Abstract
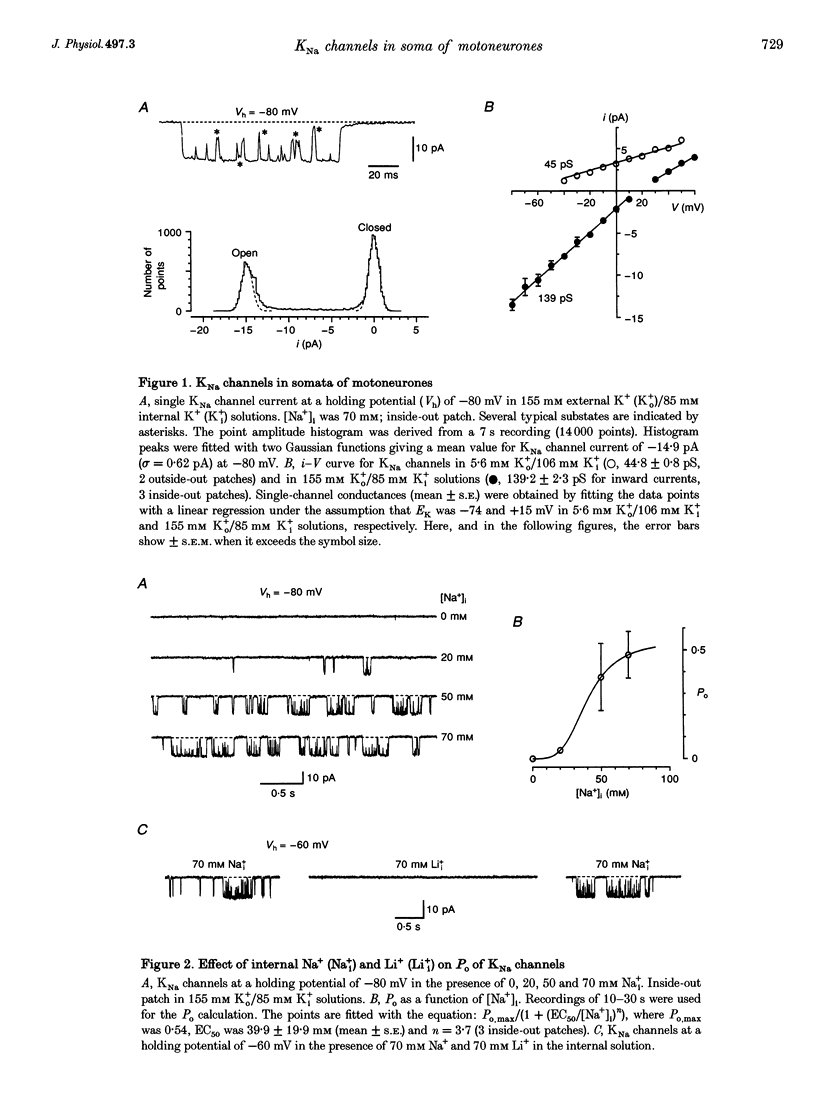
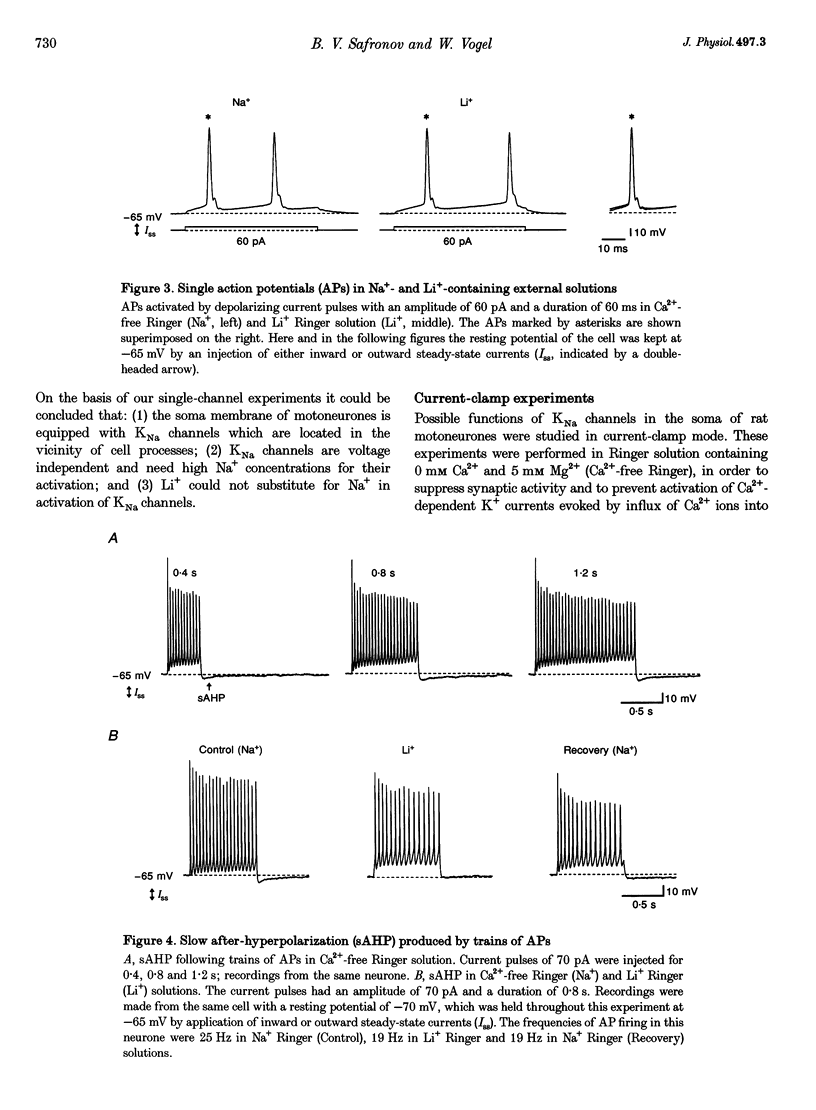
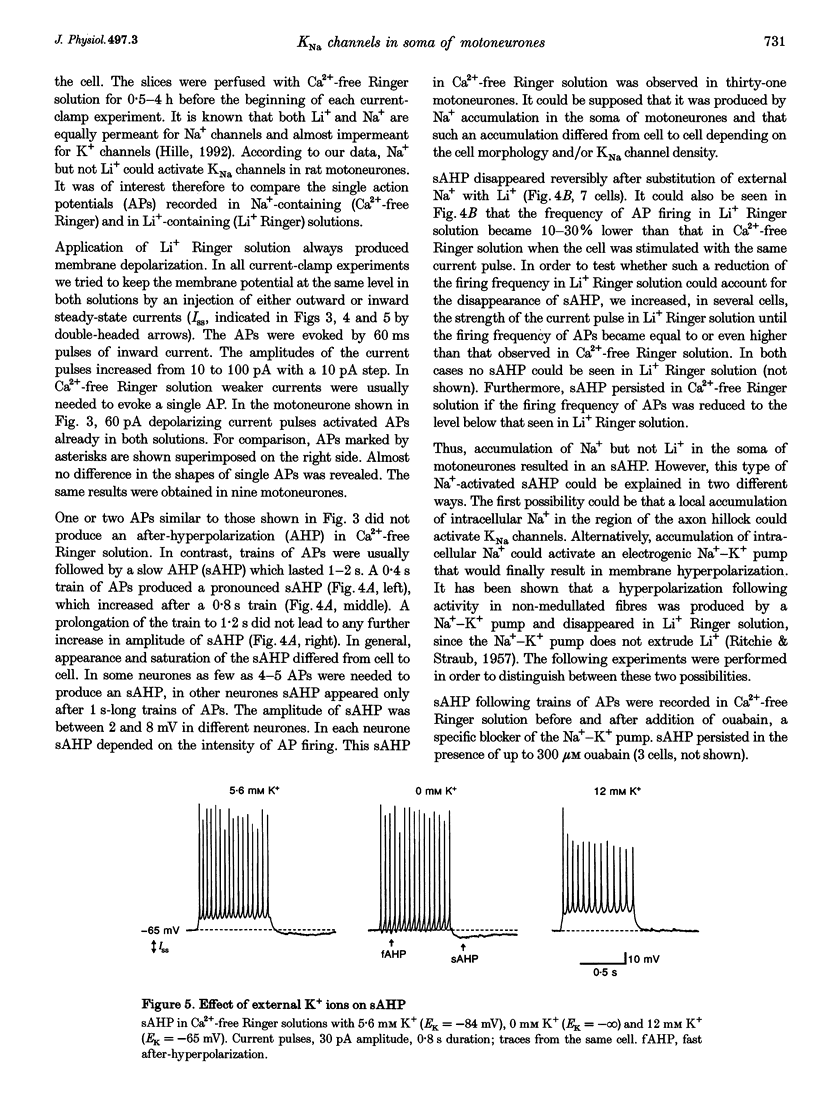
1.Properties and functions of Na(+)-activated K+ (KNa) channels in the soma of motoneurones were studied in spinal cord slices of newborn rat. KNa channels had a conductance of 44.8 pS in 5.6 mM external K+ (Ko+)/106 mM internal K+ (Ki+) solutions and 139.2 pS in 155 mM Ko+/85 mM Ki+ solutions. KNa channels were voltage independent and needed a relatively high [Na+]i to become active (EC50 = 39.9 mM). Li+ could not substitute for Na+ in activation of KNa channels. The channels were predominantly found in the vicinity of cell processes, in the regions of most probable accumulation of cytoplasmic Na+. 2. In current-clamp experiments, the shape of the single action potential (AP) recorded in Ca(2+)-free Ringer solution was not changed after substitution of external Na+ with Li+. However, 0.4-0.8 s trains of APs were followed by a slow (1-2s) after-hyperpolarization (sAHP), which reversibly disappeared when external Na+ was replaced by Li+. Na(+)-activated sAHP persisted after addition of ouabain and its amplitude was even increased in K(+)-free Ringer solution. sAHP disappeared when the membrane potential was equal to the K+ equilibrium potential. This indicated that sAHP resulted from activation of a Na(+)-dependent K+ conductance, rather than from activation of the electrogenic Na(+)-K+ pump. 3. In conclusion, KNa channels can play an important role in excitability of motoneurones. KNa channels do not make a contribution to the single AP, but they can be activated by a local accumulation of internal Na+ during trains of APs. A Na(+)-activated K+ conductance can reduce membrane excitability and contribute to regulation of AP firing in motoneurones.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dale N. A large, sustained Na(+)- and voltage-dependent K+ current in spinal neurons of the frog embryo. J Physiol. 1993 Mar;462:349–372. doi: 10.1113/jphysiol.1993.sp019559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E., Fujii J. T., Martin A. R. A Na+-activated K+ current in cultured brain stem neurones from chicks. J Physiol. 1989 Mar;410:283–296. doi: 10.1113/jphysiol.1989.sp017533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E. Na(+)-activated K+ channels and voltage-evoked ionic currents in brain stem and parasympathetic neurones of the chick. J Physiol. 1991 Apr;435:513–532. doi: 10.1113/jphysiol.1991.sp018522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E. Na(+)-activated K+ channels: a new family of large-conductance ion channels. Trends Neurosci. 1994 Apr;17(4):155–160. doi: 10.1016/0166-2236(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Dryer S. E. Properties of single Na(+)-activated K+ channels in cultured central neurons of the chick embryo. Neurosci Lett. 1993 Jan 12;149(2):133–136. doi: 10.1016/0304-3940(93)90754-9. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Dagan D., Kupper J., Levitan I. B. Na(+)-activated K+ channels are widely distributed in rat CNS and in Xenopus oocytes. Brain Res. 1992 Jul 3;584(1-2):319–321. doi: 10.1016/0006-8993(92)90913-t. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Dagan D., Kupper J., Levitan I. B. Properties and rundown of sodium-activated potassium channels in rat olfactory bulb neurons. J Neurosci. 1992 May;12(5):1964–1976. doi: 10.1523/JNEUROSCI.12-05-01964.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe P., Rimpel J., Reddy M. M., ten Bruggencate G. Changes of intracellular sodium and potassium ion concentrations in frog spinal motoneurons induced by repetitive synaptic stimulation. Neuroscience. 1982;7(12):3213–3220. doi: 10.1016/0306-4522(82)90243-3. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kubota M., Saito N. Sodium- and calcium-dependent conductances of neurones in the zebra finch hyperstriatum ventrale pars caudale in vitro. J Physiol. 1991;440:131–142. doi: 10.1113/jphysiol.1991.sp018700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee J. C., Johnston D. Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J Physiol. 1995 Aug 15;487(1):67–90. doi: 10.1113/jphysiol.1995.sp020862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov B. V., Vogel W. Single voltage-activated Na+ and K+ channels in the somata of rat motoneurones. J Physiol. 1995 Aug 15;487(1):91–106. doi: 10.1113/jphysiol.1995.sp020863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt P. C., Spain W. J., Crill W. E. Long-lasting reduction of excitability by a sodium-dependent potassium current in cat neocortical neurons. J Neurophysiol. 1989 Feb;61(2):233–244. doi: 10.1152/jn.1989.61.2.233. [DOI] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Chubb M. C., Crill W. E. Properties of persistent sodium conductance and calcium conductance of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1985 Jan;53(1):153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- Stuart G. J., Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994 Jan 6;367(6458):69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. J Physiol. 1990 Apr;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Kimitsuki T., Noma A. Conductance properties of the Na(+)-activated K+ channel in guinea-pig ventricular cells. J Physiol. 1991 Feb;433:241–257. doi: 10.1113/jphysiol.1991.sp018424. [DOI] [PMC free article] [PubMed] [Google Scholar]


