Abstract
Acute rejection (AR) remains a pivotal complication and leading cause of mortality within the first year following heart transplantation (HT). In this study, we assessed the impact of ultrasound-targeted microbubbles loaded with sirolimus (SIR-MBs) on AR in a rat HT model and delved into the underlying mechanisms. We established a rat abdominal ectopic HT model, which was stratified into three groups receiveing the PBS, SIR-MBs + ultrasound-targeted microbubble destruction (UTMD), and sirolimus, respectively. The protective effects of each treatments on survival rate, inflammatory response, autophagy and TGF-β1-Smad signaling pathway-related proteins were evaluted. Additionally, rescue experiment was performed via adding the autophagy inhibitor or TGF-β1 agonist in combination therapy. UTMD combined SIR-MBs mediated 15-fold higher local drug concentration compared to direct sirolimus administration. The infiltration of inflammatory cells in the transplanted hearts indicated that SIR-MBs combined with UTMD were effective in mitigating the inflammatory response, achieving levels significantly lower than those observed in the sirolimus group. Furthermore, after SIR-MBs combined with UTMD treatment, the expression levels of TGF-β1-Smad signaling pathway-related proteins in heart tissues also showed a significant decrease compared to the model control group. Conversely, the expressions of autophagy proteins LC3-II, Beclin-1 and β-arrestin showed an up-regulated trend. Rescue experiments also revealed that the enhancement in survival trends was markedly suppressed following the administration of CsA or SRI-011381, respectively. Collectively, our findings suggest that SIR-MBs combined with UTMD augment the local treatment efficacy for AR in rat HT models by inhibiting the TGF-β1-Smad signaling pathway, promoting autophagy, and alleviating inflammation.
Keywords: Sirolimus, Ultrasound-targeted microbubble destruction, Heart transplantation, Acute rejection, TGF-β1-Smad, Autophagy
Graphical abstract
1. Introduction
Heart transplantation (HT) is an effective treatment for end-stage heart disease (Wu et al., 2023). However, acute rejection (AR) after HT is still the most important complication and cause of death in the first year after transplantation (Awad et al., 2022). About 40 % of patients develop AR within one year of HT (Lee et al., 2021). In the one-year postoperative mortality of transplant patients, AR accounted for about 12 % (Lee et al., 2021). Studies have shown that AR is an independent risk factor for cardiac vascular disease after transplantation and can lead to irreversible cardiac dysfunction after transplantation (Wong and Keebler, 2020). Therefore, the prevention and treatment of AR is very important for the quality of life and prognosis of heart transplant patients.
At present, immunosuppressants such as tacrolimus and sirolimus are mainly used to prevent and treat AR (Kuczaj et al., 2023). Tacrolimus is a calcineurin inhibitor, and clinical studies have shown that sirolimus is significantly better than calcineurin inhibitors in alleviating graft vascular disease after HT (Sutaria et al., 2022). Sirolimus can inhibit calcium-dependent and calcium-independent post-IL-2R transduction signals and proliferation signals transmitted by non-lymphocytic cytokines such as fibroblastic growth factor (FGF), stem cell factor (SCF), and platelet-derived growth factor (PDGF) by binding to immunotropin FKBP12 (Lim, 2018). Thus blocking the progression from G1 to S phase in T lymphocytes and other cell cycles and inhibiting protein synthesis at the transcriptional level (Lim, 2018). SIR can inhibit the activation and proliferation of T lymphocytes stimulated by exogenous cytokines, and inhibit the production of antibodies by B lymphocytes, which has a strong immunosuppressive effect. Sirolimus can not only inhibit the proliferation of effector T lymphocytes, but also induce immune tolerance and alleviate rejection by promoting the production of regulatory T lymphocytes (Sallam et al., 2021). Clinically, sirolimus is often administered orally or intravenously throughout the body (Kancharla et al., 2024). However, long-term systemic administration can lead to many side effects, such as nephrotoxicity, neurotoxicity, hypertension, hyperlipidemia, and diabetic effects (Sallam et al., 2021). These side effects not only affect the quality of life of patients, but also threaten the life safety of patients after HT.
Therefore, reducing systemic drug exposure, lowering blood drug concentration, and increasing drug concentration in diseased tissues are an effective way to reduce toxic side effects. Therefore, under the safe dosage, how to increase the drug concentration in the pathological tissue and whether the increase in the drug concentration in the pathological tissue can enhance the therapeutic effect of the drug need to be further verified.
In recent years, a large number of studies have shown that ultrasonic targeted microbubble destruction (UTMD) technology is a safe and effective local targeted drug delivery strategy (Liu et al., 2023; Yang et al., 2018). UTMD combined with microbubbles (MBs) can target the delivery of drugs or genes to specific tissues or organs, such as tumors, blood vessels, and the heart (Li et al., 2022). The main mechanism is that MBs has a compressible gas core, and when MBs is exposed to ultrasound, MBs oscillates with the compressed phase and sparse phase of ultrasound (Sun et al., 2023). Under high ultrasonic pressure, MBs will collapse and collapse, release energy, generate shock wave, destroy the integrity of surrounding cell membrane and capillary wall, and increase the permeability of cells and blood vessel wall (Cai et al., 2024). At the same time that MBs ruptures, the drug or gene wrapped in MBs is released into the surrounding tissues and penetrates out of the blood vessels, increasing the local dose of the drug or gene (Cai et al., 2024).
At present, UTMD-mediated drug or gene delivery has been widely used in the biomedical field (Suzuki et al., 2010). For example, in the treatment of inflammatory diseases, Jun-ichi Suzuki et al. improved the expression of interfering RNA in the femoral artery by mediating the targeted delivery of intercellular adhesion molecule-1 interference RNA to the mouse femoral artery through UTMD, thereby inhibiting the formation of the intima and inflammatory response (Suzuki et al., 2010). Therefore, this study hypothesized that UTMD-mediated sirolimus targeted delivery to the transplanted heart could increase the concentration of sirolimus in the transplanted heart tissue and enhance the therapeutic effect.
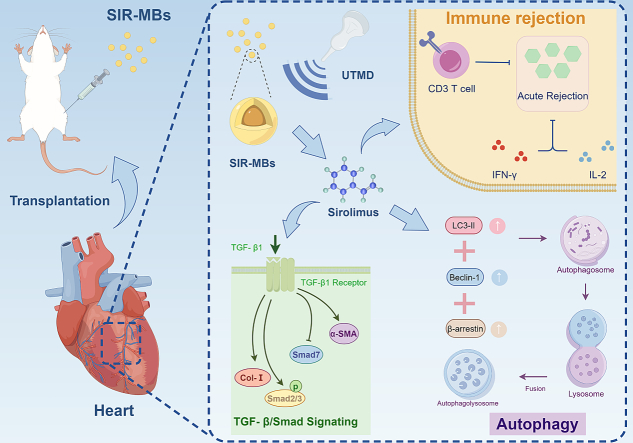
The objective of this investigation was to formulate sirolimus-loaded microbubbles (SIR-MBs) and to devise an effective local delivery strategy for sirolimus, in combination with ultrasound-targeted microbubble destruction (UTMD), to inhibit acute rejection (AR) following heart transplantation (HT) in practical applications (Fig. 1). Herein, we delved into the preparation methodology and characteristics of SIR-MBs, and examined whether SIR-MBs, when coupled with UTMD for targeted drug delivery, could elevate drug concentrations in transplanted hearts, thereby enhancing therapeutic efficacy. Furthermore, we investigated the underlying mechanisms of this combined strategy.
Fig. 1.
Schematic of the efficacy and mechanism of SIR-MBs combined with UTMD for the treatment of acute rejection of rat heart transplantation.
2. Experimental methods
2.1. Experimental materials
Distearoyl phosphatidylcholine from Avanti Corporation, USA (DSPC); Distearoyl phosphatidyl ethanolamine-polyethylene glycol (DSPE-PEG2000) was purchased from Corden, Switzerland. Sirolimus was acquired from Selleck Chemicals in the United States. Murine monoclonal LC3-II antibody was purchased from Nanotools 0231–100 / LC3-5F10, and rabbit monoclonal Beclin-1 antibody was purchased from Cell Signaling Technology. Rabbit Monoclonal β-arresting resistance (ab32099) was purchased from Abcam; β-actin antibody, purchased from Biyuntian AA128. Rabbit anti-rat TGF-β1 antibody, goat anti-rabbit TβR-I, goat anti-rat α-SMA were purchased from CST. Sprague Dawley (SD) rat, weighing 200–250 g, purchased from Shanghai Slack Laboratory Animal Co., LTD. H&E dyeing kit was purchased from Wuhan Xavier Biotechnology Co., LTD. Other conventional reagent consumables are purchased from Sinopsin Chemical Reagent Co., LTD. Or German Sigma Company. The Trizol kit was purchased from Invitrogen, USA. Reverse transcription and PCR reagents were purchased from TaKaRa, Japan. Cyclosporin A and SRI-011381 was brought from MedChemexpress (USA).
2.2. Preparation and confirmation of physical and chemical properties of SIR-MBs
The SIR-MBs were prepared by thin film hydration - mechanical oscillation method. Briefly, the solution of DSPC, DSPE-PEG2000 and Sirolimus (2:2:0.4) were added to a glass tube, nitrogen was injected, and vacuum was pumped to form a lipid film. The hydration solution (containing 10 % glycerol, 10 % 1, 2-propylene glycol and 80 % tris solution of 0.1 M, PH 7.4) is added, and the water is bathed until a uniform opalescent liquid is formed. After vacuuming, perfluoropropane gas (C3F8) was added, the mechanical oscillator shook for 30 s, and then centrifuged (1000 rpm, 5 min), remove the lower liquid, retain the upper milky viscous liquid is SIR-MBs. The particle size and polydispersity index (PDI) of SIR-MBs were measured by Brookhaven ZetaPALS analyzer using dynamic light scattering (DLS) technique. The content of SIR in the system was determined by HPLC and the drug loss rate and drug release rate were further calculated.
To determine the encapsulation efficiency, and drug loading rate of sirolimus-MBs, the first step is to prepare a sirolimus standard curve by preparing different concentrations of sirolimus standard solutions using a serial dilution method and measuring their peak areas to plot the standard curve. Subsequently, methanol is used to dissolve and disrupt the SIR-MBs, and an ultrasonic cleaner is employed for water bath treatment to facilitate the extraction of sirolimus. Prior to detection, the instrument is calibrated with the column temperature set at 50 °C, flow rate at 1 L/min, detection wavelength at 210 nm, and mobile phase consisting of acetonitrile-water-phosphoric acid (700:300:1). After recording the peak areas of the samples, the drug loading of sirolimus-MBs is calculated based on the sirolimus standard curve. The encapsulation efficiency and drug loading rate of the -MBs are then computed using the following formulas:
2.3. Preparation of rat abdominal ectopic HT model
Male Lewis rats of 180-200 g were divided into donor group and transplantation group. First, the rats in the donor group were anesthetized by injection of 1 % pentobarbital sodium at 50 mg/g. After opening in the middle of the abdomen, the intestinal tube was moved outside the abdominal cavity of the rat side, so that the abdominal aorta and inferior vena cava were clearly exposed. After heparin saline is injected, the abdominal aorta is cut for bleeding. The heart is exposed and covered and stopped with ice. After a series of preparations, the pulmonary veins were cut, the heart was removed and stored in normal saline at 4 °C. The whole operation process, to ensure that the movement is gentle, to avoid stabbing the heart.
The rats in the transplantation group were fasted for 24 h before surgery, and water was forbidden for 12 h. After anesthesia, the thoracic cavity was cut open, and the blood flow at the upper and lower ends of the abdominal aorta and inferior vena cava was blocked with hemostatic clips. The opening of the donor aorta was opposite to the opening of the recipient's abdominal aorta, the two ends of the blood vessel were fixed and the two vascular walls were sutured successively. Once one side is closed, turn the heart to the opposite side and close the other side in the same way. After arterial anastomosis, a long fusiform opening of similar size to that of the donor cardiopulmonary artery was cut on the inferior vena cava, and then the entire inferior vena cava and pulmonary artery were sutured continuously starting from the left lateral wall of the inferior vena cava. After the suture is completed, the upper and lower ends of the abdominal aorta and inferior vena cava are removed to restore blood flow to the heart. After no obvious bleeding was observed, the abdominal cavity was washed with gentamicin sulfate injection. After the intestinal tube was restored and observed for 5–10 min, the intestinal tube peristalsis was normal without bleeding or blackness, and the abdominal cavity was closed. After operation, they were kept warm with electric blanket and kept in single cage.
2.4. UTMD operation after HT in rat
After fixation, medical ultrasonic coupler was applied to the transplanted heart, and the UTMD parameters are set as follows: ultrasonic frequency = 1 MHz; duty cycle = 50 %; Ultrasonic intensity = 2 w/cm2. The concentration of MBs in each group was adjusted to 1 × 109/mL with PBS, 0.5 mL was injected by pellet through the tail vein, and then UTMD was performed.
2.5. Evaluation of vascular permeability in rat peritoneal ectopic HT model
In order to prove that UTMD can enhance the vascular permeability of the transplanted heart and the influence of different ultrasonic irradiation time on the vascular permeability of the transplanted heart, the rats were injected with EB dye solution (150 mg/kg) immediately after UTMD, and euthanized 30 min later. The rat's thoracic cavity was opened to expose the heart, and then needles were injected into the left ventricle, and hemostatic forceps were used to fix the perfusion needle. At the same time, a small incision was cut in the right auricle, and heparinized normal saline was injected slowly for perfusion, until the fluid flowing out of the right atrium became clear, and then paraformaldehyde was used for perfusion, and finally the transplanted heart was obtained. Then the transplanted heart was cut into coronal section, and the distribution of EB dye in the surface and coronal section of the transplanted heart was observed.
2.6. Detection of blood biochemical and other serological indexes in rat models of abdominal ectopic HT
Daily abdominal palpation of the transplanted heart beat, cardiac arrest was judged as AR. The whole blood or serum of rats were collected, and the blood cell classification, serum biochemical and histopathological detection were performed. Serum biochemical detection indicators were: creatinine (Cr), urea nitrogen (BUN), serum lactate dehydrogenase 1 (LDH1), creatine kinase (CK), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and other indicators detection. Blood routine indexes were detected by complete blood count instrument. The serum biochemical indexes were detected by automatic biochemical analyzer. Serum inflammation-related indicators, such as IL-6, are detected by ELISA kit, and the specific operation steps are carried out according to the instructions of the kit.
2.7. Evaluation of transplanted heart tissue damage
H&E staining was used to evaluate the histological changes of the transplanted heart after UTMD. The detection methods are as follows: (1) sampling, formaldehyde fixation: the transplanted heart was obtained, and the heart was soaked in 4 % paraformaldehyde for 24 h; (2) Dehydration: The transplanted heart tissue was immersed in different concentrations of alcohol for 30 min; After that, xylene was used for dehydration. (3) Embedding and slicing: the dehydrated tissue is soaked in paraffin, and then placed in the embedding machine for paraffin embedding; Then the short axis horizontal section of the left ventricular papillary muscle in the middle of the heart was taken, and the section thickness was 7 μm. (4) dewaxing sections to water: The tissue sections are successively added to xylene and anhydrous ethanol for dewaxing to water; (5) Hematoxylin staining nucleus: the section was placed into Harris hematoxylin for about 5 min, after washing off the dye, differentiated with 1 % hydrochloric acid alcohol for a few seconds, rinsed again and then added 0.6 % ammonia water to return to blue; (6) Eosin cytoplasm: Tissue sections were placed in eosin dye solution for 1–3 min; (7) Dehydration sealing: The slices are successively dehydrated into different concentrations of alcohol and xylene until the final sealing.
The AR response level was analyzed according to the diagnostic criteria of the International Society for Heart and Lung Transplantation (ISHLT) in 2004, as shown in Table 1.
Table 1.
The diagnostic criteria of the International Society for Heart and Lung Transplantation (ISHLT).
| Grade | Histopathological changes |
|---|---|
| 0 | Rejectin free |
| 1 | Focal and/or perivascular inflammatory cell infiltration, ≤1 myocardial lesion |
| 2 | Multifocal inflammatory cell infiltration with myocardial damage |
| 3 | Diffuse inflammatory cell infiltration, multiple myocardial lesions |
2.8. In vivo imaging assessment of transplanted heart fluorescence intensity and SIR tissue concentration detection
The rats in sirolimus group and Sirolimus-MBs + UTMD group were euthanized 30 min after administration, the transplanted hearts were obtained after perfusion of normal saline, and sirolimus in the transplanted heart tissue was extracted. Specific experimental methods were as follows: 0.2 g of heart tissue was cut, 1.0 mL of dichloromethane was added proportionately, tissue homogenization was performed at 14,000 rpm, centrifugation was performed for 10 min, the clear liquid was removed and dried with nitrogen gas, and then redissolved with 200 μL methanol. Then, the standard tissue sample curve was prepared, and the Sirolimus reserve solution of 2.0 mg/mL was prepared with methanol and diluted successively to 2000 ng/ mL, 1000 ng/mL, 200 ng/mL, 100 ng/mL, 20.0 ng/mL, 2.0 ng/mL. 0.2 ng/mL standard solution for a range of concentration gradients. The standard curve was calculated and the concentration of sirolimus in the tissue was calculated according to the standard curve.
Evaluation of fluorescence intensity in vivo imaging of transplanted hearts was also conducted after drug administration in each group of animals. Following drug administration, the hearts, livers, transplanted hearts, and other major organs of the rats were harvested. The organ surfaces were rinsed with PBS to remove bloodstains, dried with gauze, and then arranged in plastic trays for imaging using a small animal imaging system (In-vivo FX PRO, BRUKER). The transplanted hearts from different groups were manually delineated as regions of interest (ROIs), and quantitative analysis of the fluorescence intensity in the transplanted hearts was performed using software (Bruker MI SE).
2.9. Western blot analysis
The protein was quantified by BCA method, then the protein was separated by SDS-PAGE gel electrophoresis: 5 % concentrated glue and 12 % separation glue were prepared. Electrophoresis: Adjust the voltage to 80 V, turn to 100 V after 90 min, and stop when bromophenol blue runs to 0.5 cm away from the bottom of the separation rubber. Transfer film:280 mA constant flow for 1 h, turn off the power and take out PVDF film, soak in ponceum red dye solution for 10 min, if bands appear, indicating successful transfer of film, seal with TBST sealing solution containing 5 % skim milk powder for 1.5 h. Add one antibody, 4 °C refrigerator overnight. The second antibody was added at 37 °C for 2 h, and then rinsed with TBST for 10 min × 3 times before development. The protein bands were analyzed by Quantity One software, and the absorbance of target protein and beta-actin was determined, and the relative expression intensity of target protein was calculated. The formula is as follows: Relative expression intensity = A target protein/A β-actin (A represents absorbance value).
2.10. Statistical analysis
SPSS 13.0 software was used for statistical analysis. Experimental data were expressed as x ± s, and t-test was used for comparison between groups. P < 0. 05 was statistically significant.
3. Results
3.1. Preparation and evaluation of physical and chemical properties of SIR-MBs
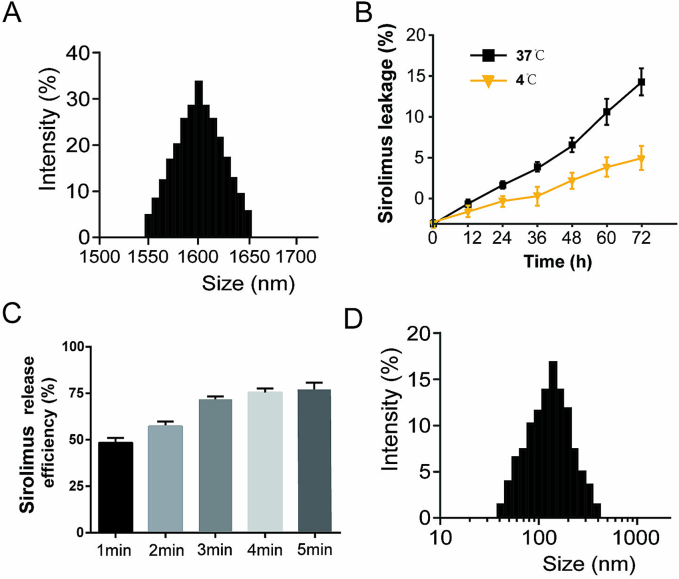
The SIR-MBs prepared by thin film hydration method was a milky suspension after mechanical oscillation. As measured by Brookhaven ZetaPALS particle size analyzer, the particle size of SIR-MBs is relatively uniform, with unipeak distribution. Under the optical microscope, SIR-MBs is a transparent spherical structure with uniform dispersion and no obvious aggregation phenomenon. The average particle size of SIR-MBs was 1.65 ± 0.32 μm, the average concentration was 4.35 × 109/mL, and the PDI was 0.16 ± 0.09 (Fig. 2A). The investigation results of drug loss at 4 °C and 37 °C were shown in Fig. 2B. When SIR-MBs was stored at 4 °C or 37 °C for 72 h, the drug loss rates were 5.0 ± 1.2 % and 15.0 ± 4.4 %, respectively. In vitro drug release of SIR-MBS is shown in Fig. 2C. UTMD can destroy SIR-MBs and release most of the SIR in it into PBS. After fixing the ultrasonic frequency, the drug release rate increases from 49.67 ± 6.64 % to 76 ± 3.61 % within 5 min with the delay of irradiation time. When the irradiation time reaches 2 min, the increase of drug release rate is not significant. The particle size distribution of SIR-MBs after UTMD destruction is shown in Fig. 2D, and the average particle size after UTMD is 156.2 ± 35.6 nm.
Fig. 2.
Characterization of SIR-MBs. (A) SIR-MBs particle size distribution diagram (B) Cumulative drug loss rate of SIR-MBs placed at 4 °C and 37 °C for different times. (C) In vitro drug release rate of SIR-MBs; (D) Particle size distribution diagram of the particles formed by SIR-MBs rupture after UTMD.
3.2. Confirmation of therapeutic conditions and preliminary safety evaluation of UTMD combined with SIR-MBs
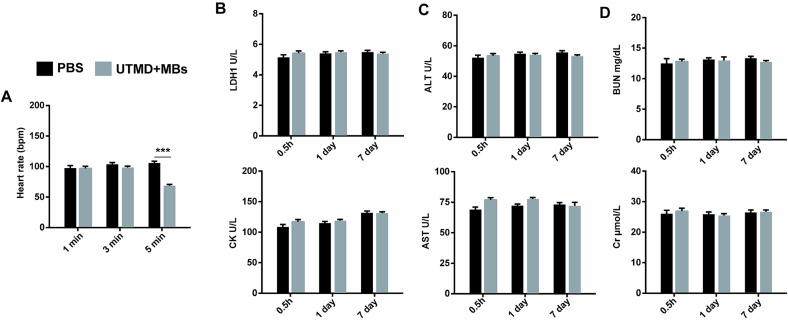
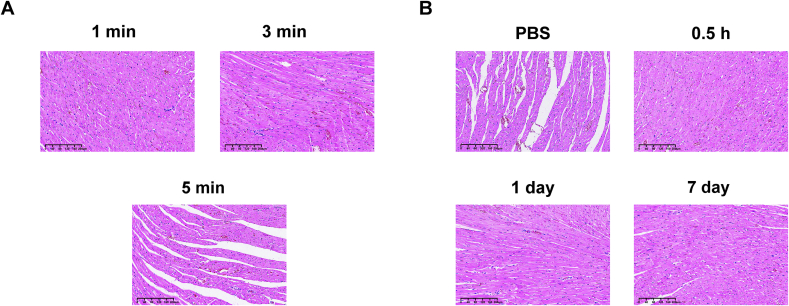
The safety of UTMD combined with SIR-MBs for different conditions in healthy SD rats were evaluated to determine appropriate therapeutic parameters. The heart rate results of rats after 1, 3 and 5 min of UTMD were shown in Fig. 3A. The heart rate of animals in the 1 and 3 min groups did not change, while the heart rate in the 5 min group showed a significant decrease (P < 0.001). The blood biochemical results showed that, compared with PBS group, there were no statistical differences in myocardial enzymes (LDH1 and CK, Fig. 3B), liver function (ALT and AST, Fig. 3C) and renal function (Cr and BUN, Fig. 3D) at 30 min, 1 day and 7 days after intervention in UTMD combined with SIR-MBs group. In addition, H&E staining results showed that, compared with PBS group, there were no significant histological changes in the important organs of animals in the UTMD combined with SIR-MBs group, including heart, liver, spleen, lung and kidney, and no obvious cell necrosis and inflammatory cell infiltration in the tissue sections of each organ after intervention.
Fig. 3.
Blood biochemical results at different time points after UTMD. (A) Changes in heart rate of transplanted heart after UTMD under ultrasonic irradiation time; (B) Serum biochemical indices LDH1 and CK; (C) Serum biochemical indices ALT and AST; (D) Serum biochemical indices BUN and Cr. ***P < 0.001, n = 10.
The histological changes of the transplanted heart after UTMD under current ultrasonic irradiation were further confirmed in a rat model of intraperitoneal ectopic HT. Histological examination results were shown in Fig. 4. No obvious erythrocyte exudation, inflammatory cell infiltration, myocardial cell necrosis and other histological damage were observed. After 3 min of ultrasonic irradiation, a small amount of scattered punctated red blood cells exudated, but no obvious inflammatory cell infiltration and myocardial cell injury were observed. When the ultrasound irradiation time was 5 min, extensive red blood cell exudation was observed in the myocardial tissue, the myocardial cell space was enlarged, and the myocardial fibers showed mild empty pack-like changes (Fig. 4A). Compared with PBS group, MBs combined with UTMD group showed no obvious histological changes in the hearts of mice in each group at 30 min, 1 day and 7 days after intervention, and no obvious cell necrosis and inflammatory cell infiltration were observed in the tissue sections of each organ (Fig. 4B).
Fig. 4.
Histological changes of transplanted heart after UTMD irradiation. (A) HE staining of transplanted heart tissue after UTMD under different ultrasonic irradiation time. (B) Cardiac HE staining results at different time points after intervention.
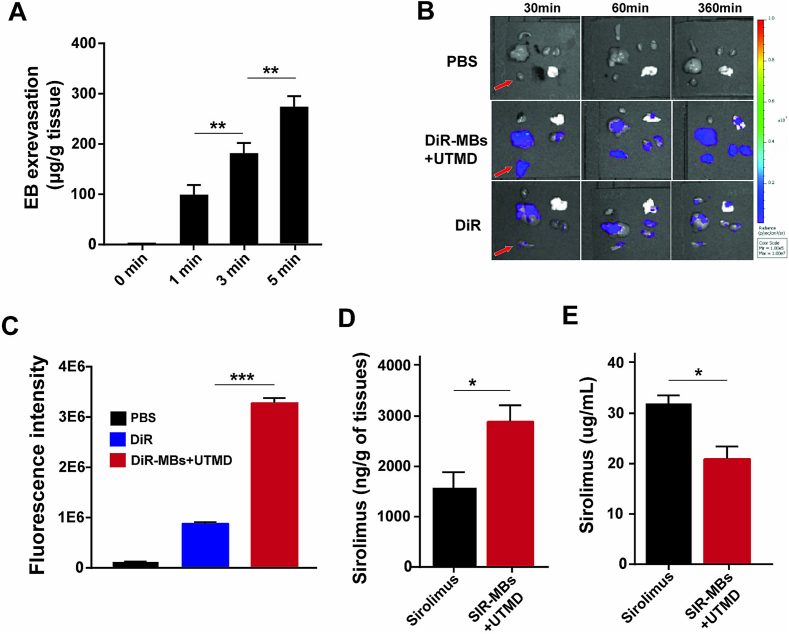
3.3. UTMD ultrasound irradiation significantly increased vascular permeability and local drug concentration in cardiac tissue
The effect of different ultrasonic irradiation time on the vascular permeability of transplanted heart was studied. EB was used as tracer to evaluate the vascular permeability of transplanted heart. With the increase of ultrasonic irradiation time, EB content in transplanted heart increased, indicating the increase of permeability (Fig. 5A). As shown in Fig. 5B, after the same amount of SIR is delivered by intravenous injection and by SIR-MBs combined with UTMD, SIR rapidly distributes to all organs in the body, mainly in the liver and spleen, followed by the lungs. To further compare the fluorescence intensity in transplanted hearts, quantitative analysis was performed. The fluorescence intensity in the transplanted heart in the SIR-MBs + UTMD group was significantly higher than that in the SIR group (P = 0.0023) (Fig. 5C). The concentration of sirolimus in the heart tissue of the intravenous sirolimus group was 1.7 μg/g, and the concentration of Sirolimus in the heart tissue of the SIR-MBs + UTMD group was 2.9 μg/g. It was significantly higher than that in sirolimus group (P = 0.027) (Fig. 5D). Interestingly, the concentration of sirolimus in peripheral blood samples in SIR-MBs was significantly lower than that in Sirolimus injection group (Fig. 5E, P < 0.05). Together, these data suggest that SIR-MBs combined with UTMD may provide better efficacy and lower toxicity.
Fig. 5.
Evaluation of myocardial tissue enrichment and drug release in heart transplantation model by drug-loaded microvesicle combined with UTMD local administration. (A) EB content in transplanted heart tissues under different ultrasonic irradiation; (B) Distribution of SIR in various organs; (C) Fluorescence intensity of different group at 360 min; sirolimus concentration in (D) cardiac tissue of transplanted heart and (E) peripheral blood. *,**,***P < 0.05, 0.01 and 0.001, n = 10.
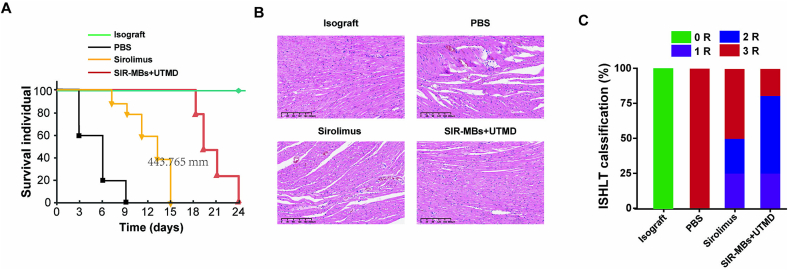
3.4. SIR-MBs combined with UTMD improves AR of HT in rats
In this experiment, the survival time of HT model rats in each group after transplantation was shown in the Fig. 6. Compared with the model control group, the survival time of all treatment groups was extended, and SIR-MBs + UTMD group was significantly better than sirolimus group (P < 0.01, Fig. 6A). H&E staining results of myocardial tissue of rats in each group showed (Fig. 6B) that the myocardial tissue of rats in the homologous transplantation group was normal without inflammatory infiltration, and was classified as grade 0 according to ISHLT standard cardiac biopsy grading. All grafts in the heterogeneous PBS group had grade 3 rejection signs, including diffuse inflammatory cell infiltration, massive coagulated cardiomyocyte necrosis, hemorrhage, edema, and obvious vasculitis. The inflammatory cell infiltration and myocardial cell necrosis in sirolimus group and SIR-MBs + UTMD group were significantly weakened compared with PBS group, and the therapeutic effect of SIR-MBs + UTMD group was significantly improved compared with sirolimus group, and the inflammatory cell infiltration and myocardial cell necrosis were further reduced. In the sirolimus group, 50 % of the grafts showed weak rejection, which fell into the category of grade 1 or 2 rejection. Compared with the sirolimus group, approximately 80 % of the grafts in the SIR-MBs + UTMD group were grade 1 or 2 rejection (Fig. 6C).
Fig. 6.
Ameliorating effect of SIR-MBs combined with UTMD on acute rejection of heart transplantation in rats. (A) survival curves of transplanted hearts in each group; (B) Evaluation of myocardial tissue HE staining; (C) Classification of acute rejection in different groups of rats (n = 10).
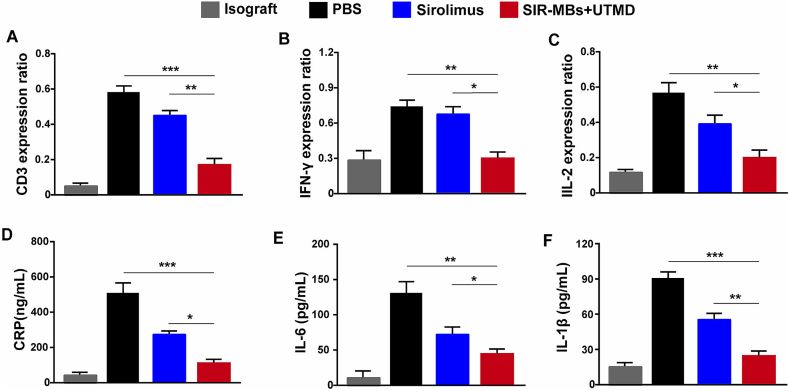
3.5. SIR-MBs combined with UTMD improves T cell infiltration and cytokine release in rat transplanted heart
In this study, we also further investigated the T cell infiltration and cytokine release in the transplanted heart tissue. In the xenotransplantation PBS group, a large number of CD3-positive T lymphocyte infiltration appeared in the myocardium of rats. Cd3-positive T lymphocyte infiltration in SIR-MBs + UTMD group and sirolimus group was significantly reduced than that in PBS group, and CD3-positive T lymphocyte infiltration in SIR-MBs + UTMD group was the least, which was significantly lower than that in sirolimus group (P < 0.001, Fig. 7A). Similarly, in the xenograft PBS group, there was a large amount of IFN-γ, IL-2, CPR, IL-6 and IL-1β infiltration in the myocardial tissue of rats (Fig. 7B-F), while the infiltration of IFN-γ and IL-2 in the SIR-MBs + UTMD group was significantly reduced compared with that in the PBS group, and the decrease was significantly lower than that in sirolimus (P < 0.05 and 0.01, respectively).
Fig. 7.
Effects of SIR-MBs combined with UTMD on T cell infiltration and cytokine release in rat transplanted hearts. (A) T cell infiltration in different groups of transplanted hearts; Secretion of (B) IFN-γ and (C)IL-2 and (D) CRP, (E) IL-6, and (F) IL-1β in the blood. *,**,***P < 0.05, 0.01 and 0.001, n = 10.
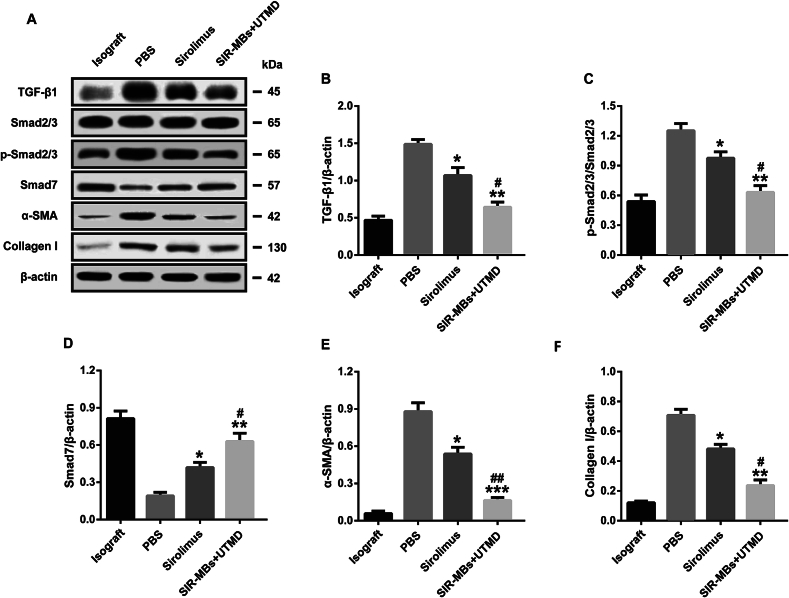
3.6. SIR-MBs combined with UTMD can inhibit TGF-β1-Smad signaling pathway activation and promote autophagy
The changes of TGF-β1-Smad signaling pathway-related proteins are shown in Fig. 8A. Compared with the tissue samples of rats in the homologous transplantation group, the relative expression levels of TGF-B1 (Fig. 8B), p-Smad2/3 (Fig. 8C), Smad7 (Fig. 8D), aSMA (Fig. 8E) and Col I proteins (Fig. 8F) in the heart tissues of rats in the xenotransplantation group were increased. The relative expression level of Smad7 protein decreased (P < 0.05); After SIR-MBs combined with UTMD or SIR-MBs, the relative expression levels of TGF-B1, p-Smad2/3, aSMA and Col I in rat heart tissues showed a downward trend, and the combined therapy group was significantly lower than that of sirolimus group (all P < 0.05), while the relative expression level of Smad7 was significantly increased (P < 0.01 vs. sirolimus group).
Fig. 8.
Effect of SIR-MBs combined with UTMD on TGF-β1-Smad signaling pathway in heart tissue of cardiac xenotransplantation rats. (A) Western blot gel image and relative expression levels of (B) TGF-B1, (C) p-Smad2/3, (D) aSMA and (E) Col I. *, **, ***P < 0.05, 0.01 and 0.001 vs. PBS group, n = 3. #, ##, ###P < 0.05, 0.01 and 0.001 vs. Sirolimus group, n = 3.
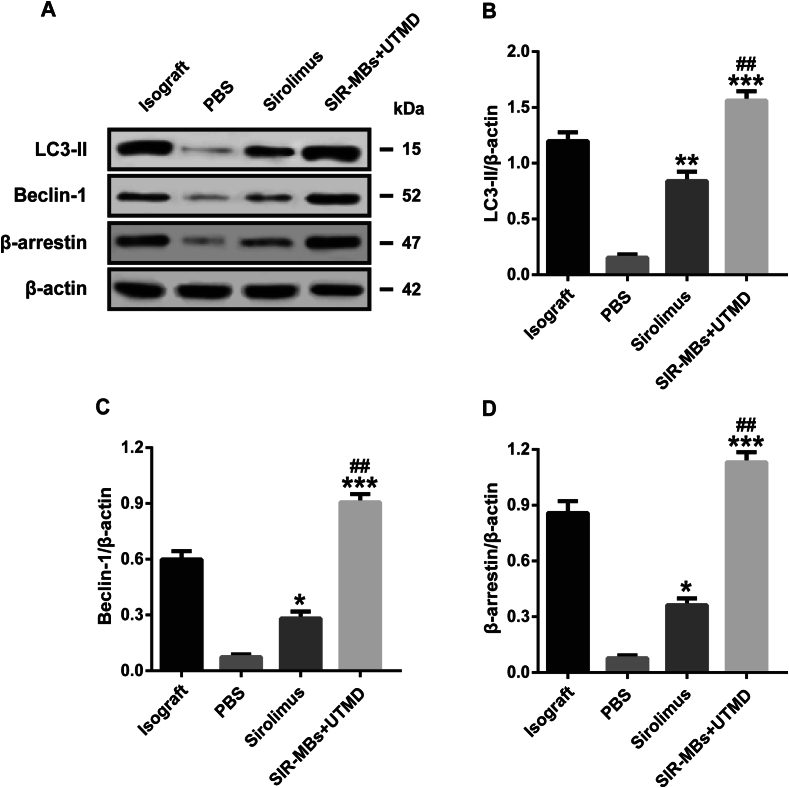
The expressions of autophagy proteins LC3-II, Beclin-1 and β-arrestin in the heart tissue of the combined therapy group showed an up-regulated trend (Fig. 9A). Moreover, the experimental results showed that the number of autophagosomes decreased significantly in transplanted heart tissues, while rapamycin pretreatment could significantly increase the number of autophagosomes, and the number of samples in SIR-MBs combined UTMD group was significantly higher than that in sirolimus group (P < 0.05, Fig. 9B-D).
Fig. 9.
Effect of SIR-MBs combined with UTMD on autophagy in cardiac tissue of cardiac xenotransplantation rats. A) Western blot gel image and relative expression levels of (B) LC3-II, (C), Beclin-1 and (D) β-arrestin. *, **, ***P < 0.05, 0.01 and 0.001 vs. PBS group, n = 3. #, ##, ###P < 0.05, 0.01 and 0.001 vs. Sirolimus group, n = 3.
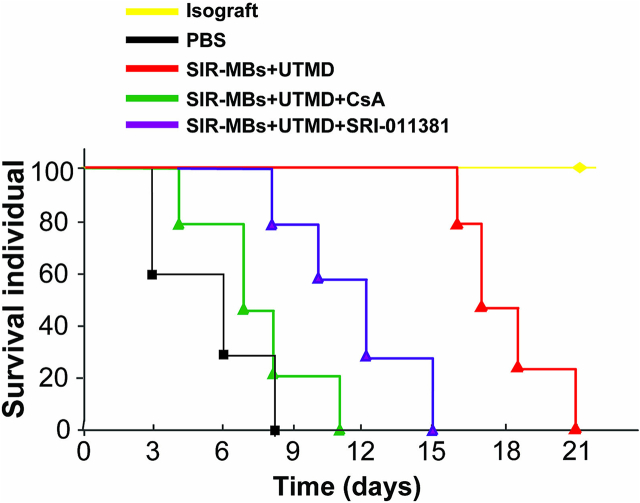
We further administered combination therapy in the rat HT model, and added the autophagy inhibitor cyclosporin A (CsA) group or SRI-011381 group to inhibit autophagy by reducing the degradation of mitochondrial autophagy related proteins, so as to investigate the effect of autophagy on the survival of animals after transplantation. The survival of the animals is shown in Fig. 10. Compared with the control group, the combination therapy can still significantly prolong the allograft, but the improvement trend of animal survival after CsA or SRI-011381 respectively is obviously inhibited. It is suggested that inhibition of autophagy and activation of TGF-β1-Smad signaling pathway weaken the protective effect of combined therapy on rat HT model.
Fig. 10.
Survival of rats undergoing heart transplantation.
4. Discussion
Acute rejection (AR) causes cardiac fibrosis, inflammation and myocardial injury through a variety of immune regulatory mechanisms, and histopathologically presents core changes such as myocardial interstitial inflammatory cell infiltration, edema, bleeding, myocardial cell damage/necrosis and capillary duct stenosis or fragmentation, leading to cardiac structural changes and functional disorders (Briasoulis et al., 2022; Choi et al., 2018). Therefore, the current clinical drugs targeting AR are mostly immunosuppressants, such as calcineurin inhibitors represented by tacrolimus and sirolimus and target protein inhibitors of rapamycin (Kerr et al., 2020). The former can effectively inhibit T cell activation and proliferation and the secretion of T cell-related inflammatory factors IL-2 and IFN-γ, thereby reducing T cell-mediated AR (Tsay and Eisen, 2019) and the risk of rejection in Heart transplantation (HT) patients (Paschier et al., 2023); The latter blocks signal transduction through different cytokine receptors, blocking the progression of T lymphocytes and other cells from G1 phase to S phase (Lim, 2018). In addition to calcineurin inhibitors, other corticosteroids such as glucocorticoids are also used clinically, but the proportion of actual use is not as high as tacrolimus or sirolimus, and the clinical side effects of glucocorticoid drugs are very significant (Dashti-Khavidaki et al., 2021).
However, long-term systemic administration of sirolimus can still lead to many side effects, such as nephrotoxicity, neurotoxicity, hypertension, hyperlipidemia, and diabetic effects, which not only affect the quality of life of patients, but also threaten the life safety of patients after HT (Sallam et al., 2021). At present, research in the industry generally believes that reducing systemic drug exposure, reducing blood drug concentration, and increasing drug concentration in diseased tissues is an effective way to reduce toxic side effects (Yang et al., 2018). In recent years, MBs has developed into a popular vehicle for delivering drugs to specific tissues, and combined ultrasonic targeted microbubble destruction (UTMD) technology such as these have shown promising applications in treating diseases such as the heart (Sun et al., 2020).
Therefore, in this study, we selected UTMD technology to carry out local targeted delivery of sirolimus microbubbles in the heart, hoping to establish an efficient local administration strategy of sirolimus, and investigate its safety and efficacy and the mechanism of enhanced effect in healthy and model animals. First of all, we prepared Sirolimus microbubbles (SIR-MBs), which has the characteristics of good biocompatibility and strong stability. Thanks to DSPC and DSPE-PEG2000 in excipients, as good phospholipid components, it can not only enhance the structural stability of microbubbles, but also enhance the biocompatibility of microbubbles. Protect it in the blood circulation is not easy to be cleared by the reticuloendothelial system. The physical and chemical parameters of SIR-MBs were further evaluated. The SIR-MBs prepared in this study was a milky suspension with uniform particle size and unipeak distribution, with an average particle size of 1.65 ± 0.32 μm and PDI of 0.16 ± 0.09. When SIR-MBs was stored at 4 °C or 37 °C for 72 h, the drug loss rates were 5.0 ± 1.2 % and 15.0 ± 4.4 %, respectively, indicating good stability.
Although some studies have shown that UTMD is a safe and non-invasive technology, for the overall security consideration when combined with SIR-MBs, we conducted a systematic evaluation of its safety at the beginning of the study (Walsh et al., 2021). We investigated the safety of UTMD combined with SIR-MBs in different conditions in healthy SD rats and rat models of abdominal ectopic HT to determine the appropriate therapeutic parameters and evaluate its safety. Our data show that SIR-MBs combined with UTMD does not cause significant damage to hematology, tissues, and organs, especially the heart, in healthy rats, suggesting that the safety of the combination therapy is fully guaranteed.
We propose to use UTMD technology to achieve instantaneous release of Sirolimus from SIR-MBs in the heart of model animals, achieving local high concentrations while avoiding continuous high concentrations in peripheral blood circulation. In order to verify this hypothesis, we first used SIR-MBs as a tracer molecule to investigate the distribution after combined with UTMD technology, and the results further proved that the fluorescence intensity of the transplanted heart in the SIR-MBs combined with UTMD group was much higher than that in the SIR group, and was stronger than that in other organs. We further used SIR-MBs combined with UTMD to investigate the local cardiac drug concentration in the rat abdominal ectopic HT model, and the results also proved that the concentration of sirolimus in the heart tissue of the SIR-MBs + UTMD group was at least 15 times higher than that of the SIrolimus group. These results prove that UTMD combined with SIR-MBs can indeed achieve local cardiac delivery and targeted drug release in microvesicles. However, whether the advantage of local drug concentration can be translated into an advantage in therapeutic effect still needs to be further investigated in model animals. Therefore, we further investigated the effect of SIR-MBs combined with UTMD on AR and animal survival in a rat model of abdominal ectopic HT.
The state of the model rats undergoing abdominal ectopic HT was relatively poor. The rats in the untreated group began to die successively on the 3rd day after surgery, while the animals in the Sirolimus group died only on the 7th day. Surprisingly, one animal in the SIR-MBs combined UTMD group died only 18 days after surgery. And the cause of death may be more infection at the wound site. Twenty-one days after operation, the survival rate of animals in SIR-MBs combined with UTMD group was the highest, which was significantly better than that in PBS group (P < 0.01) or Sirolimus group (P < 0.05). Histopathological examination of myocardial tissue of rats in each group showed that the proportion of inflammatory cell infiltration and cell necrosis in samples of SIR-MBs combined with UTMD group was the lowest, and the overall rejection grade was significantly lower, which was also consistent with the survival of animals.
We have observed in animal studies that combination therapy leads to better survival rate, cardiac histopathological scores and rejection grades, but the mechanism of action is unknown. According to previous reports, inhibition of T lymphocyte proliferation, activation and inflammatory cell secretion is the key to the treatment of AR (Wang et al., 2023; Ding et al., 2020). Therefore, in this study, we further investigated the T cell infiltration and cytokine release in the transplanted heart tissue, and the results also showed that the T cell infiltration and cytokine release were significantly lower in the SIR-MBs + UTMD group. Zhe Yang et al.'s research investigates the therapeutic effect of artemisinin on heart transplant rejection in rats (Yang et al., 2021). The results showed that it could significantly reduce graft rejection and tissue damage, and significantly prolong the survival time of transplantation (Yang et al., 2021). After further investigation of its mechanism, it was shown that artemisinin not only alleviated T cell-mediated rejection by reducing effector T cell infiltration and inflammatory cytokine secretion, and increased the levels of regulatory T cell infiltration and immune regulatory cytokine, but also weakened antibot-mediated rejection by inhibiting B cell activation and antibody production, which was consistent with the findings of this study (Yang et al., 2021).
In addition, sirolimus, as a mTOR inhibitor, also has a clear role in promoting autophagy (Kim and Guan, 2015). Autophagy, as another important intracellular signaling pathway, is also involved in the pathogenesis of cardiac fibrosis (Zhang et al., 2021). Our results show that combination therapy can significantly up-regulate the number of autophagosomes and the expression of autophagy proteins LC3-II, Beclin-1 and β-arrestin in transplanted heart tissue. Considering that low autophagy is a physiological manifestation after HT, and may in turn lead to fibrosis, which affects the survival of transplanted animals.
TGF-β superfamily consists of a subfamily of polypeptide growth factors related to structure and function, which is currently believed to regulate cell growth and differentiation (Morikawa et al., 2016). Tgf-β1 is a member of TGF-β superfamily and is the most powerful cytokine causing renal fibrosis, which can mediate a variety of signals involved in the occurrence and development of renal interstitial fibrosis, thus becoming a promoter of the process of renal interstitial fibrosis (Gifford et al., 2021). The key factor, TGF-β1 mainly relies on TGF-β superfamily signal protein Smad protein family to mediate its signal transmission, which successively binds to TβR-II and TβR-I to form a tetramer complex with kinase activity, and the activated TβR-I activation enzyme phosphorylates its downstream factors Smad2 and Smad3, making Smad2 and Smad3 It forms a complex with Smad4 that mediates TGF-β signaling from the cytoplasm into the nucleus to specifically regulate the expression of related fibrotic target genes, such as α-SMA expression (Xu et al., 2020). By binding with mTOR, Sirolimus inhibits the growth factors and cytokines that activate T and B cells, and becomes a “blocking agent” in the development of cardiac fibrosis (Alnsasra et al., 2021). After SIR-MBs combined with UTMD or SIR-MBs treatment, the relative expression levels of TGF-B1, p-Smad2/3, aSMA and Col I proteins in rat heart tissues showed a downward trend, suggesting a strong inhibitory effect on TGF-β1-smad signaling pathway.
Based on the above results, we propose that the beneficial impacts of SIR-MBs in conjunction with UTMD are mediated through the enhancement of autophagy and inhibition of the TGF-β1-Smad signaling pathway. Following transplantation, mTOR activation leads to suppressed autophagy in the heart, impeding the effective clearance of toxic protein aggregates and harmful cells. Prolonged cardiac fibrosis further sustains mTOR activation and maintains low autophagy levels. However, the combination of UTMD and SIR-MBs can effectively reverse this persistent pathological state, mitigating fibrosis progression in heart tissue and subsequently enhancing the survival rate of heart transplant recipients in rat models. To validate this potential mechanism, we conducted a salvage experiment in a rat heart transplant model. The introduction of the autophagy inhibitors CsA or SRI-011381 attenuated the protective effects of the combination therapy on the model rats. This finding suggests that inhibiting autophagy and activating the TGF-β1-Smad signaling pathway weaken the protective benefits of the combination therapy in the rat heart transplant model.
In summary, this study successfully prepared and utilized SIR-MBs to augment local drug concentration in the hearts of rat HT models through the application of UTMD. This approach aimed to achieve enhanced therapeutic efficacy while mitigating toxic side effects. Our findings indicate that the combination of SIR-MBs and UTMD can bolster protection against AR in rat HT models by inhibiting the TGF-β1-Smad signaling pathway, fostering autophagy, and modulating inflammation. The cardioprotective strategy involving UTMD and SIR-MBs holds promise for improving the clinical viability of heart transplantation in patients. However, further validation through comprehensive toxicological assessments and analysis of a larger cohort of clinical samples is necessary in the future.
Funding
This study was funded by National Major Scientific Research Instrument Development Project (82027803) and General Project of National Natural Science Foundation of China (81971623).
Ethical statement
Animal experiments were approved by the animal Experimental Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine.
Author contribution
HWB performed the experiments, analyzed the data and drafted the manuscript. LLD and HYW contributed to the study design and critical revision. TAJ supervised and administrated the projects. All authors reviewed and approved the final version of the manuscript.
CRediT authorship contribution statement
Haiwei Bao: Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Lulu Dai: Writing – review & editing, Writing – original draft, Software, Resources. Huiyang Wang: Writing – review & editing, Writing – original draft, Visualization, Validation. Tianan Jiang: Writing – review & editing, Writing – original draft, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data generated in the present study are included in the manuscript/suppl materials.
References
- Alnsasra H., et al. Impact of Sirolimus as a primary Immunosuppressant on Myocardial Fibrosis and Diastolic Function following Heart Transplantation. J. Am. Heart Assoc. 2021;10(1) doi: 10.1161/JAHA.120.018186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad M.A., Shah A., Griffith B.P. Current status and outcomes in heart transplantation: a narrative review. Rev. Cardiovasc. Med. 2022;23(1):11. doi: 10.31083/j.rcm2301011. [DOI] [PubMed] [Google Scholar]
- Briasoulis A., et al. Acute allograft rejection after heart transplantation: Outcomes of admissions and 30-day re-admissions. Hell. J. Cardiol. 2022;63:77–78. doi: 10.1016/j.hjc.2021.06.002. [DOI] [PubMed] [Google Scholar]
- Cai Q., et al. Ultrasound-targeted microbubble destruction rapidly improves left ventricular function in rats with ischemic cardiac dysfunction. Int. J. Cardiol. 2024;404 doi: 10.1016/j.ijcard.2024.131943. [DOI] [PubMed] [Google Scholar]
- Choi D.H., et al. Change in lymphocyte to neutrophil ratio predicts acute rejection after heart transplantation. Int. J. Cardiol. 2018;251:58–64. doi: 10.1016/j.ijcard.2017.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti-Khavidaki S., Saidi R., Lu H. Current status of glucocorticoid usage in solid organ transplantation. World J. Transplant. 2021;11(11):443–465. doi: 10.5500/wjt.v11.i11.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., et al. Oxidized-ATP attenuates kidney allograft rejection by inhibiting T-Cell, B-Cell, and macrophage activity. Kidney. 2020;360:106–114. doi: 10.34067/KID.0000692019. 1(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford C.C., et al. Negative regulators of TGF-β1 signaling in renal fibrosis; pathological mechanisms and novel therapeutic opportunities. Clin. Sci. (Lond.) 2021;135(2):275–303. doi: 10.1042/CS20201213. [DOI] [PubMed] [Google Scholar]
- Kancharla M., et al. Drug levels after sirolimus initiation and short-term outcomes in ambulatory heart transplantation recipients. Clin. Transpl. 2024;38(1) doi: 10.1111/ctr.15184. [DOI] [PubMed] [Google Scholar]
- Kerr S.M., et al. Assessment of rejection risk following subtherapeutic calcineurin inhibitor levels after pediatric heart transplantation. Pediatr. Transplant. 2020;24(1) doi: 10.1111/petr.13616. [DOI] [PubMed] [Google Scholar]
- Kim Y.C., Guan K.L. mTOR: a pharmacologic target for autophagy regulation. J. Clin. Invest. 2015;125(1):25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczaj A., et al. Does the induction immunotherapy (basiliximab) influence the early acute cellular rejection index after orthotopic heart transplantation?- preliminary assessment report. Transpl. Immunol. 2023;81 doi: 10.1016/j.trim.2023.101937. [DOI] [PubMed] [Google Scholar]
- Lee J.M., et al. Coronary microcirculatory dysfunction and acute cellular rejection after heart transplantation. Circulation. 2021;144(18):1459–1472. doi: 10.1161/CIRCULATIONAHA.121.056158. [DOI] [PubMed] [Google Scholar]
- Li H., et al. Highlights in ultrasound-targeted microbubble destruction-mediated gene/drug delivery strategy for treatment of malignancies. Int. J. Pharmaceut. 2022;613 doi: 10.1016/j.ijpharm.2021.121412. [DOI] [PubMed] [Google Scholar]
- Lim G.B. Transplantation: Sirolimus after heart transplantation. Nat. Rev. Cardiol. 2018;15(4):196. doi: 10.1038/nrcardio.2018.17. [DOI] [PubMed] [Google Scholar]
- Liu S., et al. Ultrasound-targeted microbubble destruction remodels tumour microenvironment to improve immunotherapeutic effect. Br. J. Cancer. 2023;128(5):715–725. doi: 10.1038/s41416-022-02076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa M., Derynck R., Miyazono K. vol. 8(5) Cold Spring Harb Perspect Biol; 2016. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschier A., et al. Tacrolimus population pharmacokinetics in adult heart transplant patients. Br. J. Clin. Pharmacol. 2023;89(12):3584–3595. doi: 10.1111/bcp.15857. [DOI] [PubMed] [Google Scholar]
- Sallam K., et al. Sirolimus adverse event profile in a non-clinical trial cohort of heart transplantation patients. Ann. Transplant. 2021;26 doi: 10.12659/AOT.923536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., et al. Ultrasound targeted microbubble destruction assisted exosomal delivery of miR-21 protects the heart from chemotherapy associated cardiotoxicity. Biochem. Biophys. Res. Commun. 2020;532(1):60–67. doi: 10.1016/j.bbrc.2020.05.044. [DOI] [PubMed] [Google Scholar]
- Sun Z., et al. Ultrasound-targeted microbubble destruction promotes PDGF-primed bone mesenchymal stem cell transplantation for myocardial protection in acute Myocardial Infarction in rats. J. Nanobiotechnol. 2023;21(1):481. doi: 10.1186/s12951-023-02204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutaria N., Sylvia L., DeNofrio D. Immunosuppression and Heart Transplantation. Handb. Exp. Pharmacol. 2022;272:117–137. doi: 10.1007/164_2021_552. [DOI] [PubMed] [Google Scholar]
- Suzuki J., et al. Ultrasound-microbubble-mediated intercellular adhesion molecule-1 small interfering ribonucleic acid transfection attenuates neointimal formation after arterial injury in mice. J. Am. Coll. Cardiol. 2010;55(9):904–913. doi: 10.1016/j.jacc.2009.09.054. [DOI] [PubMed] [Google Scholar]
- Tsay A.J., Eisen H.J. mTOR inhibitors vs calcineurin inhibitors: a Catch-22-preventing nephrotoxicity or acute allograft rejection after heart transplantation. Am. J. Transplant. 2019;19(11):2967–2968. doi: 10.1111/ajt.15578. [DOI] [PubMed] [Google Scholar]
- Walsh A.P.G., et al. Ultrasonic particles: an approach for targeted gene delivery. Adv. Drug Deliv. Rev. 2021;179 doi: 10.1016/j.addr.2021.113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., et al. Luteolin attenuates acute liver allograft rejection in rats by inhibiting T cell proliferation and regulating T cell subsets. Int. Immunopharmacol. 2023;121 doi: 10.1016/j.intimp.2023.110407. [DOI] [PubMed] [Google Scholar]
- Wong T.C., Keebler M.E. Cardiac magnetic resonance parametric mapping following heart transplantation: moving beyond acute rejection and coronary allograft vasculopathy assessment. JACC Cardiovasc. Imaging. 2020;13(7):1531–1533. doi: 10.1016/j.jcmg.2020.02.015. [DOI] [PubMed] [Google Scholar]
- Wu M.Y., Ali Khawaja R.D., Vargas D. Heart transplantation: indications, surgical techniques, and complications. Radiol. Clin. North Am. 2023;61(5):847–859. doi: 10.1016/j.rcl.2023.04.011. [DOI] [PubMed] [Google Scholar]
- Xu J., et al. Quercetin regulates fibrogenic responses of endometrial stromal cell by upregulating miR-145 and inhibiting the TGF-β1/Smad2/Smad3 pathway. Acta Histochem. 2020;122(7) doi: 10.1016/j.acthis.2020.151600. [DOI] [PubMed] [Google Scholar]
- Yang F., et al. Preparation of cationic lipid-coated ultrasound contrast agents and noninvasive gene transfection via ultrasound-targeted microbubble destruction. Curr. Pharm. Des. 2018;24(30):3587–3595. doi: 10.2174/1381612824666181011120031. [DOI] [PubMed] [Google Scholar]
- Yang Z., et al. Artemisinin attenuates transplant rejection by inhibiting multiple lymphocytes and prolongs cardiac allograft survival. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.634368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., et al. Knockout RAGE alleviates cardiac fibrosis through repressing endothelial-to-mesenchymal transition (EndMT) mediated by autophagy. Cell Death Dis. 2021;12(5):470. doi: 10.1038/s41419-021-03750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated in the present study are included in the manuscript/suppl materials.