Abstract
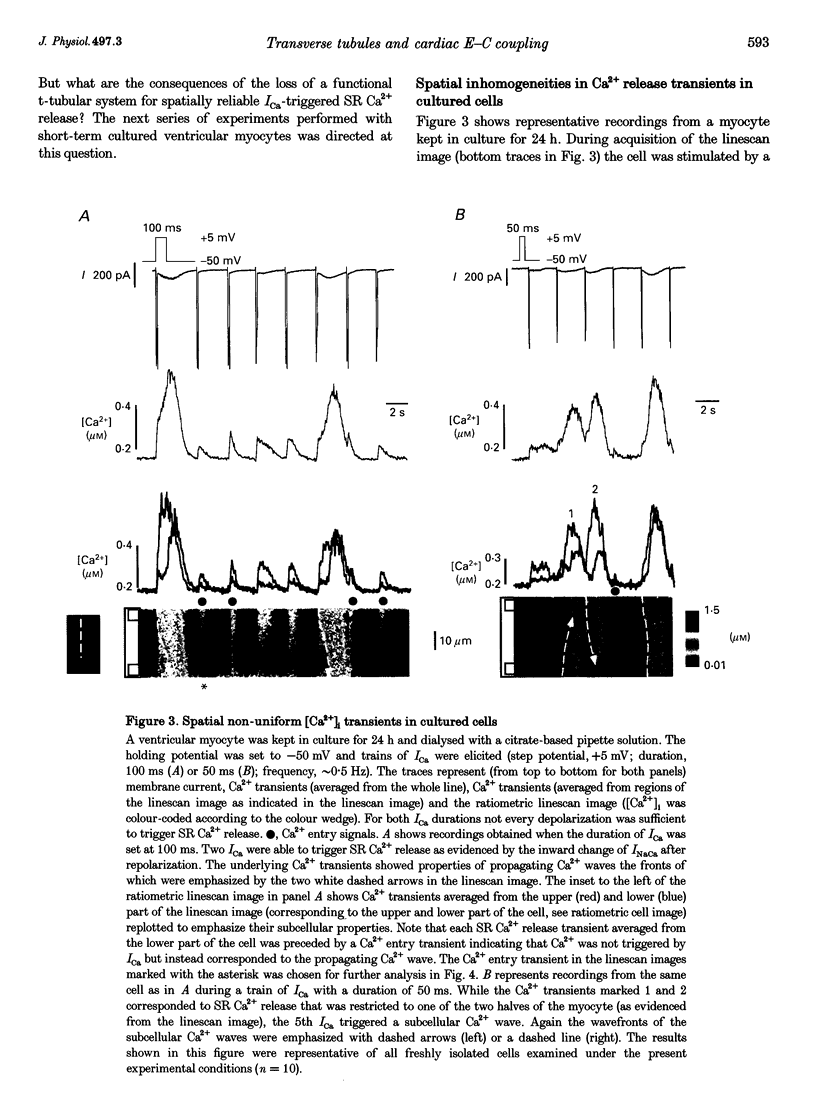
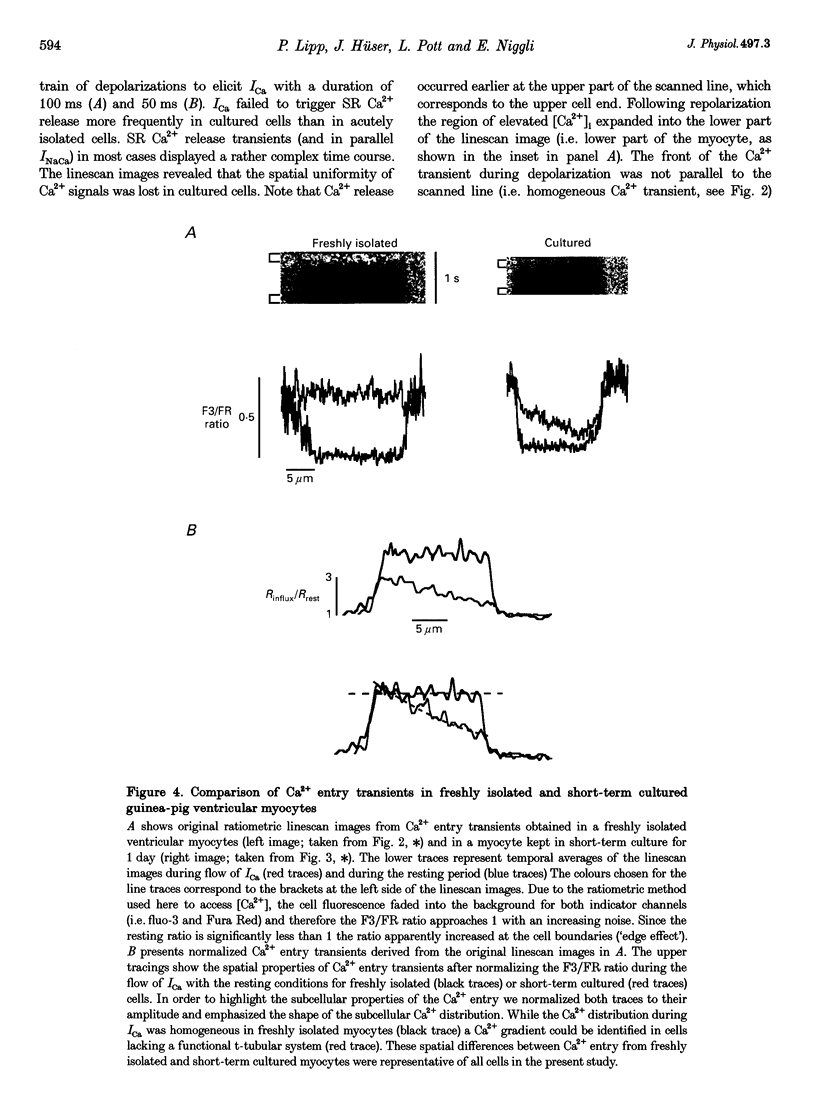
1. Ratiometric confocal microscopy and the whole-cell patch clamp technique were used to simultaneously record intracellular Ca2+ transients and membrane currents from guinea-pig ventricular myocytes. Intracellular dialysis with the low-affinity Ca2+ buffer citrate enabled us to record and analyse Ca2+ transients caused by Ca2+ influx alone and by additional Ca2+ release from the sarcoplasmic reticulum (SR) in the same cell. 2. In freshly isolated adult myocytes (used within 1-4 h of isolation) both types of Ca2+ transients ('Ca2+ entry' and 'Ca2+ release' transients) were spatially uniform regardless of the Ca2+ current (ICa) duration. In contrast, Ca2+ transients in short-term cultured (1-2 days) myocytes exhibited marked spatial inhomogeneities. ICa frequently evoked Ca2+ waves that propagated from either or both ends of the cardiac myocyte. Reduction of the ICa duration caused Ca2+ release that was restricted to one of the two halves of the cell. 3. Analysis of the Ca2+ entry signals in freshly isolated and short-term cultured myocytes indicated that the spatial properties of the Ca2+ influx signal were responsible for the spatial properties of the triggered Ca2+ release from the SR. In freshly isolated ventricular myocytes Ca2+ influx was homogeneous while in short-term cultured cells pronounced Ca2+ gradients could be found during Ca2+ influx. Spatial non-uniformities in the amplitude of local Ca2+ entry transients were likely to cause subcellularly restricted Ca2+ release. 4. The changes in the spatial properties of depolarization-induced Cai2+ signals during short-term culture were paralleled by a decrease (to 65%) in the total cell capacitance. In addition, staining the sarcolemma with the membrane-selective dye Di-8-ANEPPS revealed that, in cultured myocytes, t-tubular membrane connected functionally to the surface membrane was reduced or absent. 5. These results demonstrate that the short-term culture of adult ventricular myocytes results in the concomitant loss of functionally connected t-tubular membrane. The lack of the t-tubular system subsequently caused spatially non-uniform SR Ca2+ release. Evidence is presented to show that in ventricular myocytes lacking t-tubules non-uniform SR Ca2+ release was, most probably, the result of inhomogeneous Ca2+ entry during ICa. These findings directly demonstrate the functional importance of the t-tubular network for uniform ventricular Ca2+ signalling.
Full text
PDF

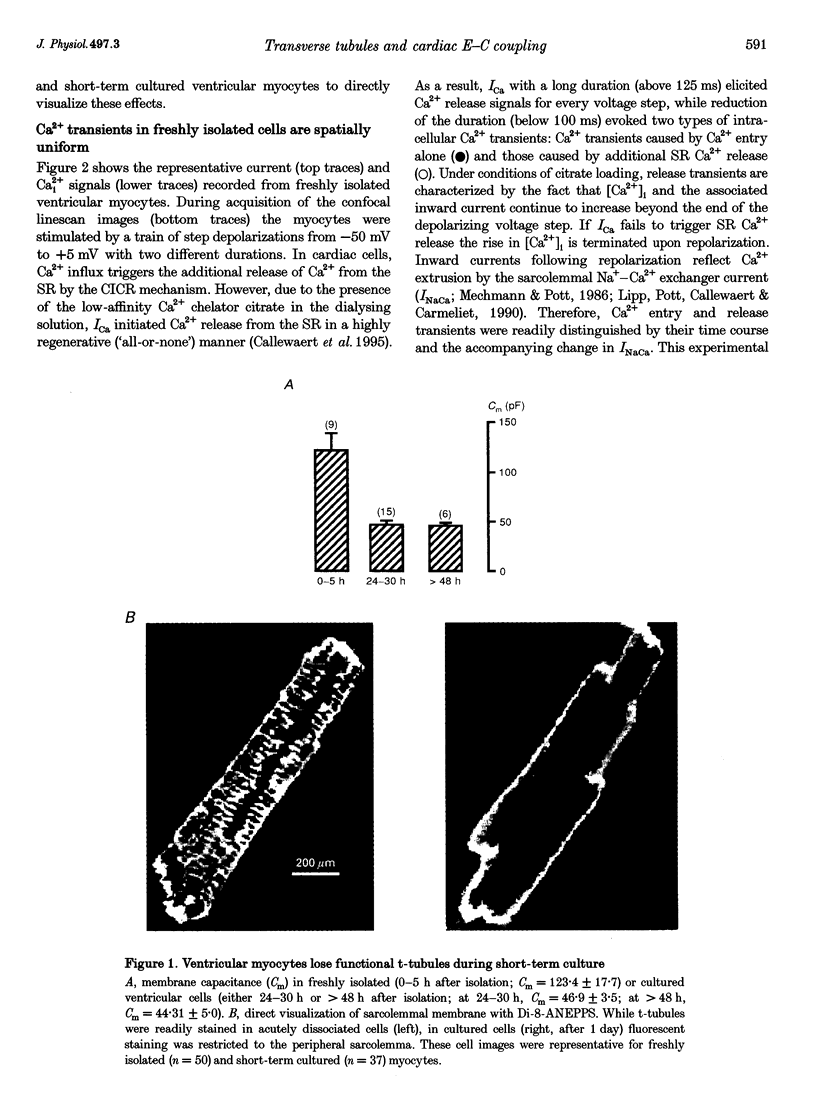
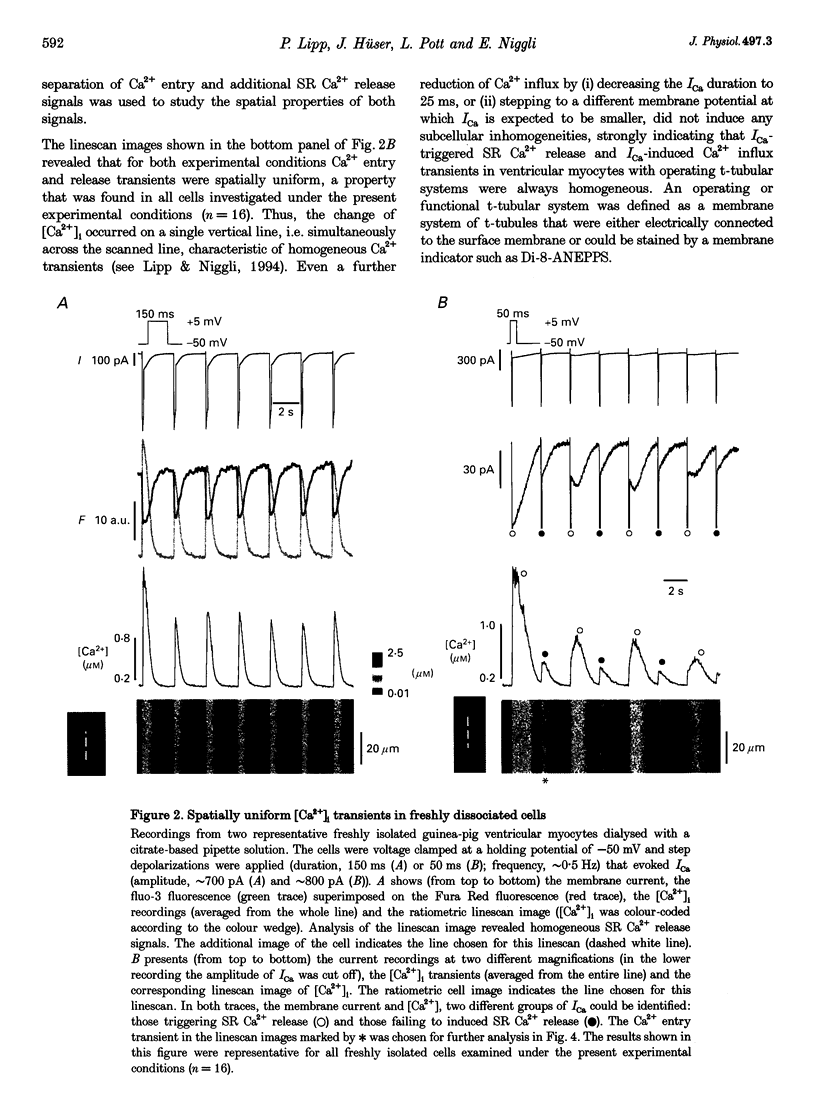



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callewaert G. Excitation-contraction coupling in mammalian cardiac cells. Cardiovasc Res. 1992 Oct;26(10):923–932. doi: 10.1093/cvr/26.10.923. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J. 1994 Nov;67(5):1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl S. L., Felix K., Caswell A. H., Brandt N. R., Ball W. J., Jr, Vaghy P. L., Meissner G., Ferguson D. G. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J Cell Biol. 1995 May;129(3):673–682. doi: 10.1083/jcb.129.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Cannell M. B., Lederer W. J. Propagation of excitation-contraction coupling into ventricular myocytes. Pflugers Arch. 1994 Oct;428(3-4):415–417. doi: 10.1007/BF00724526. [DOI] [PubMed] [Google Scholar]
- Decker M. L., Behnke-Barclay M., Cook M. G., Lesch M., Decker R. S. Morphometric evaluation of the contractile apparatus in primary cultures of rabbit cardiac myocytes. Circ Res. 1991 Jul;69(1):86–94. doi: 10.1161/01.res.69.1.86. [DOI] [PubMed] [Google Scholar]
- Delcarpio J. B., Claycomb W. C., Moses R. L. Ultrastructural morphometric analysis of cultured neonatal and adult rat ventricular cardiac muscle cells. Am J Anat. 1989 Dec;186(4):335–345. doi: 10.1002/aja.1001860403. [DOI] [PubMed] [Google Scholar]
- Hüser J., Lipsius S. L., Blatter L. A. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J Physiol. 1996 Aug 1;494(Pt 3):641–651. doi: 10.1113/jphysiol.1996.sp021521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Lüscher C., Niggli E. Photolysis of caged compounds characterized by ratiometric confocal microscopy: a new approach to homogeneously control and measure the calcium concentration in cardiac myocytes. Cell Calcium. 1996 Mar;19(3):255–266. doi: 10.1016/s0143-4160(96)90026-3. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Modulation of Ca2+ release in cultured neonatal rat cardiac myocytes. Insight from subcellular release patterns revealed by confocal microscopy. Circ Res. 1994 May;74(5):979–990. doi: 10.1161/01.res.74.5.979. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Ratiometric confocal Ca(2+)-measurements with visible wavelength indicators in isolated cardiac myocytes. Cell Calcium. 1993 May;14(5):359–372. doi: 10.1016/0143-4160(93)90040-d. [DOI] [PubMed] [Google Scholar]
- Lipp P., Pott L., Callewaert G., Carmeliet E. Simultaneous recording of Indo-1 fluorescence and Na+/Ca2+ exchange current reveals two components of Ca2(+)-release from sarcoplasmic reticulum of cardiac atrial myocytes. FEBS Lett. 1990 Nov 26;275(1-2):181–184. doi: 10.1016/0014-5793(90)81467-3. [DOI] [PubMed] [Google Scholar]
- Mechmann S., Pott L. Identification of Na-Ca exchange current in single cardiac myocytes. Nature. 1986 Feb 13;319(6054):597–599. doi: 10.1038/319597a0. [DOI] [PubMed] [Google Scholar]
- Mitcheson J. S., Hancox J. C., Levi A. J. Action potentials, ion channel currents and transverse tubule density in adult rabbit ventricular myocytes maintained for 6 days in cell culture. Pflugers Arch. 1996 Apr;431(6):814–827. doi: 10.1007/s004240050073. [DOI] [PubMed] [Google Scholar]
- Shacklock P. S., Wier W. G., Balke C. W. Local Ca2+ transients (Ca2+ sparks) originate at transverse tubules in rat heart cells. J Physiol. 1995 Sep 15;487(Pt 3):601–608. doi: 10.1113/jphysiol.1995.sp020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu T., Wier W. G. High temporal resolution video imaging of intracellular calcium. Cell Calcium. 1990 Feb-Mar;11(2-3):111–120. doi: 10.1016/0143-4160(90)90064-2. [DOI] [PubMed] [Google Scholar]
- Trafford A. W., Díaz M. E., O'Neill S. C., Eisner D. A. Comparison of subsarcolemmal and bulk calcium concentration during spontaneous calcium release in rat ventricular myocytes. J Physiol. 1995 Nov 1;488(Pt 3):577–586. doi: 10.1113/jphysiol.1995.sp020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibo M., Bravo G., Godfraind T. Postnatal maturation of excitation-contraction coupling in rat ventricle in relation to the subcellular localization and surface density of 1,4-dihydropyridine and ryanodine receptors. Circ Res. 1991 Mar;68(3):662–673. doi: 10.1161/01.res.68.3.662. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Egan T. M., López-López J. R., Balke C. W. Local control of excitation-contraction coupling in rat heart cells. J Physiol. 1994 Feb 1;474(3):463–471. doi: 10.1113/jphysiol.1994.sp020037. [DOI] [PMC free article] [PubMed] [Google Scholar]