10: the worst pain).
Given the similar levels of pain reduction across the success rates, it suggests that procedural success of rupture did not immediately impact the amount of pain relief, or that the sample sizes might be too small to detect a significant effect. There were not enough observations for analysis in the group that subsequently underwent surgery.
3.7. Association of imaging features and treatment outcomes
Cyst size in greatest bi-axial dimension had a mean size of 46.4 mm. Size was not found to be associated with treatment success of IPCR or IPCR +DF. However, small cysts were more often found to require subsequent surgery (mean of 44.2 mm vs 50.0 mm, p = .012) (Table 2). Forty-two of the 87 FSCs with corresponding MRI demonstrated a bright T2 signal, forty demonstrated an intermediate signal, and five demonstrated a dark signal on T2-weighted imaging. Intrinsic cyst intensity was not found to be associated with success of IPCR, IPCR +DF, or subsequent surgery.
4. Discussion
Our study is, to our knowledge, one of the first to evaluate the effectiveness of FSC rupture using a combination of IPCR, followed by the DF technique in cases of IPCR failure, by employing CT-guidance. We found a lower rate of rupture using IPCR under CT-guidance compared to what has been previously reported using fluoroscopy in a large cohort (70.0% vs 81% [8]). However, we found a higher rate of successful FSC rupture when IPCR was followed by DF (90.6%), and combining the IPCR and DF techniques resulted in a higher rate of success than fluoroscopically guided IPCR alone rates, which has been described in prior meta-analyses (55.8%) [9].
Rates of success with IPCR combined with DF in our study were overall similar to a previously reported IPCR alone CT-guided study, which reported an 87% success rate [5]. Though our study utilized slightly different techniques (e.g. needles used in the Cambron et al. study included a 14-gauge to penetrate covering facet osteophytes), our high rate of success emphasizes the value of CT-guided intervention to not only allow for repeated attempts until unequivocal rupture with the less invasive initial IPCR approach, but also the flexibility to transition to the more invasive DF technique, which we have shown increases the likelihood of FSC rupture.
Prior studies have examined radiologic FSC features, and association with subsequent surgical conversion in one study of 45 patients found no correlation between cyst signal intensity, size, facet effusion, spondylolisthesis, canal stenosis, or facet edema with eventual surgery [7]. In comparison, our study, with a larger cohort, interestingly found an association between smaller cyst size and the need for subsequent surgery. This result was somewhat surprising, and we surmise that smaller cysts may perhaps have an easier time reforming than larger cysts, which in turn, results in persistent symptoms for the patient. However, it is not certain from these findings that the symptoms requiring FSC treatment and subsequent surgery were caused by these small cysts, and our assumptions based on this correlation are therefore limited. Cambron et al. found in their study of 110 patients that cysts with higher intrinsic T2 intensity were more likely to rupture using the IPCR technique, and they hypothesized that the higher proportion of fluid within the cyst improves ease of IPCR compared to lower intensity cysts, which may be more characteristically gelatinous or calcified. They also discussed that increased T2 intensity led to fewer subsequent surgeries compared to the cohort with more hypointense cysts. Our study, however, did not find these same associations, potentially limited by our lower sampling power with a smaller patient cohort.
Rates of surgical intervention following IPCR + DF were found to be lower in our CT-guided cohort than previously reported in a large IPCR by fluoroscopy study in 101 patients (36.0% vs 54% [8]), and more similar to other fluoroscopically-guided studies demonstrating a conversion rate of 20–38.7% [7,9,16].
Strengths of our study include the ability to unequivocally confirm FSC rupture with CT-guidance. Single and bi-planar views with fluoroscopy may not be as definitive, as we suspect there may be a component of false positive ruptures when assessing rupture under fluoroscopic guidance versus CT. This may partially account for the discrepancy between CT confirmed success, and previously reported fluoroscopic success.
One limitation of our study includes the retrospective nature of our investigation with absence of patient-reported outcomes, and inconsistent collection of pre and post pain scores, which limited our analyses. There is also a lack of repeat MR imaging in some patients that went underwent repeat rupture, as clinicians might order a repeat intervention upon return of symptomatology with the assumption of recurrence instead of obtaining post-procedural MR imaging, which partially limits our determination of associated imaging variables. Additionally, we could only track subsequent surgery in this patient cohort if it occurred at our institution which may under-represent this percentage.
5. Conclusion
Confirmed FSC rupture via indirect technique under CT-guidance was lower compared to previously reported fluoroscopic-guided studies in the literature, but the rates of successful rupture increased with the addition of the direct fenestration technique. Our findings emphasize the benefits of flexibility afforded to the operator with a CT-guided approach. CT guided direct fenestration may be indicated to treat FSCs that fail fluoroscopic or CT-controlled indirect rupture.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.inpm.2024.100447.
Contributor Information
Allison Y. Yang, Email: yangay@iu.edu.
Troy A. Hutchins, Email: Troy.Hutchins@hsc.utah.edu.
Lubdha M. Shah, Email: Lubdha.Shah@hsc.utah.edu.
Lacey Woods, Email: Lacey.Woods@hci.utah.edu.
Ghazaleh Safazadeh, Email: Ghazaleh.Safazadeh@hci.utah.edu.
Blair A. Winegar, Email: Blair.Winegar@hsc.utah.edu.
Anna Hudson, Email: Aewillis10@gmail.com.
Miriam E. Peckham, Email: Miriam.Peckham@hsc.utah.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Ramhmdani S., Ishida W., Perdomo-Pantoja A., Witham T.F., Lo S.L., Bydon A. Synovial cyst as a marker for lumbar instability: a systematic review and meta-analysis. World Neurosurg. 2019;122:e1059–e1068. doi: 10.1016/j.wneu.2018.10.228. Epub 2018/11/12. doi: 10.1016/j.wneu.2018.10.228. PubMed PMID: 30415048. [DOI] [PubMed] [Google Scholar]
- 2.Shah V.N., von Fischer N.D., Chin C.T., Yuh E.L., Amans M.R., Dillon W.P., et al. Long-term effectiveness of direct CT-guided aspiration and fenestration of symptomatic lumbar facet synovial cysts. AJNR Am J Neuroradiol. 2018;39(1):193–198. doi: 10.3174/ajnr.A5428. Epub 2017/11/11. doi: 10.3174/ajnr.A5428. PubMed PMID: 29122762; PubMed Central PMCID: PMC7410714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boody B.S., Savage J.W. Evaluation and treatment of lumbar facet cysts. J Am Acad Orthop Surg. 2016;24(12):829–842. doi: 10.5435/JAAOS-D-14-00461. Epub 2016/10/30. doi: 10.5435/jaaos-d-14-00461. PubMed PMID: 27792054. [DOI] [PubMed] [Google Scholar]
- 4.Lutz G.E., Nicoletti M.R., Cyril G.E., Harrison J.R., Lutz C., Solomon J.L., et al. Percutaneous rupture of zygapophyseal joint synovial cysts: a prospective assessment of nonsurgical management. Pm r. 2018;10(3):245–253. doi: 10.1016/j.pmrj.2017.07.078. Epub 2017/08/12. doi: 10.1016/j.pmrj.2017.07.078. PubMed PMID: 28797833. [DOI] [PubMed] [Google Scholar]
- 5.Cambron S.C., McIntyre J.J., Guerin S.J., Li Z., Pastel D.A. Lumbar facet joint synovial cysts: does T2 signal intensity predict outcomes after percutaneous rupture? AJNR Am J Neuroradiol. 2013;34(8):1661–1664. doi: 10.3174/ajnr.A3441. Epub 2013/03/02. doi: 10.3174/ajnr.A3441. PubMed PMID: 23449657; PubMed Central PMCID: PMC3801423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bykowski J.L., Wong W.H. Role of facet joints in spine pain and image-guided treatment: a review. AJNR Am J Neuroradiol. 2012;33(8):1419–1426. doi: 10.3174/ajnr.A2696. Epub 2011/09/24. doi: 10.3174/ajnr.A2696. PubMed PMID: 21940805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell J., Bhatia M., Hadeed M.M., George J., Hill A., Novicoff W.M., et al. Fluoroscopically guided facet cyst rupture: rate of conversion to surgery and risk factor analysis. Clin Spine Surg. 2021;34(7):E410. doi: 10.1097/BSD.0000000000001146. e4. Epub 2021/02/27. doi: 10.1097/bsd.0000000000001146. PubMed PMID: 33633003. [DOI] [PubMed] [Google Scholar]
- 8.Martha J.F., Swaim B., Wang D.A., Kim D.H., Hill J., Bode R., et al. Outcome of percutaneous rupture of lumbar synovial cysts: a case series of 101 patients. Spine J. 2009;9(11):899–904. doi: 10.1016/j.spinee.2009.06.010. Epub 2009/08/12. doi: 10.1016/j.spinee.2009.06.010. PubMed PMID: 19664971. [DOI] [PubMed] [Google Scholar]
- 9.Shuang F., Hou S.X., Zhu J.L., Ren D.F., Cao Z., Tang J.G. Percutaneous resolution of lumbar facet joint cysts as an alternative treatment to surgery: a meta-analysis. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0111695. Epub 2014/11/13. doi: 10.1371/journal.pone.0111695. PubMed PMID: 25389771; PubMed Central PMCID: PMC4229115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner A.L. CT fluoroscopy-guided epidural injections: technique and results. AJNR Am J Neuroradiol. 2004;25(10):1821–1823. Epub 2004/12/01. PubMed PMID: 15569755; PubMed Central PMCID: PMC8148733. [PMC free article] [PubMed] [Google Scholar]
- 11.Lee B., Lee S.E., Kim Y.H., Park J.H., Lee K.H., Kang E., et al. Evaluation of contrast flow patterns with cervical interlaminar epidural injection: comparison of midline and paramedian approaches. Medicina (Kaunas) 2020;57(1) doi: 10.3390/medicina57010008. Epub 2020/12/31. doi: 10.3390/medicina57010008. PubMed PMID: 33374193; PubMed Central PMCID: PMC7823639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy N.S., Maus T.P., Aprill C. The retrodural space of Okada. AJR Am J Roentgenol. 2011;196(6):W784–W789. doi: 10.2214/AJR.10.5751. Epub 2011/05/25. doi: 10.2214/ajr.10.5751. PubMed PMID: 21606270. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz A.O., Tekchandani L. Improved outcomes with direct percutaneous CT guided lumbar synovial cyst treatment: advanced approaches and techniques. J Neurointerventional Surg. 2014;6(10):790–794. doi: 10.1136/neurintsurg-2013-010891. Epub 2013/11/28. doi: 10.1136/neurintsurg-2013-010891. PubMed PMID: 24280130. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70(4):213–220. doi: 10.1037/h0026256. Epub 1968/10/01. doi: 10.1037/h0026256. PubMed PMID: 19673146. [DOI] [PubMed] [Google Scholar]
- 15.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. Epub 1977/03/01. PubMed PMID: 843571. [PubMed] [Google Scholar]
- 16.Eshraghi Y., Desai V., Cajigal Cajigal C., Tabbaa K. Outcome of percutaneous lumbar synovial cyst rupture in patients with lumbar radiculopathy. Pain Physician. 2016;19(7):E1019–E1025. Epub 2016/09/28. PubMed PMID: 27676672. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.