Abstract
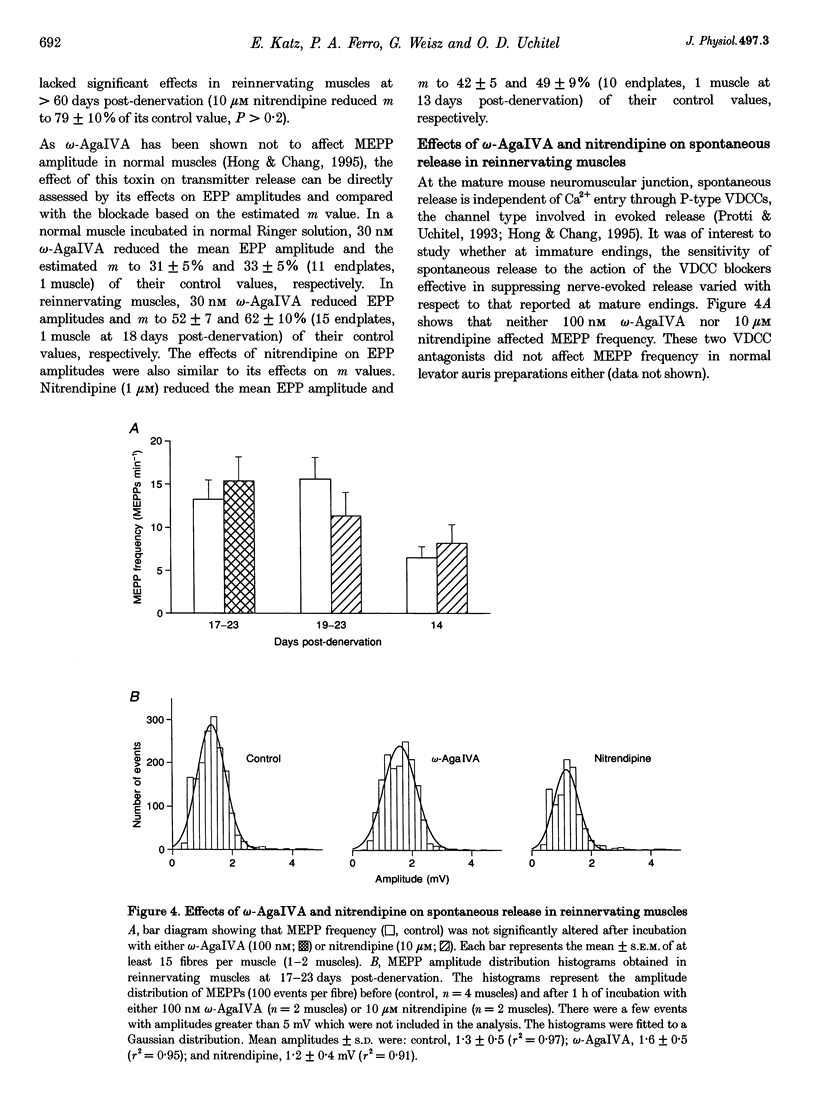
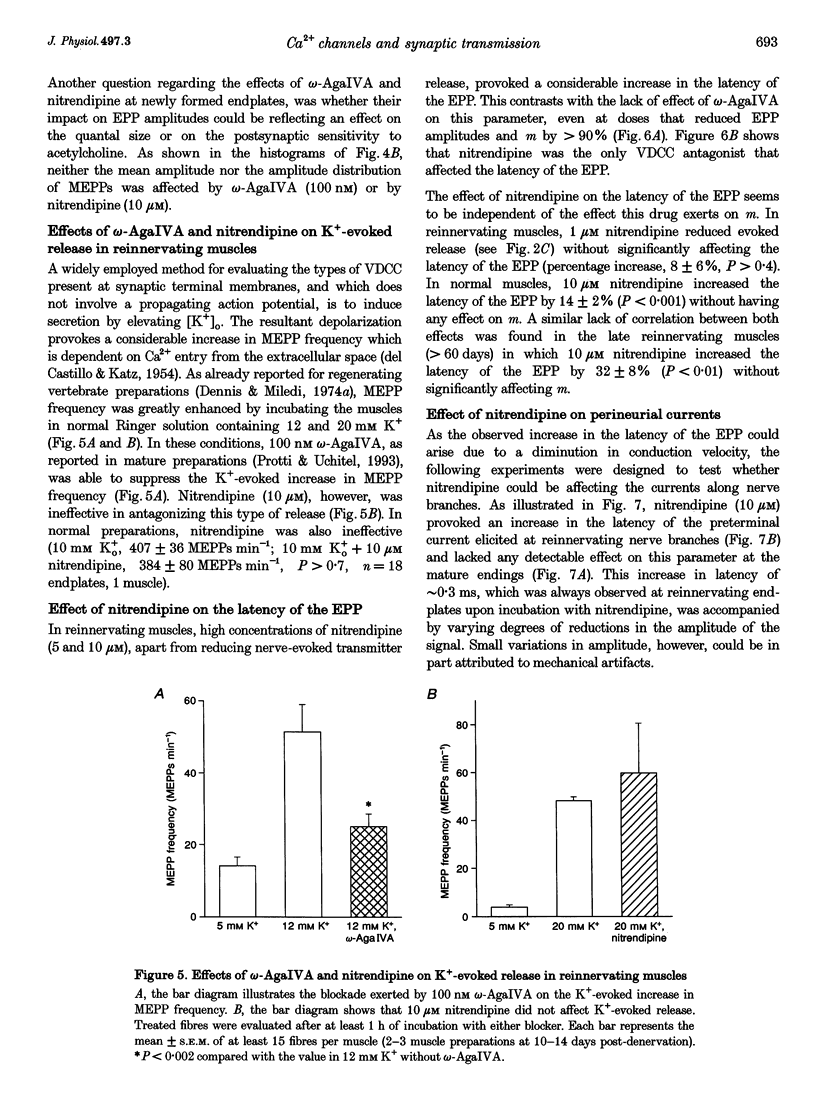
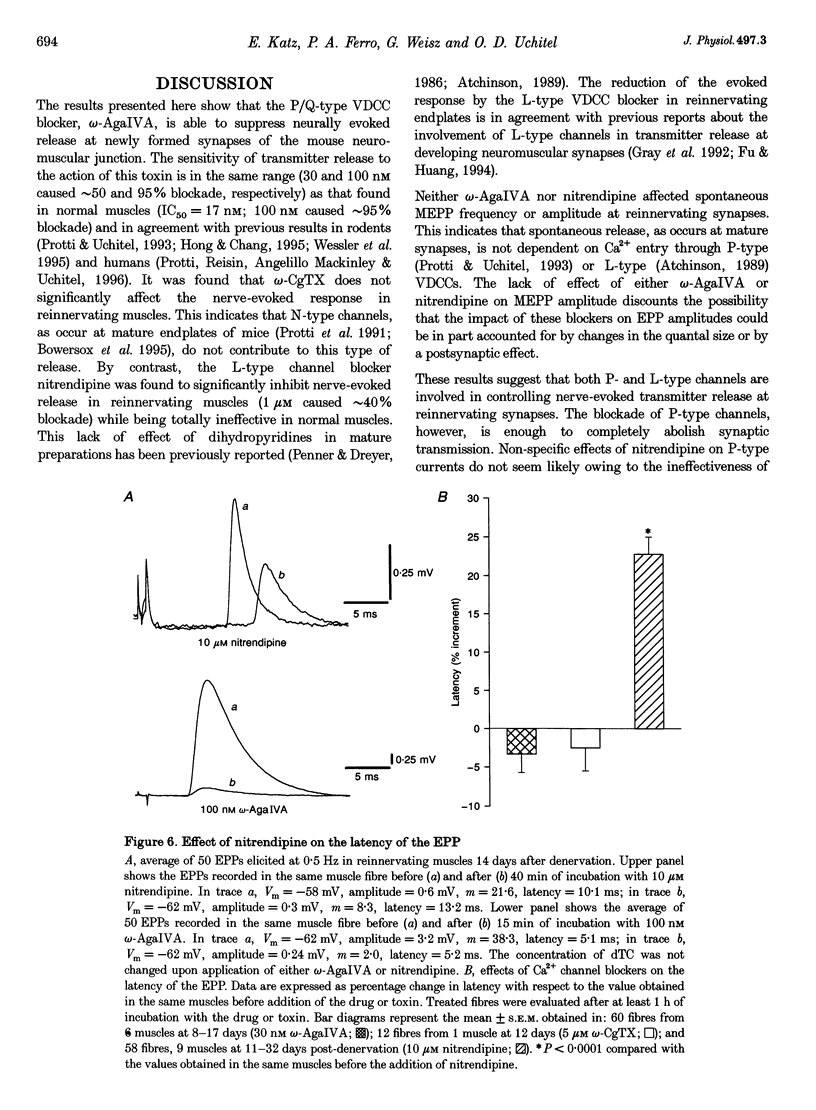
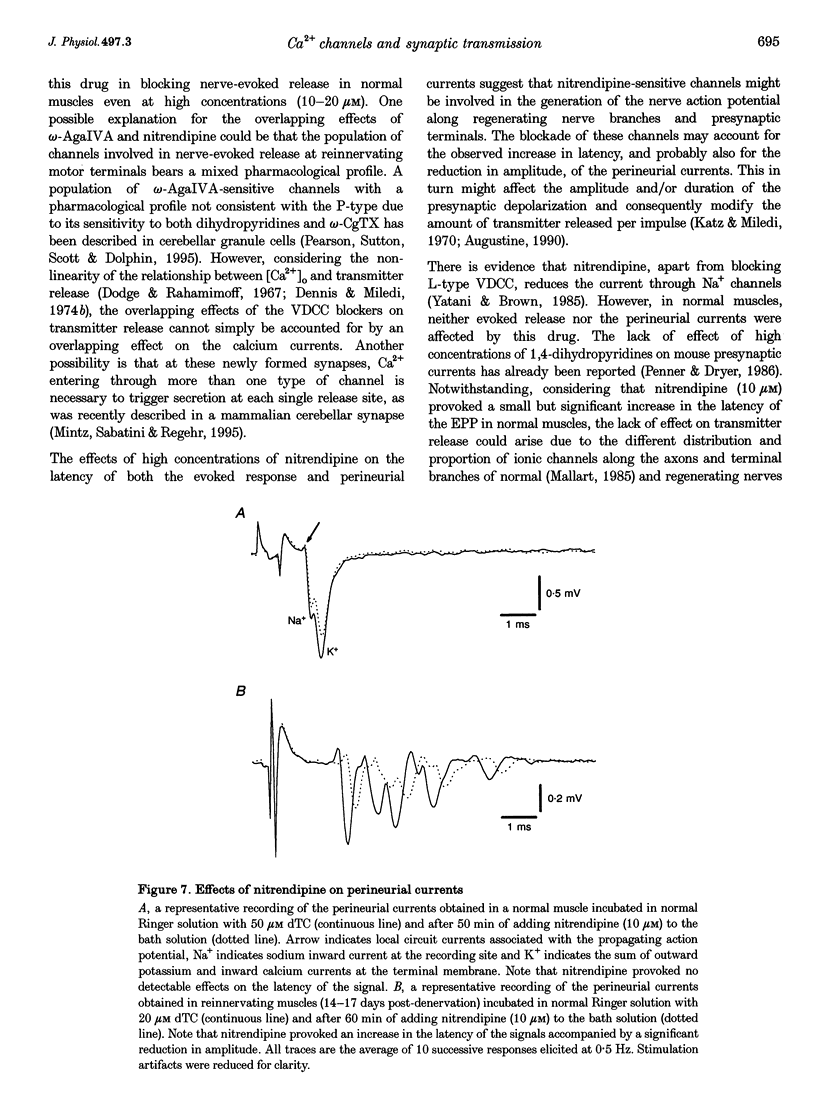
1. The involvement of the different types of voltage-dependent calcium channels (VDCCs) in synaptic transmission at the mature and newly formed mammalian neuromuscular junction was studied by evaluating the effects of L-, P/Q- and N-type VDCC antagonists on transmitter release in normal and reinnervating levator auris preparations of adult mice. 2. Nerve-evoked transmitter release was blocked by omega-agatoxin IVA (omega-AgaIVA), a P/Q-type VDCC blocker, both in normal and reinnervating endplates (100 nM omega-AgaIVA caused > 90% inhibition). The N-type VDCC antagonist omega-conotoxin GVIA (omega-CgTX; 1 and 5 microM), as occurs in normal preparations, did not significantly affect this type of release during reinnervation. Nitrendipine (1-10 microM), an L-type VDCC blocker, strongly antagonized release in reinnervating muscles (approximately 40-69% blockade) and lacked any effect in normal preparations. 3. In reinnervating muscles, spontaneous release was not dependent on Ca2+ entry through either P- or L-type VDCCs. Neither 100 nM omega-AgaIVA nor 10 microM nitrendipine affected the miniature endplate potential (MEPP) frequency or amplitude. 4. At the newly formed endplates, K(+)-evoked release was dependent on Ca2+ entry through VDCCs of the P-type family (100 nM omega-AgaIVA reduced approximately 70% of the K(+)-evoked MEPP frequency). L-type VDCCs were found not to participate in this type of release (10 microM nitrendipine lacked any effect). 5. In reinnervating muscles, the L-type VDCC blocker, nitrendipine (10 microM), provoked a significant increase (approximately 25%) in the latency of the evoked endplate potential (EPP). This drug also caused an increase (approximately 0.3 ms) in the latency of the presynaptic currents. The P/Q- and Ny-type VDCC blockers did not affect the latency of the EPP. 6. These results show that at newly formed mouse neuromuscular junctions, as occurs in mature preparations, VDCCs of the P-type family play a prominent role in evoked transmitter release whereas N-type channels are not involved in this process. In addition, signal conduction and transmitter release become highly sensitive to nitrendipine during reinnervation. This suggests that L-type VDCCs may play a role in synaptic transmission at the immature mammalian neuromuscular junction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angaut-Petit D., Mallart A. Electrical activity of mouse motor endings during muscle reinnervation. Neuroscience. 1985 Dec;16(4):1047–1056. doi: 10.1016/0306-4522(85)90115-0. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D., Mallart A. Ionic channel distribution in regenerating mouse motor endings. J Physiol (Paris) 1985;80(4):307–311. [PubMed] [Google Scholar]
- Angelov D. N., Neiss W. F., Streppel M., Andermahr J., Mader K., Stennert E. Nimodipine accelerates axonal sprouting after surgical repair of rat facial nerve. J Neurosci. 1996 Feb 1;16(3):1041–1048. doi: 10.1523/JNEUROSCI.16-03-01041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison W. D. Dihydropyridine-sensitive and -insensitive components of acetylcholine release from rat motor nerve terminals. J Pharmacol Exp Ther. 1989 Nov;251(2):672–678. [PubMed] [Google Scholar]
- Augustine G. J. Regulation of transmitter release at the squid giant synapse by presynaptic delayed rectifier potassium current. J Physiol. 1990 Dec;431:343–364. doi: 10.1113/jphysiol.1990.sp018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M., Taylor R. S. The formation of synapses in reinnervated mammalian striated muscle. J Physiol. 1973 Sep;233(3):481–500. doi: 10.1113/jphysiol.1973.sp010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Sears T. A., Sherratt R. M. The effects of 4-aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibres. J Physiol. 1981;313:301–315. doi: 10.1113/jphysiol.1981.sp013666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowersox S. S., Miljanich G. P., Sugiura Y., Li C., Nadasdi L., Hoffman B. B., Ramachandran J., Ko C. P. Differential blockade of voltage-sensitive calcium channels at the mouse neuromuscular junction by novel omega-conopeptides and omega-agatoxin-IVA. J Pharmacol Exp Ther. 1995 Apr;273(1):248–256. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Characteristics of transmitter release at regenerating frog neuromuscular junctions. J Physiol. 1974 Jun;239(3):571–594. doi: 10.1113/jphysiol.1974.sp010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Non-transmitting neuromuscular junctions during an early stage of end-plate reinnervation. J Physiol. 1974 Jun;239(3):553–570. doi: 10.1113/jphysiol.1974.sp010582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W. M., Huang F. L. L-type Ca2+ channel is involved in the regulation of spontaneous transmitter release at developing neuromuscular synapses. Neuroscience. 1994 Jan;58(1):131–140. doi: 10.1016/0306-4522(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Hong S. J., Chang C. C. Inhibition of acetylcholine release from mouse motor nerve by a P-type calcium channel blocker, omega-agatoxin IVA. J Physiol. 1995 Jan 15;482(Pt 2):283–290. doi: 10.1113/jphysiol.1995.sp020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970 May;207(3):789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2918–2922. doi: 10.1073/pnas.73.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Hillman D. E., Cherksey B. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992 Sep;15(9):351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- Lüscher C., Lipp P., Lüscher H. R., Niggli E. Control of action potential propagation by intracellular Ca2+ in cultured rat dorsal root ganglion cells. J Physiol. 1996 Jan 15;490(Pt 2):319–324. doi: 10.1113/jphysiol.1996.sp021146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A. Electric current flow inside perineurial sheaths of mouse motor nerves. J Physiol. 1985 Nov;368:565–575. doi: 10.1113/jphysiol.1985.sp015876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I. M., Sabatini B. L., Regehr W. G. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995 Sep;15(3):675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Venema V. J., Swiderek K. M., Lee T. D., Bean B. P., Adams M. E. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992 Feb 27;355(6363):827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Momiyama A., Takahashi T. Calcium channels responsible for potassium-induced transmitter release at rat cerebellar synapses. J Physiol. 1994 Apr 15;476(2):197–202. doi: 10.1113/jphysiol.1994.sp020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Miljanich G. P., Ramachandran J., Adams M. E. Calcium channel diversity and neurotransmitter release: the omega-conotoxins and omega-agatoxins. Annu Rev Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- Pearson H. A., Sutton K. G., Scott R. H., Dolphin A. C. Characterization of Ca2+ channel currents in cultured rat cerebellar granule neurones. J Physiol. 1995 Feb 1;482(Pt 3):493–509. doi: 10.1113/jphysiol.1995.sp020535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Dreyer F. Two different presynaptic calcium currents in mouse motor nerve terminals. Pflugers Arch. 1986 Feb;406(2):190–197. doi: 10.1007/BF00586682. [DOI] [PubMed] [Google Scholar]
- Protti D. A., Reisin R., Mackinley T. A., Uchitel O. D. Calcium channel blockers and transmitter release at the normal human neuromuscular junction. Neurology. 1996 May;46(5):1391–1396. doi: 10.1212/wnl.46.5.1391. [DOI] [PubMed] [Google Scholar]
- Protti D. A., Szczupak L., Scornik F. S., Uchitel O. D. Effect of omega-conotoxin GVIA on neurotransmitter release at the mouse neuromuscular junction. Brain Res. 1991 Aug 23;557(1-2):336–339. doi: 10.1016/0006-8993(91)90156-p. [DOI] [PubMed] [Google Scholar]
- Protti D. A., Uchitel O. D. Transmitter release and presynaptic Ca2+ currents blocked by the spider toxin omega-Aga-IVA. Neuroreport. 1993 Dec 13;5(3):333–336. doi: 10.1097/00001756-199312000-00039. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C. Development of voltage-dependent and ligand-gated channels in excitable membranes. Prog Brain Res. 1994;102:169–179. doi: 10.1016/S0079-6123(08)60538-5. [DOI] [PubMed] [Google Scholar]
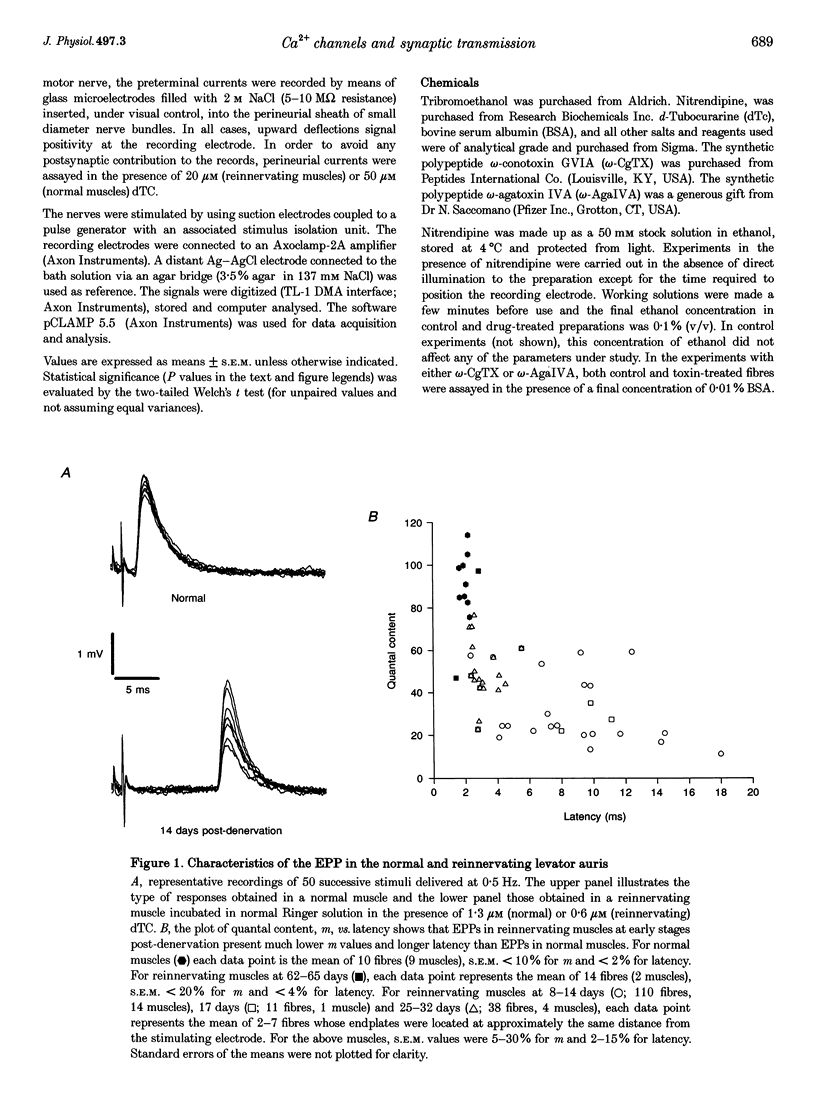
- Tonge D. A. Physiological characteristics of re-innervation of skeletal muscle in the mouse. J Physiol. 1974 Aug;241(1):141–153. doi: 10.1113/jphysiol.1974.sp010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchitel O. D., Protti D. A., Sanchez V., Cherksey B. D., Sugimori M., Llinás R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler I., Dooley D. J., Lohr B. P-type Ca2+ channels trigger stimulus-evoked [3H]acetylcholine release from mammalian motor endplates. Eur J Pharmacol. 1995 May 4;278(1):83–86. doi: 10.1016/0014-2999(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Yatani A., Brown A. M. The calcium channel blocker nitrendipine blocks sodium channels in neonatal rat cardiac myocytes. Circ Res. 1985 Jun;56(6):868–875. doi: 10.1161/01.res.56.6.868. [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Randall A. D., Ellinor P. T., Horne W. A., Sather W. A., Tanabe T., Schwarz T. L., Tsien R. W. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993 Nov;32(11):1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
- Zhu P. H., Vrbová G. The role of Ca2+ in the elimination of polyneuronal innervation of rat soleus muscle fibres. Eur J Neurosci. 1992;4(5):433–437. doi: 10.1111/j.1460-9568.1992.tb00893.x. [DOI] [PubMed] [Google Scholar]