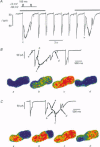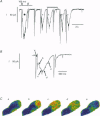Abstract
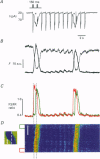
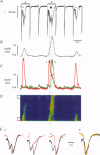
1. Spatiotemporal aspects of subcellular Ca2+ signalling were studied in cultured adult guinea-pig atrial myocytes. A mixture of the Ca2+ indicators fluo-3 and Fura Red in combination with laser-scanning confocal microscopy was used for [Ca2+]i measurements while membrane currents were recorded simultaneously. 2. In citrate-loaded atrial myocytes not every Ca2+ current (ICa) could trigger Ca2+ release from the sarcoplasmic reticulum (SR). Two types of Ca2+ signals could be observed: Ca2+ transients resulting from (i) Ca2+ influx alone and (ii) additional Ca2+ release. 3. Ca2+ release elicited by voltage steps of 100-150 ms duration was either apparently homogeneous or propagated as Ca2+ waves through the entire cell. With brief ICa (50-75 ms), Ca2+ waves with limited subcellular propagation were observed frequently. These waves always originated from either end of the myocyte. 4. The time course of changes in Na(+)-Ca2+ exchange current (INaCa) depended on the subcellular properties of the underlying Ca2+ transient and on the particular cell geometry. Apparently homogeneous Ca2+ release was accompanied by an inward change of INaCa the onset phase of which was fused with ICa. Changes in INaCa caused by a Ca2+ wave propagating through the entire cell showed a W shape, which could be attributed to differences of the fractional surface-to-volume ratio in different cell segments during propagation of the Ca2+ wavefront. Those waves with limited spreading only activated a small component of INaCa. 5. The different subcellular patterns of Ca2+ release signals can be explained by spatial inhomogeneities in the positive feedback of the SR. This depends on the local SR Ca2+ loading state under the control of the local Ca2+ influx during activation of ICa. Due to the higher surface-to-volume ratio at the two ends of the myocyte, SR loading and therefore the positive feedback in Ca(2+)-induced Ca2+ release may be higher at the ends, locations where Ca2+ waves are preferentially triggered. 6. We conclude that the individual cell geometry may be an important determinant of subcellular Ca2+ signalling not only in cardiac muscle cells but presumably also in other types of cells that depend on Ca2+ signalling. In addition, the cell geometry in combination with varying subcellular Ca2+ release patterns can greatly affect the time course of Ca(2+)-activated membrane currents.
Full text
PDF


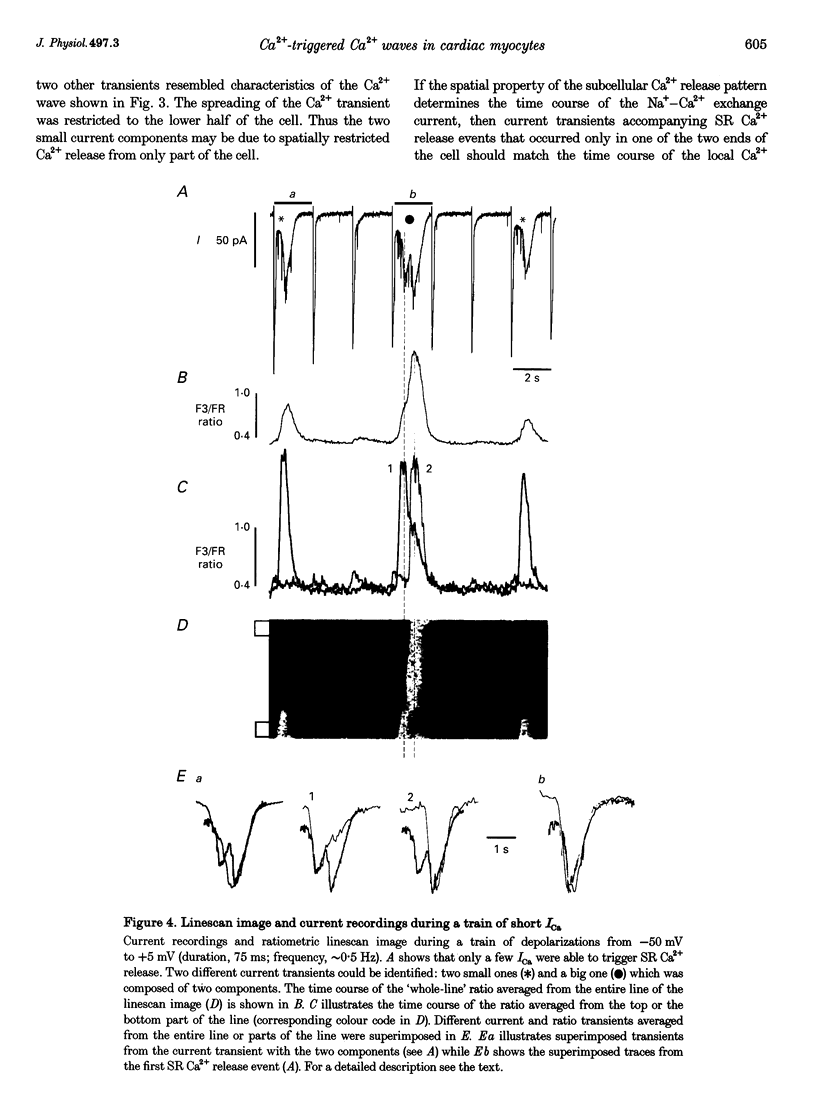
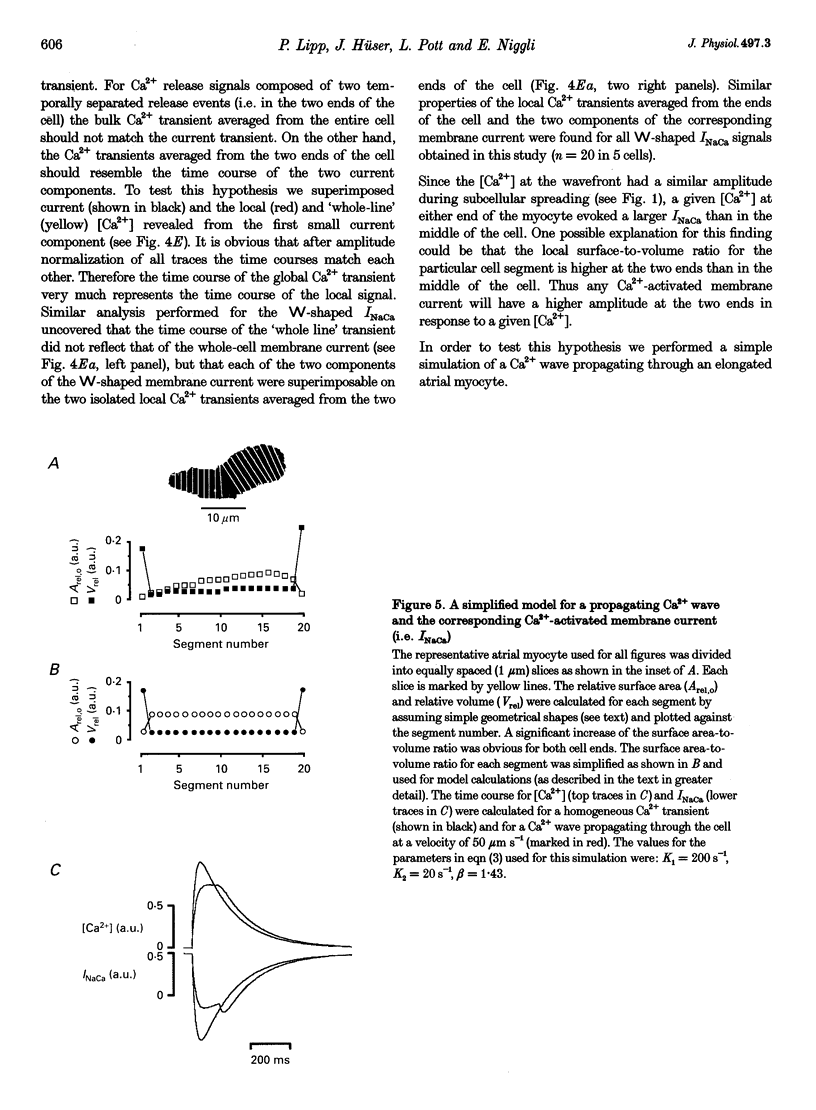




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bechem M., Pieper F., Pott L. Guinea-pig atrial cardioballs. Basic Res Cardiol. 1985;80 (Suppl 1):19–22. doi: 10.1007/978-3-662-11041-6_3. [DOI] [PubMed] [Google Scholar]
- Bechem M., Pott L. Removal of Ca current inactivation in dialysed guinea-pig atrial cardioballs by Ca chelators. Pflugers Arch. 1985 May;404(1):10–20. doi: 10.1007/BF00581485. [DOI] [PubMed] [Google Scholar]
- Beuckelmann D. J., Wier W. G. Sodium-calcium exchange in guinea-pig cardiac cells: exchange current and changes in intracellular Ca2+. J Physiol. 1989 Jul;414:499–520. doi: 10.1113/jphysiol.1989.sp017700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T., Lipp P., Pott L. Measurement of Ca2(+)-release-dependent inward current reveals two distinct components of Ca2+ release from sarcoplasmic reticulum in guinea-pig atrial myocytes. Pflugers Arch. 1991 Feb;417(6):638–644. doi: 10.1007/BF00372963. [DOI] [PubMed] [Google Scholar]
- Callewaert G. Excitation-contraction coupling in mammalian cardiac cells. Cardiovasc Res. 1992 Oct;26(10):923–932. doi: 10.1093/cvr/26.10.923. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Sipido K. R., Carmeliet E., Pott L., Lipp P. Intracellular citrate induces regenerative calcium release from sarcoplasmic reticulum in guinea-pig atrial myocytes. Pflugers Arch. 1995 Apr;429(6):797–804. doi: 10.1007/BF00374803. [DOI] [PubMed] [Google Scholar]
- Cheng H., Cannell M. B., Lederer W. J. Propagation of excitation-contraction coupling into ventricular myocytes. Pflugers Arch. 1994 Oct;428(3-4):415–417. doi: 10.1007/BF00724526. [DOI] [PubMed] [Google Scholar]
- Hüser J., Lipsius S. L., Blatter L. A. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J Physiol. 1996 Aug 1;494(Pt 3):641–651. doi: 10.1113/jphysiol.1996.sp021521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc N., Hume J. R. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990 Apr 20;248(4953):372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Niggli E., Hadley R. W. Sodium-calcium exchange in excitable cells: fuzzy space. Science. 1990 Apr 20;248(4953):283–283. doi: 10.1126/science.2326638. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Microscopic spiral waves reveal positive feedback in subcellular calcium signaling. Biophys J. 1993 Dec;65(6):2272–2276. doi: 10.1016/S0006-3495(93)81316-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Ratiometric confocal Ca(2+)-measurements with visible wavelength indicators in isolated cardiac myocytes. Cell Calcium. 1993 May;14(5):359–372. doi: 10.1016/0143-4160(93)90040-d. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Sodium current-induced calcium signals in isolated guinea-pig ventricular myocytes. J Physiol. 1994 Feb 1;474(3):439–446. doi: 10.1113/jphysiol.1994.sp020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Pott L., Callewaert G., Carmeliet E. Calcium transients caused by calcium entry are influenced by the sarcoplasmic reticulum in guinea-pig atrial myocytes. J Physiol. 1992 Aug;454:321–338. doi: 10.1113/jphysiol.1992.sp019266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Pott L., Callewaert G., Carmeliet E. Simultaneous recording of Indo-1 fluorescence and Na+/Ca2+ exchange current reveals two components of Ca2(+)-release from sarcoplasmic reticulum of cardiac atrial myocytes. FEBS Lett. 1990 Nov 26;275(1-2):181–184. doi: 10.1016/0014-5793(90)81467-3. [DOI] [PubMed] [Google Scholar]
- Lipp P., Pott L. Effects of intracellular Ca2+ chelating compounds on inward currents caused by Ca2+ release from sarcoplasmic reticulum in guinea-pig atrial myocytes. Pflugers Arch. 1991 Oct;419(3-4):296–303. doi: 10.1007/BF00371110. [DOI] [PubMed] [Google Scholar]
- Niggli E., Lipp P. Subcellular features of calcium signalling in heart muscle: what do we learn? Cardiovasc Res. 1995 Apr;29(4):441–448. [PubMed] [Google Scholar]
- Niggli E., Lipp P. Subcellular restricted spaces: significance for cell signalling and excitation-contraction coupling. J Muscle Res Cell Motil. 1993 Jun;14(3):288–291. doi: 10.1007/BF00123093. [DOI] [PubMed] [Google Scholar]
- Pott L., Mechmann S. Large-conductance ion channel measured by whole-cell voltage clamp in single cardiac cells: modulation by beta-adrenergic stimulation and inhibition by octanol. J Membr Biol. 1990 Aug;117(2):189–199. doi: 10.1007/BF01868685. [DOI] [PubMed] [Google Scholar]
- Sham J. S., Cleemann L., Morad M. Gating of the cardiac Ca2+ release channel: the role of Na+ current and Na(+)-Ca2+ exchange. Science. 1992 Feb 14;255(5046):850–853. doi: 10.1126/science.1311127. [DOI] [PubMed] [Google Scholar]
- Takamatsu T., Wier W. G. High temporal resolution video imaging of intracellular calcium. Cell Calcium. 1990 Feb-Mar;11(2-3):111–120. doi: 10.1016/0143-4160(90)90064-2. [DOI] [PubMed] [Google Scholar]
- Trafford A. W., Lipp P., O'Neill S. C., Niggli E., Eisner D. A. Propagating calcium waves initiated by local caffeine application in rat ventricular myocytes. J Physiol. 1995 Dec 1;489(Pt 2):319–326. doi: 10.1113/jphysiol.1995.sp021053. [DOI] [PMC free article] [PubMed] [Google Scholar]