Abstract
This study aimed to investigate the impact of diurnal and seasonal variations in photon flux density (PPFD) and air temperature on PSII efficiency in three sweet potato leaf-color cultivars: green (G), yellow-green (Y), and purple (P). The cultivars were exposed to full sunlight and measurements were taken from November to March. The maximal quantum yield of PSII photochemistry for the dark-adapted state (Fv/Fm) indicated Y's increased sensitivity to low temperatures at predawn, followed by G and P. Both quantum yield of PSII photochemistry for the dark and light-adapted state (ΔF/Fm') depressions were correlated with increased PPFD, with regression slopes in the order of Y > G > P. On high-light and low-temperature days, Fv/Fm values deviated below regression lines, with differences ranked as Y > G > P. These findings suggest that Y exhibits the highest sensitivity to high light and low temperatures, followed by G and then P in terms of PSII efficiency.
Keywords: leaf pigments, chlorophyll fluorescence, nonphotochemical quenching, photoinhibition, photoprotection
Highlights
Predawn Fv/Fm in sweet potatoes: yellow-green (Y) variety is the most sensitive
Noon and 17:00 h: Fv/Fm and ΔF/Fm' dips with high light, Y > G > P
On high light days, Fv/Fm values deviated below regression lines, with differences ranked as Y > G > P
Introduction
There are usually multiple varieties having differing leaf colors in vegetables and foliage plants. Different leaf colors are due to physical leaf structures (Chen et al. 2017) and pigment content chemistry (Li et al. 2019). Leaf color is typically green when chlorophyll (Chl) predominates in plant leaves. When leaves have less Chl content, they are usually yellow-green (Peng et al. 2002, Weng et al. 2011, Yang et al. 2020). Leaves containing anthocyanins appear red or purple (Lin et al. 2019, Stetsenko et al. 2020). Leaf pigment content not only affects appearance but also affects photosynthetic performance (Peng et al. 2002, Weng et al. 2011, Yang et al. 2020). The quality of light, referring to the wavelength and spectral components, exerts profound effects on the process of photosynthesis in plants (Lazar et al. 2022). The color of plant leaves impacts their utilization of the light spectrum because the pigments influencing leaf color also affect the absorption and utilization of light.
Chl is an essential pigment for photosynthesis, as it absorbs light energy and converts it to chemical energy, especially in the red and blue ranges of the spectrum (Gitelson et al. 2003). Red light primarily participates in photosynthesis, promoting biomass synthesis. Blue light is effective in stomata regulation, as well as biomass accumulation (Li and Kubota 2009). However, when leaves absorb more energy than they can utilize, this excessively absorbed energy may lead to photosystem damage (Demmig-Adams and Adams 1996, Kato et al. 2003, Adams et al. 2004). Plants can employ several mechanisms to protect the photosystem, such as xanthophyll-dependent nonphotochemical quenching of the excited Chl states (NPQ), to dissipate the excess energy as heat (Demmig-Adams and Adams 1996, Jahns et al. 2009), and utilize antioxidants to reduce the oxidative stress caused by reactive oxygen species from excessively absorbed energy (Smirnoff 2000). Carotenoids also absorb blue light, causing around 90% attenuation of blue and red light in the top 20% of the leaf, while a significant portion of green light penetrates deeper (Cui et al. 1991, Lazar et al. 2022). Anthocyanins are water-soluble, nonphotochemically active pigments belonging to the flavonoid family of plant secondary metabolites. Anthocyanins are ubiquitous and can be found in flowers, fruits, stems, and leaves in the vegetative and reproductive organs of plants (Tanaka and Ohmiya 2008). Anthocyanins typically exhibit their absorption peaks in the blue-light region of the visible spectrum. Anthocyanins play a vital regulatory role in plant photosynthesis and survival by providing additional light absorption (Gould 2004). The function of anthocyanin in plant leaves involves protection against various biotic and abiotic stresses. Conditions such as high irradiance and low temperature can cause damage to plant PSII, anthocyanin contributes to plant protection by both diminishing excess light through absorption and by acting as an antioxidant to shield the photosystem from oxidative damage (Zhang et al. 2019, Stetsenko et al. 2020).
Whether excess energy is absorbed depends on the amount of light energy absorbed and consumed. When light intensity increases, a cell can decrease the fraction of absorbed energy to protect itself against photodamage. When CO2 exchange is inhibited, such as during low temperatures, energy consumption is reduced (Baker 1993, Leegood 1995). Thus, both high irradiance and low temperature can cause damage to the PSII of plants (Weng et al. 2013, Huang et al. 2016, Lin et al. 2021). A reduction of Chl content reduces the ability of leaves to absorb photons as well as changes in the light-harvesting apparatus and the efficiency with which absorbed photons are subsequently used in photosynthesis (Peng et al. 2002, Goh et al. 2009, Yang et al. 2020). However, the photoprotective mechanisms of leaf-color mutants are diverse. Some mutants show more efficiency in photoprotection than wild types of the same species. Yet, the opposite trend is also found in other mutants (Peng et al. 2002, Goh et al. 2009, Yang et al. 2020). Our previous studies (Weng et al. 2011) found that compared to green cultivars of the same species yellow-green foliage cultivars of four vegetables showed lower Chl and total carotenoid values, smaller PSII antenna size, and lower photosynthetic capacities. Due to a lower ability in xanthophyll cycle-dependent energy quenching (qE), yellow-green sweet potato (Ipomoea batatas L.) cultivars showed more photoinhibition under high irradiance, particularly in low temperatures (Weng et al. 2011, Lin et al. 2021).
Sweet potato is an important root crop and leafy vegetable in the subtropical and tropical regions of the world. It is relatively tolerant to environmental stresses; however, it is sensitive to low-temperature conditions (Noh et al. 2009). In Taiwan (21°53'N–25°18'N), sweet potato is often cultivated through the winter. In subtropical regions, chilling temperatures (i.e., < 10°C) coincide frequently with bright mornings as a result of rapid heat dissipation by the emission of long-wave radiation during clear nights. Thus, high-light and low-temperature stresses may occur simultaneously. In addition, high-light irradiances exert adverse effects on sweet potato plants during warmer seasons. Temperature and light changes evoke a variety of photosynthetic responses that can vary among different sweet potato species with different leaf colors (Weng et al. 2011, Lin et al. 2021). Such photo- and temperature responses are of practical importance in recent plant cultivation technologies. There are many sweet potato cultivars with different leaf colors, including yellow-green, green, and purple. Our previous studies indicated that the PSII efficiency of a yellow-green cultivar was more sensitive to low temperature and high light than the green cultivar. It was reported that in many cases, seasonal variation in PSII efficiency can reflect the capacity of photosynthetic CO2 exchange (Corcuera et al. 2005, Weng et al. 2005), and seasonal variations in lutein, neoxanthin, and β-carotenoid can reflect the capacity of photoprotection (Corcuera et al. 2005). In addition, under dynamic or diurnal variations in light intensity, photosynthetic rates are closely correlated with the value obtained by light intensity × PSII efficiency (Wong et al. 2012, Sun and Wang 2018, Alemu and Roro 2020, He and Qin 2020, Huang et al. 2021).
PSII efficiency can be assessed quickly and easily by Chl fluorescence quenching analysis and is a reliable method for assessing changes in the functioning of PSII under different environmental and physiological conditions (Roháček and Barták 1999, Maxwell and Johnson 2000). It has been widely used to monitor functional changes such as seasonal and diurnal variations in the photosynthesis apparatus under different conditions, as well as in responses to stresses (Demmig-Adams and Adams 1996, Verhoeven et al. 1999, Close et al. 2001, Weng et al. 2005, Li et al. 2021). However, most experiments with sweet potato cultivars having different leaf colors have been made under artificial conditions. Moreover, the purple cultivar has not been explored (Weng et al. 2011, Lin et al. 2021). The response in the PSII efficiency of sweet potato cultivars to natural diurnal and seasonal variations in air temperature and PPFD under natural conditions is not clear, so in this study, we used three cultivars having green, yellow-green, and purple leaves to elucidate these variable responses.
Materials and methods
Plant materials and growth conditions
Three sweet potato [Ipomoea batatas (L.) Lam] cultivars having green (Taoyuan No. 2), yellow-green (cv. CN1927), and purple leaves (CYY 8467) were used. Plant materials propagated from cuttings were planted into pots (16-cm diameter, 12-cm depth) filled with sandy loam and placed outdoors to receive regular water and fertilizers (1/2 strength of Hoagland's nutrient solution) and full sunlight on the campus of National Chung-Hsing University, Taichung, Taiwan (24°08'N, 120°40'E, 70 m a.s.l.). The mean monthly air temperature was 17–26°C during the growing season (October–March).
Air temperature and photosynthetic photon flux density
PPFD and air temperature were measured with an LI-190SA sensor (LI-COR, Lincoln, NE, USA) and copper constantan thermocouples, respectively. Sensors were connected to a data logger (CR10, Campbell Scientific, Logan, UT, USA) that collected data automatically every 2 min, and the averaged values for each hour were recorded.
Leaf pigments
Leaves were collected in November for the determination of Chl content using the methods described by Yang et al. (1998). Leaf discs were excised using a standard hole punch, immediately sealed in prelabeled aluminum envelopes, and placed in liquid nitrogen. Sixteen pieces of each color leaf were selected for measuring pigments. Tissues were stored at –80°C until analysis and then extracted in 80% (v/v) aqueous acetone. Supernatants were obtained by centrifuging mixtures at 3,000 rpm for 10 min. Supernatants were assayed for the absorbance of Chl a and Chl b at 440.5, 663.6, and 646.6 nm in a 10-mm quartz cuvette (Hellma, Germany), with a U-2000 type spectrophotometer (Hitachi, Tokyo, Japan). Chl a, Chl b, and carotenoid (Car) concentrations were calculated using the following equations (Yang et al. 1998):
Chl a [μg m–2] = (12.25 A663.6 – 2.55 A646.6) × volume of supernatant [mL]/leaf area [m2]
Chl b [μg m–2] = (20.31 A646.6 – 4.91 A663.6) × volume of supernatant [mL]/leaf area [m2]
Car [μg m–2] = (4.69 A440.5 × volume of supernatant [mL]/ leaf area [m2]) – 0.267 Chl (a+b) [μg m–2]
The anthocyanin content of extracts was measured based on the protocol of Mancinelli et al. (1975). A mixture of 99% methanol containing 1% HCl (v/v) was added to powdered samples and incubated for 1 h at room temperature. The mixture was then centrifuged at 4°C and 3,000 rpm for 5 min to obtain a supernatant, followed by measuring absorbances at 530 and 657 nm on a spectrophotometer. The following equation (Mancinelli et al. 1975) was used:
Anthocyanin [mmol m–2] = (A530 – 0.33 × A657/31.6) × volume of supernatant [mL]/leaf area [m2]
Chl fluorescence measurements
Chl fluorescence was determined by a PAM-2000 fluorometer (Walz, Effeltrich, Germany) with a low-intensity beam from light-emitting diodes (excitation wavelength at 655 nm, detection above 700 nm, 1.6 kHz). The minimum fluorescence intensities (F0, dark-adapted state; F0', light-adapted state) and the maximum fluorescence intensities induced by a brief saturation light [0.8 s, > 5,000 μmol(photon) m–2 s–1] (Fm, dark-adapted state; Fm', light-adapted state) were detected, respectively, and used to calculate the quantum yield of photochemistry as Fv/Fm = (Fm – F0)/Fm and ΔF/Fm' = (Fm' – F0')/Fm' (Kitajima and Butler 1975, Genty et al. 1989). Fv/Fm – ΔF/Fm' represents the difference between the maximal quantum yield of PSII photochemistry for the dark-adapted state at predawn and the efficient quantum yield of PSII photochemistry for the light-adapted state at noon. Nonphotochemical quenching (NPQ) was calculated as Fm/Fm' – 1 (Maxwell and Johnson 2000). From November to March, the maximal quantum yield of PSII photochemistry for the dark-adapted state (Fv/Fm) at predawn, noon, and dusk, and the efficient quantum yield of PSII photochemistry for the light-adapted state (ΔF/Fm') at noon, were measured every 1 to 5 d. At predawn, potted materials were moved to a dark room before sunrise (5:40 h). Then Fv/Fm was measured at 6:00 h. After the measurement of Fv/Fm, potted materials were put outdoors to receive full sunlight until noon. At noon (12:00–12:20 h), the ΔF/Fm' was measured by the same fluorometer under sunlight. Materials were then moved to a darkroom for 20 min, and then Fv/Fm was measured from the same leaves that were used to measure ΔF/Fm'. Measurements were taken in the darkroom to avoid underestimating Fv/Fm, because large F0 values could have resulted from the high leaf temperatures that could have occurred when leaves were clipped under high illumination (Weng 2006). After the measurement of Fv/Fm, materials were moved outdoors again until measurements of Fv/Fm were taken at dusk. At 17:00 h, potted materials were moved to the darkroom for 20 min and then Fv/Fm was measured.
Statistical analysis
In this study, one leaf from each pot was designated as a replicate, with four replicates measured in total. The study aimed to compare the pigment content [Chl (a+b), carotenoid, and anthocyanin] across three leaf colors. The data were analyzed using analysis of variance (ANOVA), followed by the Least Significant Difference (LSD) test, with significance determined at p<0.05. Chl fluorescence data were examined through linear or logarithmic regression models, depending on statistical significance (p<0.05). Correlation coefficients (r for linear models) and coefficients of determination (r² for nonlinear models) were calculated using Sigma Plot 9.01 (Systat Software, Point Richmond, CA, USA). The normality of the data was assessed using the Kolmogorov–Smirnov's test.
Results
The pigment contents of the yellow-green (Y), green (G), and purple (P) sweet potato leaves are shown in Table 1. The Chl (a+b), Car, and Ant values observed in P leaves were 0.41 g m–2, 0.058 g m–2, and 11.8 mmol m–2, respectively. Significantly lower Chl (a+b) and Car contents in Y leaves were compared to the others, and P had the highest Chl (a+b), followed by G. The Car of P and G was higher than that in Y, but there was no significant difference between P and G. Y and G were not tested for Ant, so no such data are presented. For the Chl a/b ratio, the Y leaves had the highest values (4.99), followed by G leaves (3.2) (Table 1). The different contents of Chl, Chl a/b, Car, and Ant may lead to variations in photosynthetic efficiency among three colored leaves.
Table 1. Contents of chlorophyll (a+b), carotenoids, and anthocyanin in yellow-green, green, and purple sweet potato leaves. Each point represents the mean of four leaves. Data are mean ± SE. Different letters indicate significant differences in the LSD analyses of Chl (a+b) and Car.
| Leaf color | Chl (a+b) [g m–2] | Chl a/b ratio | Carotenoids [g m–2] | Anthocyanin [mmol m–2] |
| Yellow-green | 0.096 ± 0.007c | 4.99 ± 0.89a | 0.037 ± 0.002b | Not determined |
| Green | 0.337 ± 0.024b | 3.20 ± 0.25b | 0.060 ± 0.002a | Not determined |
| Purple | 0.413 ± 0.019a | 2.58 ± 0.18c | 0.058 ± 0.003a | 11.802 ± 0.910 |
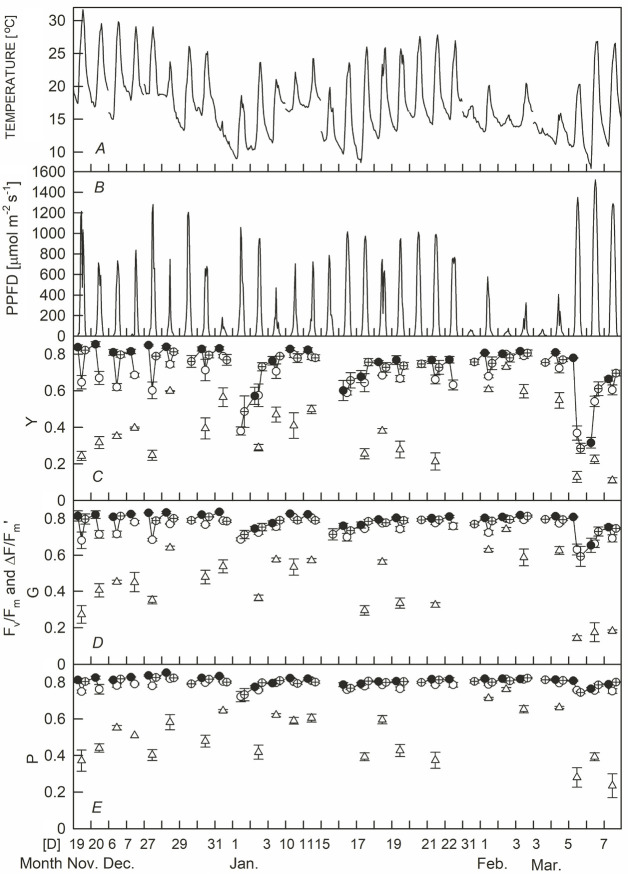
During the experiment period (19 November to March), predawn temperatures ranged from 18.9°C (27 December) to 7.5°C (6 March). Temperatures drop significantly when cold fronts strike in winter in Taiwan. The temperature at noon was between 31.7°C (19 November) and 12.9°C (3 March). The average PPFD of 11:00–12:00 h was the highest at 1,440 μmol m–2 s–1 (6 March), and the lowest was 35 μmol m–2 s–1 (3 March) (Fig. 1A,B). PPFD was mainly affected by the amount of cloud cover at that time. Because sunlight raises temperatures during the day, a day with strong sunlight has a large day–night temperature difference. During the experiment, the difference between daily maximum and minimum temperatures ranged from 19.3°C (6 March) to 1.7°C (2 February). Fig. 1C–E shows the Fv/Fm of the three leaf-color sweet potato cultivars at predawn, noon, and dusk, and the ΔF/Fm' under light at noon. The results show that, in the morning, Fv/Fm decreased with increasing PPFD and recovered gradually with decreasing PPFD in the afternoon. The predawn Fv/Fm ranges of Y, G, and P were 0.32–0.85, 0.65–0.84, and 0.77–0.85, respectively. At noon, these values were 0.37–0.80, 0.63–0.81, and 0.75–0.82, and at dusk were 0.28–0.82, 0.59–0.81, and 0.73–0.83, respectively. The variation range of Fv/Fm was the largest for Y and smallest for P. It is worth noting that Y showed a higher Fv/Fm value at noon than at predawn on 6 March, when there was a cold predawn as well as a bright and warmer noon. The noon ΔF/Fm' of the three leaf-color sweet potato cultivars were 0.11–0.76, with Y showing the lowest values (0.11–0.73), then G (0.14–0.74), and P the highest (0.23–0.76).
Fig. 1. Temperature (A), photosynthetic photon flux density (B), and photosystem II efficiency of yellow-green (C), green (D), and purple-leaf sweet potato (E) at predawn (●), noon (○ and △), and dusk (⊕). Circle and triangle symbols indicate the quantum yield of PSII photochemistry for dark (Fv/Fm) and light-adapted (ΔF/Fm') state, respectively. The error bar is SE (n = 4).
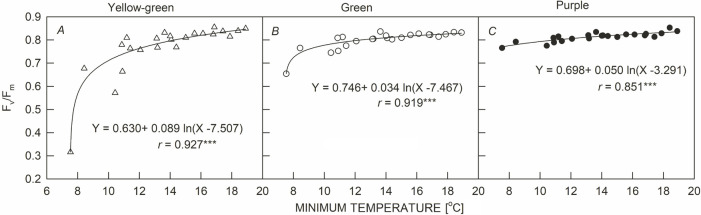
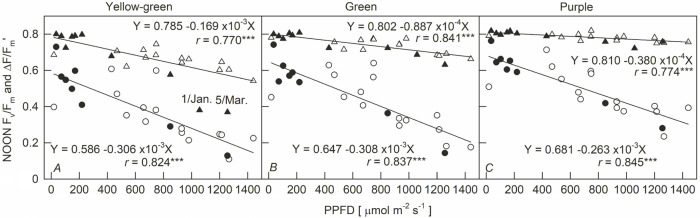
The predawn Fv/Fm values of the three leaf-color sweet potato cultivars showed a significant positive logarithmic curve with the daily minimum temperature (r = 0.851–0.927, p <0.001) (Fig. 2). In warmer predawns, the Fv/Fm of the three sweet potato cultivars were all above 0.8. Y dropped slowly below 16°C and then dropped sharply below 10°C. G and P decreased slowly below 14°C and 12°C, respectively. G dropped sharply below 8°C, while P did not drop sharply until 7.5°C. This shows that the Fv/Fm of the three sweet potato cultivars decreased when the predawn temperature dropped to a certain level. The temperature at which the Fv/Fm begins to decrease, and the degree of decrease, was the highest for Y, then G, and then P. The Fv/Fm of the three sweet potato cultivars decreased with the increase in PPFD at noon, and there was a significant negative linear correlation (r = 0.770–0.841, p <0.001, Fig. 3, triangle symbols). The noon Fv/Fm values of the three sweet potato cultivars were around 0.8 for the low PPFD. However, the slope of the Fv/Fm decrease was as follows: Y was the largest (0.169 × 10–3), G second (0.887 × 10–4), and P the lowest (0.380 × 10–4) as PPFD increased. The Fv/Fm of Y showed more drastic depressions on 1 January and 5 March, as those two days had low temperatures (< 20°C) and high PPFD values (> 1,000 μmol m–2 s–1). The relationship between the PPFD and Fv/Fm of G and P was not affected by low temperatures at noon (Fig. 3B,C).
Fig. 2. Relationship between the maximal quantum yield of PSII photochemistry for dark-adapted state (Fv/Fm) and daily minimum air temperature at predawn on sweet potato plants having different leaf colors (△: yellow-green leaves, ○: green leaves, ●: purple leaves). Each line represents the mean of 23 points. ***: p<0.001.
Fig. 3. Relationship between the maximal quantum yield of PSII photochemistry for dark-adapted state (Fv/Fm) and photosynthetic photon flux density (PPFD) for sweet potatoes with different leaf colors at noon. Triangle and circle symbols are for Fv/Fm and ΔF/Fm' at noon, respectively. Closed and open symbols indicate that the average temperature (11:00–12:00 h) was below and above 20°C, respectively. Each line represents the mean of 24 points. ***: p<0.001.
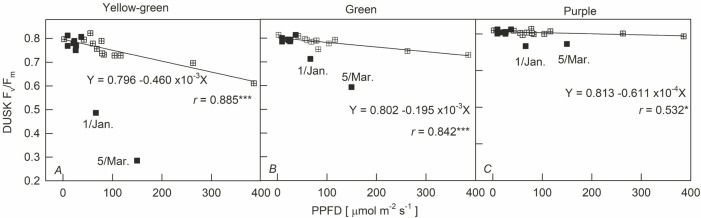
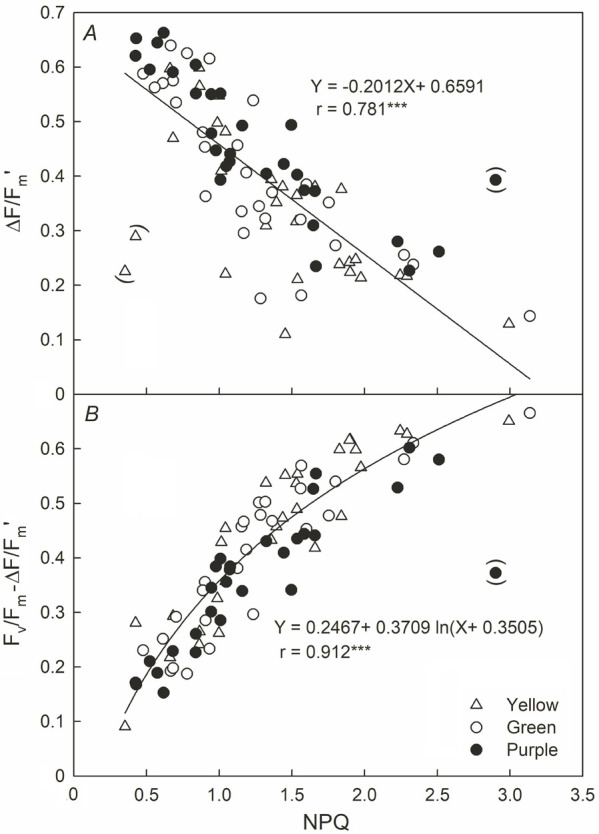
The noon ΔF/Fm' decreased linearly with the increase in PPFD (Fig. 3, circle symbols), but the difference in the slope of the decline between the different leaf colors was about 1.2 times (0.263–0.308 × 10–3). The difference in the slope of Fv/Fm decreasing with the increase in PPFD was about 4.4 times (0.380 × 10–5–0.169 × 10–4). The ΔF/Fm' was still decreasing under 100 μmol m–2 s–1 low light, and the degree of decrease was Y > G > P. The dusk Fv/Fm showed a positive linear regression with PPFD (16:00–17:00 h), its decreasing slope being Y > G > P (Fig. 4). However, the Fv/Fm was much lower than the regression line on the two days having high light and low temperature (1 January and 5 March), and the degree of deviation between the Fv/Fm value and the regression line was Y > G > P. These results were similar to Fig. 3A. At low PPFD, the Fv/Fm values of the three cultivars were restored to nearly 0.8. In addition, the decrease of PSII in sweet potato leaves was also investigated by NPQ dissipation in this study. Fig. 5 shows that both ΔF/Fm' and Fv/Fm – ΔF/Fm' values were negatively correlated with NPQ (r = –0.781 and –0.912) in the leaves of sweet potato, respectively.
Fig. 4. Relationship between the maximal quantum yield of PSII photochemistry for dark-adapted state (Fv/Fm) in sweet potatoes having different leaf colors and photosynthetic photon flux density (PPFD) at dusk. Closed and open symbols indicate that the average temperature (16:00–17:00 h) was below and above 20°C, respectively. Each line represents the mean of 24 points. * and ***: p<0.02 and 0.001, respectively.
Fig. 5. Relationship between nonphotochemical quenching (NPQ) and the efficient quantum yield of PSII photochemistry for the light-adapted state (ΔF/Fm') in sweet potatoes having different leaf colors. ***: p<0.001. The brackets indicate that the measurements were taken during a cold current, resulting in extremely low temperatures at dawn and high light but low temperatures at noon (the data was not included in the calculation).
Discussion
Seasonal variation of Fv/Fm at predawn
When leaves are exposed to light, PSII efficiency decreases with increasing irradiance and decreasing temperature (Weng et al. 2013, Acebron et al. 2021, Lin et al. 2021). In prior research concerning Chl fluorescence induction parameters, the use of mean and standard deviation for expressing parameters has been discouraged (Lazár and Nauš 1998). Therefore, in this study, data measured from a single leaf were utilized to perform analyses using linear or logarithmic regression models. In the present study, our work revealed a negative correlation between ΔF/Fm' and NPQ in sweet potato. Similarly, Lin et al. (2021) also indicated that NPQ is negatively correlated with Fv/Fm and ΔF/Fm', respectively, in sweet potato under different light intensities. These results suggested that the depression in PSII efficiency in sweet potato under high light is mainly due to xanthophyll-dependent NPQ dissipating excess energy as heat (Demmig-Adams and Adams 1996, Jahns et al. 2009, Lin et al. 2021). Within the xanthophyll cycle, violaxanthin (V) is de-epoxidized first into antheraxanthin (A) and then into zeaxanthin (Z) (Hager and Holocher 1994). Nevertheless, PSII efficiency gradually recovers when sunshine mellows in the afternoon (Verhoeven et al. 1999, Santanoo et al. 2019, Acebron et al. 2021) due to the conversion of Z to A, and then to V (Demmig-Adams and Adams 1996, Verhoeven et al. 1999). However, after high-light illumination, particularly at low temperatures, high contents of A + Z may be maintained for a long time. Therefore, at predawn in winter, large amounts of A + Z are retained and low values of intrinsic PSII efficiency are sustained (Verhoeven et al. 1999, Adams et al. 2004, Weng et al. 2006). The depression of predawn Fv/Fm during winter is due to chronic photoinhibition and is related to a slowly reversible loss of PSII reaction center functioning (Long et al. 1994, Close et al. 2001). Previous studies (Blennow et al. 1998, Weng et al. 2005, 2006) have pointed out that predawn Fv/Fm in several plant species is closely related to the daily minimum temperature. We also found that, in subtropical Taiwan, tropical species show a greater depression in predawn Fv/Fm, and accumulate more A + Z with decreasing temperature, than species distributed over a broad altitude range (Weng et al. 2006). The same tendency is observed in Fig. 2. On warmer days, predawn Fv/Fm was above 0.8, indicating that no chronic photoinhibition occurred in PSII (Björkman and Demmig 1987, Bolhàr-Nordenkampf et al. 1989). However, lower Fv/Fm was observed in the cool predawn, and predawn FV/Fm values of Y were most sensitive to low temperature, followed by G and then P. This may be due to the low night temperature delay of the epoxidation of Z to A and V, and lead to the downregulation of PSII (Verhoeven et al. 1999, Adams et al. 2004, Weng et al. 2006).
Effect of light intensity and air temperature on Fv/Fm and ΔF/Fm' at noon
Generally, the factors that reduce the efficiency of PSII include increasing light intensity and low temperature. PSII efficiency is the highest in the early morning before sunrise, then decreases with increasing light intensity, and reaches a minimum mostly at noon (Demmig-Adams et al. 1996, Verhoeven et al. 1999, Santanoo et al. 2019, Yang et al. 2020, Acebron et al. 2021). The decrease in PSII efficiency with increasing light intensity occurs drastically in low temperatures (Huang et al. 2016, Santanoo et al. 2019). This same tendency is also seen in Figs. 1 and 3.
Under illumination, the depression of PSII efficiency is correlated with an increase in NPQ (Demmig-Adams et al. 1996, Müller et al. 2001). At least four different components of NPQ are defined by their formatting and dark relaxation kinetics. The fastest and most important component of NPQ is qE (Müller et al. 2001, Johnson and Ruban 2011). This is closely related to: (1) the thylakoid membrane proton gradient (ΔpH), (2) the xanthophylls pool ratio change, and (3) the PsbS protein of PSII (Müller et al. 2001, Lavaud and Lepetit 2013). Nilkens et al. (2010) reported that after the completion of the reaction in subunit PsbS, zeaxanthin (Z) is combined with PsbS to dissipate H+. Therefore, the part after the reaction of qE is called qZ and is similar to the slower NPQ response identified by Maxwell and Johnson (2000). The third component of NPQ is qT (phosphorylation shift-dependent quenching) (Quick and Stitt 1989), which shows the phosphorylation shift of the light-harvesting complex (LHC) II between PSII and PSI. The slowest reaction component of NPQ is qI (photoinhibitory quenching), which is a slowly inducible and reversible component, takes several hours to relax and is related to the photoinhibition of photosynthesis (Müller et al. 2001). It is also known as sustained NPQ and is associated with zeaxanthin retention (Demmig-Adams et al. 2006).
In this study, ΔF/Fm' was measured under sunlight, and Fv/Fm was measured after 20 min of dark adaption. By noon, leaves had been exposed to sunlight for several hours, thus, the depression of ΔF/Fm' included the formation of qE, qZ + qT, and qI. While the depression of Fv/Fm was mainly due to the qI, Fv/Fm is widely used as an indicator of photoinhibition (Adams et al. 2004, Gorbe and Calatayud 2012). Fig. 3 shows that the intercept of the Fv/Fm–PPFD regression in the three cultivars was nearly 0.8. This indicates that, under low PPFD, no chronic photoinhibition can be found (Björkman and Demmig 1987, Bolhàr-Nordenkampf et al. 1989) for these three cultivars. However, the slope of the Fv/Fm–PPFD regression was Y > G > P. As a result, under high PPFD, Y showed the greatest degree of photoinhibition, followed by G and then P. For the response of Fv/Fm to temperature among the three cultivars, only the Fv/Fm of Y showed a more drastic depression to lower temperature (< 20°C) at higher PPFD (> 1,000 μmol m–2 s–1). The other two cultivars had no notable differences between high and low temperatures in their Fv/Fm–PPFD relationships. Fig. 3 also shows that under all levels of PPFD, the depression of ΔF/Fm' was Y > G > P. This indicates that Y shows the highest degree of qE + (qZ + qT) + qI, followed by G and then P. It could be considered that, under the same level of PPFD, the difference between ΔF/Fm' and Fv/Fm values is mainly due to the components of qE + (qZ + qT). The qE is the pH- (energy-) dependent nonphotochemical quenching of Chl fluorescence (ChlF). The qZ is nonphotochemical quenching of ChlF dependent on zeaxanthin, and the qT is lowering of ChlF due to the state-transition (Müller et al. 2001, Nilkens et al. 2010). Fig. 3 shows that, at low PPFD, the degree of qE + (qZ + qT) is Y > G > P, but it is P ≈ G > Y at high PPFD.
Our previous studies indicate that, compared to G, Y shows lower abilities in qE and PSII efficiency under high irradiances, particularly during low temperatures (Weng et al. 2011, Lin et al. 2021). The results shown in Fig. 3 generally agree with the results of our previous study (Weng et al. 2011, Lin et al. 2021). In addition, Fig. 3 shows that P has the lowest qI, and qE + (qZ + qT), as does G under high light. Fig. 1 also shows that G has the highest Fv/Fm in the cool predawn. These results indicate that P had the lowest photoinhibition in high-light levels and low temperatures. The leaves of P contain anthocyanin (Table 1), which plays a light attenuation role and/or antioxidant role in protecting the photosystem (Zhang et al. 2019, Stetsenko et al. 2020). The protective effects of anthocyanin are related to its ability, via screening and internal light trapping, to reduce the amount of excessive solar radiation reaching the photosynthetic apparatus (Liakopoulos et al. 2006, Gitelson et al. 2009). Anthocyanins absorb mostly green light, they also absorb blue and red light, decreasing the absorption of Chl (Landi et al. 2021). In addition, a high content of Ant in young leaves is correlated with low pools of xanthophyll-cycle components (Manetas et al. 2002). In this study, P resisted photoinhibition whether it was a shading or a protective effect in this study.
Generally, Fv/Fm is lower at noon than at predawn (Demmig-Adams et al. 1996, Verhoeven et al. 1999, Santanoo et al. 2019, Yang et al. 2020, Acebron et al. 2021). However, the Fv/Fm of Y was higher at noon than predawn on 6 March. Fig. 1 shows that the temperature was as low as 7.5°C in the predawn on 6 March due to radiation cooling at night on 5 March. In Taiwan, air temperature varies considerably in the winter, with chilling temperatures (i.e., < 10°C) coinciding frequently with bright mornings (i.e., high incident sunlight) because of rapid heat dissipation by emission of long-wave radiation during clear nights. Nevertheless, air temperatures can increase to almost 25°C at midday in winter. Fig. 1B shows that 6 March was a clear sunny day, the temperature rose to 27.8°C at noon (Fig. 1A), and the Fv/Fm increased to the general level (corresponding to the Fv/Fm–PPFD regression shown in Fig. 3). This indicates that the depression of Fv/Fm is related to the reversible loss of the PSII function in a cold predawn (Long et al. 1994, Close et al. 2001).
Recovery of Fv/Fm at dusk
Afternoon PSII efficiency recovers gradually with decreasing light intensity, and reaches similar predawn values at sunset in warmer seasons (Verhoeven et al. 1999, Santanoo et al. 2019, Acebron et al. 2021). In this study, leaves were dark-treated at 17:00 h, although the sun had not yet set. Fv/Fm was still significantly negatively correlated with PPFD at this time (Fig. 4). The slope of the Fv/Fm–PPFD regression was Y > G > P. At high light and low temperature on 1 January and 5 March, the Fv/Fm of the three cultivars was lower than the regression line (Fig. 4). The differences between measured values and regression lines were Y > G > P. From the (1) slopes of the Fv/Fm–PPFD regressions and (2) differences between measured values and regression lines, the sensitivities of Fv/Fm to light intensity and low temperature at dusk were Y > G > P. These also indicated that the response of predawn Fv/Fm values to temperature was also Y > G > P (Fig. 1). However, the Fv/Fm of Y deviated downward while G and P did not at noon on 1 January and 5 March (Fig. 3).
Conclusion
The results of this study indicated that the pigment analysis showed that P leaves had the highest Chl (a+b) and carotenoid contents, while Y leaves exhibited significantly lower contents. Notably, Y leaves possessed the highest Chl a/b ratio. Predawn temperature fluctuations significantly impacted PSII efficiency (Fv/Fm) across the leaf types. Fv/Fm values decreased with lower predawn temperatures and increased with higher PPFD at noon. Y leaves demonstrated the most significant Fv/Fm variation, suggesting greater sensitivity to temperature and light changes than G and P leaves. A significant negative correlation between nonphotochemical quenching (NPQ) and Fv/Fm indicated that high light-induced PSII efficiency depression in sweet potato leaves was primarily due to xanthophyll-dependent NPQ, which dissipates excess energy as heat. The study also found that P leaves' Fv/Fm was the least affected under high light and low temperatures, likely attributable to the protective role of anthocyanin. Conversely, Y leaves showed an unusual increase in Fv/Fm at noon compared to predawn under specific conditions of cold predawns followed by bright, warmer noons, implying a reversible loss of PSII function. In summary, this research sheds light on the adaptive responses of sweet potato leaves to environmental stressors, emphasizing the intricate relationship between pigment content, temperature, light intensity, and PSII efficiency. These findings contribute to a deeper understanding of plant photoprotective mechanisms and offer valuable information for optimizing sweet potato cultivation practices.
Acknowledgments
This work was financially supported in part by the ‘Innovation and Development Center of Sustainable Agriculture’ from the Featured Areas Research Center Program within the Higher Education Sprout Project by the Ministry of Education (MOE) of Taiwan and by the Ministry of Science and Technology (MOST) in Taiwan (grant no. MOST-108-2621-B-034-002).
Abbreviations
- A
antheraxanthin
- Car
carotenoid
- Chl
chlorophyll
- Fv/Fm
maximal quantum yield of PSII photochemistry for dark-adapted state
- G
green
- NPQ
nonphotochemical quenching
- P
purple
- qE
energy quenching
- qI
photoinhibitory quenching
- V
violaxanthin
- Y
yellow-green
- Z
zeaxanthin
- ΔF/Fm'
the efficient quantum yield of PSII photochemistry for the light-adapted state
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Acebron K., Matsubara S., Jedmowski C. et al. : Diurnal dynamics of nonphotochemical quenching in Arabidopsis npq mutants assessed by solar-induced fluorescence and reflectance measurements in the field. – New Phytol. 229: 2104-2119, 2021. 10.1111/nph.16984 [DOI] [PubMed] [Google Scholar]
- Adams W.W., Zarter C.R., Ebbert V., Demmig-Adams B.: Photoprotective strategies of overwintering evergreens. – BioScience 54: 41-49, 2004. 10.1641/0006-3568(2004)054[0041:PSOOE]2.0.CO;2 [DOI] [Google Scholar]
- Alemu S.T., Roro A.G: Ultraviolet-B, end of day light and exclusion effect on photosynthetic efficiency of sweet potato (Ipomoea batatas L.) based on altitude. – J. Hortic. Postharvest Res. 3: 1-10, 2020. 10.22077/jhpr.2019.2882.1101 [DOI] [Google Scholar]
- Baker N.R.: Chilling stress and photosynthesis. – In: Foyer C.H., Mullineaux P.M. (eds.): Cause of Photooxidative Stress and Amelioration of Defense Systems in Plants. Pp. 127-154. CRC Press, New York: 1993. 10.1201/9781351070454 [DOI] [Google Scholar]
- Björkman O., Demmig B.: Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. – Planta 170: 489-504, 1987. 10.1007/BF00402983 [DOI] [PubMed] [Google Scholar]
- Blennow K., Lang A.R.G., Dunne P., Ball M.C.: Cold-induced photoinhibition and growth of seedling snow gum (Eucalyptus pauciflora) under differing temperature and radiation regimes in fragmented forests. – Plant Cell Environ. 21: 407-416, 1998. 10.1046/j.1365-3040.1998.00291.x [DOI] [Google Scholar]
- Bolhàr-Nordenkampf H.R., Long S.P., Baker N.R. et al. : Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: a review of current instrumentation. – Funct. Ecol. 3: 497-514, 1989. 10.2307/2389624 [DOI] [Google Scholar]
- Chen Y.-S., Chesson P., Wu H.-W. et al. : Leaf structure affects a plant’s appearance: combined multiple-mechanisms intensify remarkable foliar variegation. – J. Plant Res. 130: 311-325, 2017. 10.1007/s10265-016-0890-4 [DOI] [PubMed] [Google Scholar]
- Close D.C., Beadle C.L., Hovenden M.J.: Cold-induced photoinhibition and foliar pigment dynamics of Eucalyptus nitens seedlings during establishment. – Aust. J. Plant Physiol. 28: 1133-1141, 2001. 10.1071/PP01039 [DOI] [Google Scholar]
- Corcuera L., Morales F., Abadía A., Gil-Pelegrín E.: Seasonal changes in photosynthesis and photoprotection in a Quercus ilex subsp. ballota woodland located in its upper altitudinal extreme in the Iberian Peninsula. – Tree Physiol. 25: 599-608, 2005. 10.1093/treephys/25.5.599 [DOI] [PubMed] [Google Scholar]
- Cui M., Vogelmann T.C., Smith W.K.: Chlorophyll and light gradients in sun and shade leaves of Spinacia oleracea. – Plant Cell Environ. 14: 493-500, 1991. 10.1111/j.1365-3040.1991.tb01519.x [DOI] [Google Scholar]
- Demmig-Adams B., Adams W.W.: The role of xanthophyll cycle carotenoids in the protection of photosynthesis. – Trends Plant Sci. 1: 21-26, 1996. 10.1016/S1360-1385(96)80019-7 [DOI] [Google Scholar]
- Demmig-Adams B., Adams W.W., Barker D.H. et al. : Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. – Physiol. Plantarum 98: 253-264, 1996. 10.1034/j.1399-3054.1996.980206.x [DOI] [Google Scholar]
- Demmig-Adams B., Ebbert V., Mellman D.L. et al. : Modulation of PsbS and flexible vs sustained energy dissipation by light environment in different species. – Physiol. Plantarum 127: 670-680, 2006. 10.1111/j.1399-3054.2006.00698.x [DOI] [Google Scholar]
- Genty B., Briantais J.-M., Baker N.R.: The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. – BBA-Gen. Subjects 990: 87-92, 1989. 10.1016/S0304-4165(89)80016-9 [DOI] [Google Scholar]
- Gitelson A.A., Chivkunova O.B., Merzlyak M.N.: Nondestructive estimation of anthocyanins and chlorophylls in anthocyanic leaves. – Am. J. Bot. 96: 1861-1868, 2009. 10.3732/ajb.0800395 [DOI] [PubMed] [Google Scholar]
- Gitelson A.A., Gritz Y., Merzlyak M.N.: Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. – J. Plant Physiol. 160: 271-282, 2003. 10.1078/0176-1617-00887 [DOI] [PubMed] [Google Scholar]
- Goh C.-H., Jang S., Jung S. et al. : Rice phot1a mutation reduces plant growth by affecting photosynthetic responses to light during early seedling growth. – Plant Mol. Biol. 69: 605-619, 2009. 10.1007/s11103-008-9442-1 [DOI] [PubMed] [Google Scholar]
- Gorbe E., Calatayud A.: Applications of chlorophyll fluorescence imaging technique in horticultural research: a review. – Sci. Hortic.-Amsterdam 138: 24-35, 2012. 10.1016/j.scienta.2012.02.002 [DOI] [Google Scholar]
- Gould K.S.: Nature's Swiss army knife: the diverse protective roles of anthocyanins in leaves. – J. Biomed. Biotechnol. 2004: 314-320, 2004. 10.1155/S1110724304406147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A., Holocher K.: Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light-dependent) pH decrease. – Planta 192: 581-589, 1994. https://link.springer.com/article/10.1007/BF00203597 [Google Scholar]
- He J., Qin L.: Growth and photosynthetic characteristics of sweet potato (Ipomoea batatas) leaves grown under natural sunlight with supplemental LED lighting in a tropical greenhouse. – J. Plant Physiol. 252: 153239, 2020. 10.1016/j.jplph.2020.153239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.-Y., Wong S.-L., Weng J.-H.: Rapid light-response curve of chlorophyll fluorescence in terrestrial plants: relationship to CO2 exchange among five woody and four fern species adapted to different light and water regimes. – Plants-Basel 10: 445, 2021. 10.3390/plants10030445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Hu H., Zhang S.-B.: Photosynthesis and photosynthetic electron flow in the alpine evergreen species Quercus guyavifolia in winter. – Front. Plant Sci. 7: 1511, 2016. 10.3389/fpls.2016.01511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahns P., Latowski D., Strzalka K.: Mechanism and regulation of the violaxanthin cycle: The role of antenna proteins and membrane lipids. – BBA-Bioenergetics 1787: 3-14, 2009. 10.1016/j.bbabio.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Johnson M.P., Ruban A.V.: Restoration of rapidly reversible photoprotective energy dissipation in the absence of PsbS protein by enhanced ΔpH. – J. Biol. Chem. 286: 19973-19981, 2011. 10.1074/jbc.M111.237255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M.C., Hikosaka K., Hirotsu N. et al. : The excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms determines the rate of photoinactivation in photosystem II. – Plant Cell Physiol. 44: 318-325, 2003. 10.1093/pcp/pcg045 [DOI] [PubMed] [Google Scholar]
- Kitajima M., Butler W.L.: Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. – BBA-Bioenergetics 376: 105-115, 1975. 10.1016/0005-2728(75)90209-1 [DOI] [PubMed] [Google Scholar]
- Landi M., Agati G., Fini A. et al. : Unveiling the shade nature of cyanic leaves: a view from the ‘blue absorbing side’ of anthocyanins. – Plant Cell Environ. 44: 1119-1129, 2021. 10.1111/pce.13818 [DOI] [PubMed] [Google Scholar]
- Lavaud J., Lepetit B.: An explanation for the inter-species variability of the photoprotective non-photochemical chlorophyll fluorescence quenching in diatoms. – BBA-Bioenergetics 1827: 294-302, 2013. 10.1016/j.bbabio.2012.11.012 [DOI] [PubMed] [Google Scholar]
- Lazár D., Nauš J.: Statistical properties of chlorophyll fluorescence induction parameters. – Photosynthetica 35: 121-127, 1998. 10.1023/A:1006886202444 [DOI] [Google Scholar]
- Lazar D., Stirbet A., Björn L.O., Govindjee G.: Light quality, oxygenic photosynthesis and more. – Photosynthetica 60: 25-58, 2022. 10.32615/ps.2021.055 [DOI] [Google Scholar]
- Leegood R.C.: Effects of temperature on photosynthesis and photorespiration. – In: Smirnoff N (ed.): Environment and Plant Metabolism: Flexibility and Acclimation. Pp. 45-62. BIOS Scientific Publishers, Oxford: 1995. [Google Scholar]
- Li Q., Kubota C.: Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. – Environ. Exp. Bot. 67: 59-64, 2009. 10.1016/j.envexpbot.2009.06.011 [DOI] [Google Scholar]
- Li S., Zhao L., Sun N. et al. : Photosynthesis product allocation and yield in sweetpotato with different irrigation levels at mid-season. – Agr. Water Manage. 246: 106708, 2021. 10.1016/j.agwat.2020.106708 [DOI] [Google Scholar]
- Li Y., Sun Y., Jiang J., Liu J.: Spectroscopic determination of leaf chlorophyll content and color for genetic selection on Sassafras tzumu. – Plant Methods 15: 73, 2019. 10.1186/s13007-019-0458-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakopoulos G., Nikolopoulos D., Klouvatou A. et al. : The photoprotective role of epidermal anthocyanins and surface pubescence in young leaves of grapevine (Vitis vinifera). – Ann. Bot.-London 98: 257-265, 2006. 10.1093/aob/mcl097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.-H., Lin K.-H., Jiang J.-Y. et al. : Comparisons between yellow and green leaves of sweet potato cultivars in chlorophyll fluorescence during various temperature regimes under high light intensities. – Sci. Hortic.-Amsterdam 288: 110335, 2021. 10.1016/j.scienta.2021.110335 [DOI] [Google Scholar]
- Lin K.H., Huang M.Y, Weng J.H., Kao S.C.: Photosynthetic activity of red and green leaf sectors in Coleus blumei plants as sensed by chlorophyll fluorescence. – Photosynthetica 57: 659-667, 2019. 10.32615/ps.2019.051 [DOI] [Google Scholar]
- Long S.P., Humphries S., Falkowski P.G.: Photoinhibition of photosynthesis in nature. – Annu. Rev. Plant Physiol. Plant Mol. Biol. 45: 633-662, 1994. 10.1146/annurev.pp.45.060194.003221 [DOI] [Google Scholar]
- Mancinelli A.L., Yang C.P.H., Lindquist P. et al. : Photocontrol of anthocyanin synthesis: III. The action of streptomycin on the synthesis of chlorophyll and anthocyanin. – Plant Physiol. 55: 251-257, 1975. 10.1104/pp.55.2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetas Y., Drinia A., Petropoulou Y.D.: High contents of anthocyanins in young leaves are correlated with low pools of xanthophyll cycle components and low risk of photoinhibition. – Photosynthetica 40: 349-354, 2002. 10.1023/A:1022614722629 [DOI] [Google Scholar]
- Maxwell K., Johnson G.N.: Chlorophyll fluorescence – a practical guide. – J. Exp. Bot. 51: 659-668, 2000. 10.1093/jexbot/51.345.659 [DOI] [PubMed] [Google Scholar]
- Müller P., Li X.-P., Niyogi K.K.: Non-photochemical quenching: A response to excess light energy. – Plant Physiol. 125: 1558-1566, 2001. 10.1104/pp.125.4.1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilkens M., Kress E., Lambrev P.: Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated understeady-state conditions in Arabidopsis. – BBA-Bioenergetics 1797: 466-475, 2010. 10.1016/j.bbabio.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Noh S.A., Park S.H., Huh G.H. et al. : Growth retardation and differential regulation of expansin genes in chilling-stressed sweetpotato. – Plant Biotechnol. Rep. 3: 75-85, 2009. 10.1007/s11816-008-0077-0 [DOI] [Google Scholar]
- Peng C.-L., Duan J., Lin G., Gilmore A.M.: Correlation between photoinhibition sensitivity and the rates and relative extents of xanthophyll cycle de-epoxidation in chlorine mutants of barley (Hordeum vulgare L.). – Photosynthetica 40: 503-508, 2002. 10.1023/A:1024383431403 [DOI] [Google Scholar]
- Quick W.P., Stitt M.: An examination of factors contributing to non-photochemical quenching of chlorophyll fluorescence in barley leaves. – BBA-Bioenergetics 977: 287-296, 1989. 10.1016/S0005-2728(89)80082-9 [DOI] [Google Scholar]
- Roháček K., Barták M.: Technique of the modulated chlorophyll fluorescence: basic concepts, useful parameters, and some applications. – Photosynthetica 37: 339-363, 1999. 10.1023/A:1007172424619 [DOI] [Google Scholar]
- Santanoo S., Vongcharoen K., Banterng P. et al. : Seasonal variation in diurnal photosynthesis and chlorophyll fluorescence of four genotypes of cassava (Manihot esculenta Crantz) under irrigation conditions in a tropical savanna climate. – Agronomy 9: 206, 2019. 10.3390/agronomy9040206 [DOI] [Google Scholar]
- Smirnoff N.: Ascorbic acid: metabolism and functions of a multifaceted molecule. – Curr. Opin. Plant Biol. 3: 229-235, 2000. https://www.sciencedirect.com/science/article/pii/S1369526600800709 [PubMed] [Google Scholar]
- Stetsenko L.A., Pashkovsky P.P., Voloshin R.A. et al. : Role of anthocyanin and carotenoids in the adaptation of the photosynthetic apparatus of purple- and green-leaved cultivars of sweet basil (Ocimum basilicum) to high-intensity light. – Photosynthetica 58: 890-901, 2020. 10.32615/ps.2020.048 [DOI] [Google Scholar]
- Sun D., Wang Q.: Linear relationships between photosynthetic rate and photochemical energy expressed by PAR × Fv/Fm. – Am. J. Plant Sci. 9: 125-138, 2018. 10.4236/ajps.2018.92011 [DOI] [Google Scholar]
- Tanaka Y., Ohmiya A.: Seeing is believing: engineering anthocyanin and carotenoid biosynthetic pathways. – Curr. Opin. Biotech. 19: 190-197, 2008. 10.1016/j.copbio.2008.02.015 [DOI] [PubMed] [Google Scholar]
- Verhoeven A.S., Adams W.W., Demmig-Adams B.: The xanthophyll cycle and acclimation of Pinus ponderosa and Malva neglecta to winter stress. – Oecologia 118: 277-287, 1999. 10.1007/s004420050728 [DOI] [PubMed] [Google Scholar]
- Weng J.-H.: Underestimate of PS2 efficiency in the field due to high leaf temperature resulting from leaf clipping and its amendment. – Photosynthetica 44: 467-470, 2006. 10.1007/s11099-006-0052-3 [DOI] [Google Scholar]
- Weng J.-H., Chen Y.-N., Liao T.-S.: Relationships between chlorophyll fluorescence parameters and photochemical reflectance index of tree species adapted to different temperature regimes. – Funct. Plant Biol. 33: 241-246, 2006. 10.1071/FP05156 [DOI] [PubMed] [Google Scholar]
- Weng J.-H., Chien L.-F., Jiang C.-Y. et al. : A comparison between yellow-green and green cultivars of four vegetable species in pigments, ascorbate, photosynthesis, energy dissipation and photoinhibition. – Photosynthetica 49: 361-370, 2011. 10.1007/s11099-011-0046-7 [DOI] [Google Scholar]
- Weng J.-H., Liao T.-S., Sun K.-H. et al. : Seasonal variation in photosynthesis of Picea morrisonicola grown in sub-alpine of subtropical Taiwan. – Tree Physiol. 25: 973-979, 2005. 10.1093/treephys/25.8.973 [DOI] [PubMed] [Google Scholar]
- Weng J.-H., Wong S.-L., Lin R.-J., Jhaung L.-H.: Quantitative effects of temperature and light intensity on PSII efficiency of mango leaves under artificial and natural conditions. – J. For. Res. 18: 371-378, 2013. 10.1007/s10310-012-0359-9 [DOI] [Google Scholar]
- Wong S.-L., Chen C.-W., Huang H.-W., Weng J.-H.: Using combined measurements of gas exchange and chlorophyll fluorescence to investigate the photosynthetic light responses of plant species adapted to different light regimes. – Photosynthetica 50: 206-214, 2012. 10.1007/s11099-012-0027-5 [DOI] [Google Scholar]
- Yang C.-M., Chang K.-W., Yin M.-H., Huang H.-M.: Methods for the determination of chlorophylls and their derivatives. – Taiwania 43: 116-122, 1998. https://dn790002.ca.archive.org/0/items/taiwania-43-116-122/taiwania-43-116-122_text.pdf [Google Scholar]
- Yang S.H., Wei J.J., Yan F. et al. : Differences in leaf anatomy, photosynthesis, and photoprotective strategies in the yellow-green leaf mutant and wild type of Rosa beggeriana Schrenk. – Photosynthetica 58: 1167-1177, 2020. 10.32615/ps.2020.059 [DOI] [Google Scholar]
- Zhang Q., Zhai J., Shao L. et al. : Accumulation of anthocyanins: an adaptation strategy of Mikania micrantha to low temperature in winter. – Front. Plant Sci. 10: 1049, 2019. 10.3389/fpls.2019.01049 [DOI] [PMC free article] [PubMed] [Google Scholar]