The development of targeted immunotherapy for acute myeloid leukemia (AML) has been challenging due to the paucity of tumor-specific antigens, on-target off-leukemia hematological toxicity, and an immunosuppressive tumor microenvironment.1-4 Repurposing immunotherapies that have been used to target other hematological malignancies could, in case of a shared target antigen, represent a promising opportunity to expand the immunotherapeutic options for AML. CD19, a B-cell marker highly expressed in B-cell precursor acute lymphoblastic leukemia (BCP-ALL), is one such shared target antigen. Indeed, CD19-expression is also characteristic of t(8;21)(q22;q22) AML, the most common translocation in children with this disease. The success of CD19-directed immunotherapies in BCP-ALL,5-7 as well as in two adults with relapsed CD19+ t(8;21) AML,8,9 prompted us to investigate CD19 as an immunotherapy target in pediatric AML. This study had been approved by the Biobank Data Access Committee of the Princess Máxima Center.
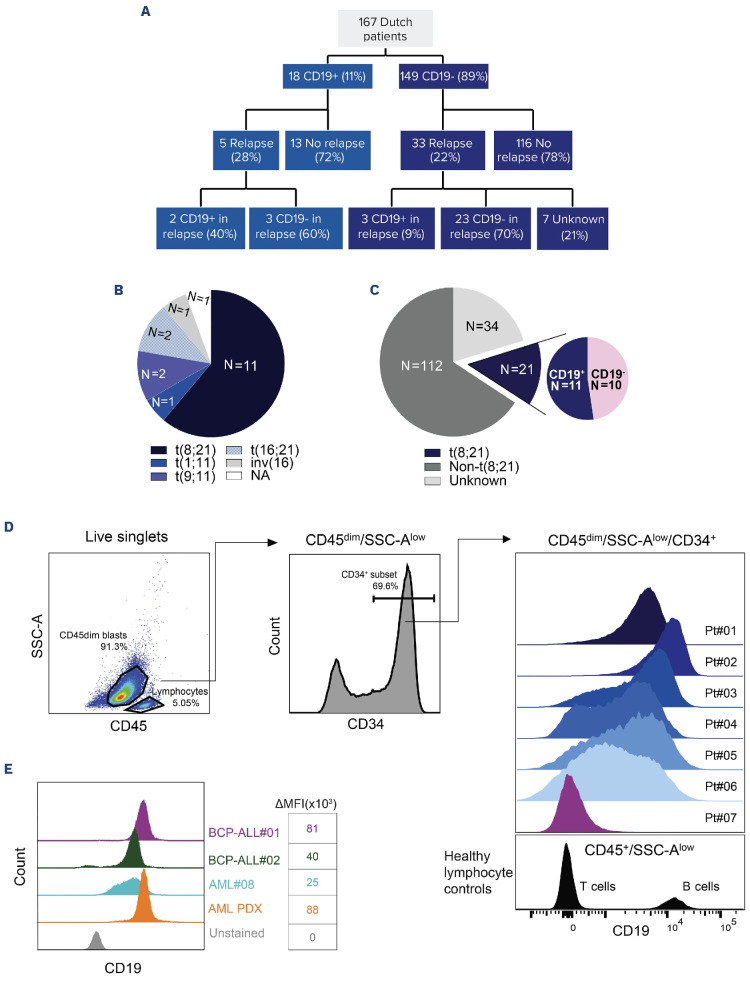
Using diagnostic flow cytometry records of 167 newly diagnosed de novo pediatric AML patients, we identified 18 patients with CD19+ AML (11%; 78% male; median age: 10.5 years; range: 1-17) (Figure 1A). Among these patients, ten had weak CD19-expression (difference [Δ] in log (CD19 mean fluorescent intensity [MFI]) between leukemia and T cells [as healthy CD19- controls] of 0.5 to 1). In eight patients, the Δlog MFI was greater than 1. was greater than 1. Regarding cytogenetic alterations, 61% (N=11) of CD19+ patients carried the translocation (8;21) (q22;q22) (Figure 1B). Other cytogenetic alterations included t(9;11)(p22;q23) (N=2), t(16;21)(q24;q22) (N=2), t(1;11)(q21;q23) (N=1), inv(16)(p13;q22) (N=1), and unknown (N=1; Figure 1B). In the entire cohort, 21 of 167 patients had the (8;21) translocation, indicating that 52% of patients with this translocation had CD19+ AML (Figure 1C). All 18 patients with CD19+ AML achieved complete remission by the second induction course, of which five (28%) relapsed (Figure 1A). Of these relapsed patients, two retained CD19-positivity and are alive, while three lost CD19-expression, of which one deceased. This was the only death among patients with CD19+ AML at diagnosis. Intriguingly, three patients with CD19- AML at diagnosis gained CD19-positivity at relapse (of 33 relapses in the CD19- AML group; Figure 1A). Their cytogenetic alterations were del(9q) (N=1), del(7q), del(12p), +1q (N=1), and unknown (N=1), with one fatal outcome. These data demonstrate enrichment of CD19-expression in pediatric t(8;21) AML at diagnosis, which can also emerge at relapse in cases that initially had CD19-AML.
Figure 1.
CD19-expression among pediatric acute myeloid leukemia patients. (A) Incidence of CD19-positivity among newly diagnosed and relapsed pediatric acute myeloid leukemia (AML) patients. (B) Cytogenetic alterations observed in CD19+ pediatric AML patients. NA: cytogenetic data not available. (C) Incidence of the t(8;21) subtype across the total cohort, and the incidence of CD19-positivity among all t(8;21) patients. (D) Gating strategy to identify CD19+ populations and their expression levels among CD45dimSSC-AlowCD34+ blasts in the bone marrow (BM) for 7 t(8;21) AML patients. CD19-expression on these blasts was compared to the CD19 levels on healthy T cells (as CD19- control). The CD19-expression of B cells is shown as a CD19+ reference. (E) Comparison of CD19-expression between 1 primary AML sample (patient#08), AML patient-derived xenograft (PDX) (RL048), and 2 primary BCP-ALL samples (patients#01-02). The difference in median fluorescence intensity (ΔMFI) in this experiment was calculated by subtracting the MFI of CD19 in stained samples from the CD19 MFI in corresponding unstained samples.
To further characterize the extent of CD19-expression in pediatric t(8;21) AML, we re-analyzed diagnostic bone marrow mononuclear cell (BMMC) flow cytometry data available for six CD19+ patients (#01-06) and one CD19-patient (#07) with t(8;21) AML. We examined CD19-expression levels on CD45dimSSC-AlowCD3-CD20-CD34+ myeloid blasts, using CD19-bone marrow (BM) T cells (from patient#01) as healthy negative controls (Figure 1D). In two patients (patients#01-02), the CD19-expression showed a unimodal pattern which approximated that of B cells. This is indicative of high CD19-expression in the majority of leukemic cells. In contrast, the other four patients (patients#03-06) exhibited lower CD19-expression levels with a non-unimodal pattern (Figure 1D). We then compared CD19-expression levels in CD19+ t(8;21) AML samples (RL048 patient-derived xenograft [PDX]10 and BMMC from patient#08) with two primary BCP-ALL BMMC samples. While the CD19 MFI in AML patient#08 was lower compared to both BCP-ALL samples, the MFI of the AML PDX sample was equal to (patient#02) or even higher (patient#01) than that of the BCP-ALL samples (Figure 1E). Next, we analyzed the CD19-expression on different AML subpopulations in AML samples (patient#01, patient#08, and RL048 PDX). Importantly, nearly all CD34+CD38- immature progenitors expressed CD19 (Online Supplementary Figure S1A-C). Furthermore, in patient#01, we identified CD19 to be expressed on putative leukemic stem cells (LSC; CD34+CD38-CD45RA+) but not on normal stem cells (CD34+CD38-CD45RA-; Online Supplementary Figure S1A). Additionally, virtually all CD34+CD38+ cells, including CD34+CD38+CD11b+ and CD34+C-D38+CD11b- cells, were positive for CD19 (Online Supplementary Figure S1A-C). In summary, although the CD19-expression level among CD19+ AML patients was heterogeneous, in those with unimodal and high CD19-expression, CD19 was expressed on both more and less mature AML cells. These findings encouraged exploration of the ex vivo killing efficiency of immunotherapies targeting CD19, including blinatumomab (CD19-directed bispecific T-cell engager) and CD19-directed chimeric antigen receptor (CAR) T cells.
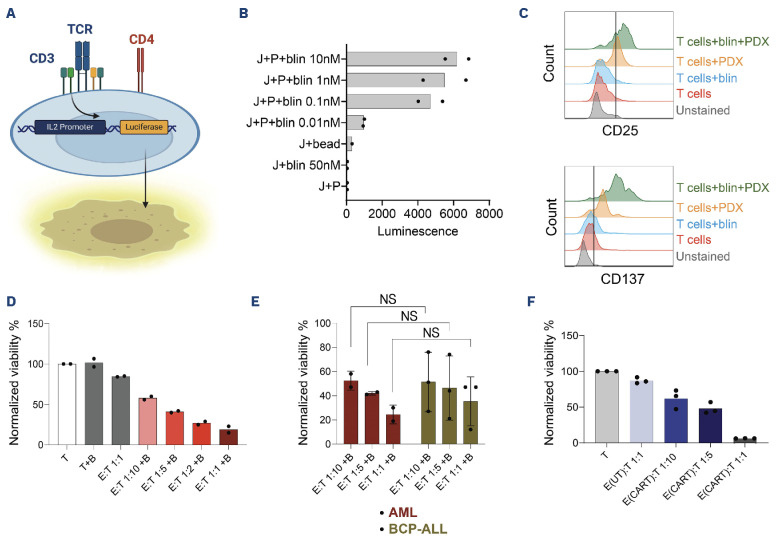
We next wondered whether blinatumomab-mediated AML T-cell contact could induce T-cell activation. Using genetically engineered Jurkat cells (Figure 2A), we observed a dose-dependent increase in the luminescent signal in a co-culture of CD19+ AML PDX (RL048) and Jurkat cells, indicating blinatumomab-mediated CD3-signaling in T cells (Figure 2B). This finding was further validated using an ex vivo co-culture of healthy donor T cells with CD19+ AML PDX cells. The addition of blinatumomab led to significant upregulation of the T cell activation markers CD25 (50% marker positivity) and CD137 (90% marker positivity; Figure 2C). Next, to determine whether AML cells were sensitive to blinatumomab-mediated T-cell cytotoxicity, we treated ex vivo co-cultures with 1 nM blinatumomab, which resulted in 40% AML PDX cell killing at a low effector-to-target (E:T) ratio of 1:10 and almost 90% killing at an E:T ratio of 1:1 (Figure 2D). Notably, absence of allogeneic T cells, or blinatumomab alone, led to no or negligible background killing. We subsequently compared the blinatumomab-mediated T-cell killing efficiency between AML and BCP-ALL samples in ex vivo co-cultures with healthy donor T cells. Intriguingly, although we observed substantial variation in the killing efficiency among the three BCP-ALL samples, the observed AML cell killing was comparable to that of BCP-ALL cells at each E:T ratio (P>0.05; Figure 2E). We also assessed whether CD19+ t(8;21) AML cells were sensitive to CAR T-cell-mediated cytotoxicity. Similar to blinatumomab, co-culturing CD19-directed CAR T cells containing a 4-1BB transactivation domain with primary AML cells at a low E:T ratio of 1:10 led to 40% killing of AML cells within 48 hours, while AML cells were nearly completely eradicated at an E:T-ratio of 1:1 (Figure 2F). AML cell viability remained constant in co-cultures with untransduced T cells from the same donor, again indicating negligible background killing (Figure 2F). Taken together, these findings demonstrate that CD19-directed immunotherapies induce efficient killing of CD19+ AML cells ex vivo.
Figure 2.
T-cell activation and acute myeloid leukemia cell cytotoxicity mediated by blinatumomab and chimeric antigen receptor T cells. (A) Illustration of the engineered Jurkat T-cell activation bioassay. (B) The luminescent signal intensity upon addition of blinatumomab (blin) concentrations to CD19+ acute myeloid leukemia (AML) patient-derived xenograft (PDX) (P) and Jurkat cells (J; N=2 technical replicates). bead: CD3/CD28 Dynabeads. (C) Induction of activation markers in primary T cells upon addition of blinatumomab (blin) and/or CD19+ AML PDX cells. (D) Effect of 1 nM blinatumomab (marked as B) on the viability of AML PDX cells (T; Target cells) at various effector-to-target (E:T) ratios using healthy donor T cells (E; Effector cells) after 48 hours. Data points represent technical replicates. (E) The viability of AML (patient#08 and PDX) and primary BCP-ALL BMMC samples (N=3 different patients) after 48 hours of co-culture with healthy donor T cells and blinatumomab (marked as B). Data represent mean ± standard deviation. A two-way ANOVA followed by Šídák’s multiple comparisons test was performed between each E:T ratio in AML versus BCP-ALL (NS: not significant, P>0.05). (F) The viability of primary AML cells (patient#08) after 48 hours of co-culture with CD19-directed chimeric antigen receptor (CAR) T cells or untransduced T cells (UT; as control) at different E:T ratios. Data points represent technical replicates.
In order to improve our understanding of the extent of immunosuppression in the BM of pediatric CD19+ AML: t(8;21) AML patients, we characterized the tumor immune microenvironment using immunogenomic computational approaches applied to diagnostic BM bulk RNA-sequencing data. Accordingly, we deconvoluted the immune cell abundance in the BM of treatment-naïve CD19+ t(8;21) AML (N=5), CD19- t(8;21) AML (N=5), other AML genotypes (N=30), and non-leukemic controls (N=4)11 using CIBERSORTx12 and the TIDE algorithm.13 As we did not detect differences between CD19+ and CD19- t(8;21) AML (Online Supplementary Figure S2A-J), we considered these cases in aggregate for subsequent comparisons (t(8;21)-group). Similarly, we found no differences in the abundance of microenvironmental subsets between both AML groups and non-leukemic controls, which was also the case for two RNA-based metrics related to immune (dys)function14,15 (Online Supplementary Figure S2K-R). Comparing the BM of pediatric t(8;21) AML (N=10) to that of pediatric BCP-ALL (N=209), we found that BCP-ALL cases had a significantly higher abundance of myeloid-derived suppressor cells (MDSC; Online Supplementary Figure S2O). Furthermore, BCP-ALL cases were enriched for T- and NK-cell exhaustion and senescence (Online Supplementary Figure S2L-N), potentially reflecting a prior T- and NK-cell response rendered dysfunctional. On the other hand, cancer-associated fibroblasts (CAF), memory B cells, and plasma cells were significantly increased in t(8;21) AML compared to BCP-ALL (Online Supplementary Figure S2P-R). Altogether, our immunogenomic approach revealed that the BM microenvironment in pediatric t(8;21) AML is similar to non-leukemic controls but, at least in part, distinct from pediatric BCP-ALL.
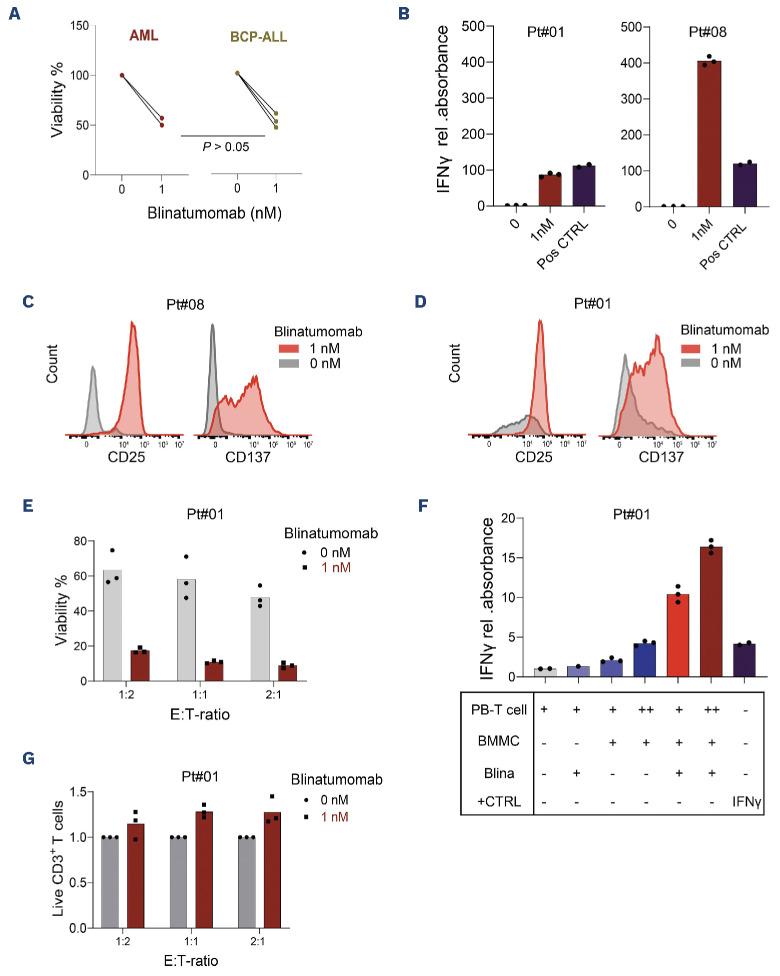
Following the deconvolution of the abundance and function of T cells in the pediatric t(8;21) AML BM, we evaluated the functionality of autologous T cells in AML and BCP-ALL BMMC samples. Addition of blinatumomab to AML BMMC (from patients#01 and #08, containing 4% and 8% T cells, respectively) led, in 48 hours, to a 50% reduction in cell viability (Figure 3A). This was comparable to that observed in BCP-ALL samples (all contained 3% autologous CD3 T cells; P>0.05; Figure 3A). Moreover, we found that addition of blinatumomab to AML BMMC samples induced a significant increase in IFN-γ secretion compared to BMMC without blinatumomab, with the extent proportional to the abundance of T cells in the BM (Figure 3B). In addition, the T-cell activation markers CD25 and CD137 on BM T cells increased substantially in response to blinatumomab (Figure 3C), together providing support for the activation of T cells. For patient#01, matched peripheral blood (PB) was also available, which allowed for a co-culture of PB-derived T cells with autologous BMMC. The autologous PB T cells showed substantial activation upon treatment with blinatumomab (Figure 3D) and demonstrated effective AML cell killing (Figure 3E), which was accompanied by substantial IFN-γ secretion (Figure 3F) and an increased T-cell abundance after treatment (Figure 3G). Overall, these findings demonstrate that autologous T cells from AML patients are capable to induce cytotoxicity upon binding to T-cell engagers, encouraging the exploitation of CD19 as an immunotherapy target in pediatric CD19+ t(8;21) AML.
Figure 3.
Functionality and cytotoxicity of T cells from t(8;21) patients. (A) Cytotoxicity of autologous bone marrow-derived T cells upon direct addition of 1 nM blinatumomab to bone marrow mononuclear cells (BMMC) from acute myeloid leukemia (AML) (N=2) and BCP-ALL (N=3) samples after 48 hours. A t test was performed to compare the viability of blinatumomab-treated AML versus BCP-ALL samples. (B) Relative (rel.) interferon (IFN)-γ measurement in the supernatant of two BMMC AML samples. Positive control (Pos CTRL): 125 pg/mL of recombinant IFN-γ protein (N=3 technical replicates for each patient). (C, D) Changes of the activation markers on blinatumomab-treated autologous CD3+ T cells, either present within BMMC sample (C; patient#08) or derived from peripheral blood (PB) and co-cultured with autologous BMMC (D; patient#01). (E) Viability of AML BMMC sample (patient#01) following 48 hours of co-culture with autologous PB-derived CD3+ T cells and 1 nM blinatumomab at various effector-to-target (E:T) ratios (N=3 technical replicates). (F) Rel. IFN-γ measurement in the supernatant of BMMC from patient#01 upon co-culture with autologous PB-derived T cells at different E:T ratios. The + and ++ for PB and BMMC in the table beneath indicate E:T ratios of 1:1 and 2:1, respectively. Absorbance values were normalized to the corresponding value of CD3+ T cells alone; Pos CTRL: 125 pg/mL recombinant IFN-γ, N=3 technical replicates. (G) Ratio of CD3+ T-cell numbers after blinatumomab treatment to the corresponding numbers before treatment, in the autologous BMMC and PB-derived T-cell co-cultures (patient#01, 48 hours).
Taken together, our study highlights the potential of CD19-directed immunotherapies for pediatric CD19+ AML. Given that immunotherapies work best at a favorable E:T ratio,1 these therapies may be particularly effective in the minimal residual disease setting before allogeneic stem cell transplantation. Additionally, they could serve as alternatives to intensive chemotherapy, for instance in case of severe chemotherapy-induced complications, or as life-prolonging treatments when curative options are no longer viable.
Supplementary Material
Acknowledgments
We thank the patients, parents, and caregivers for their participation. Appreciation goes to Anja de Jong (Princess Máxima Center) for flow cytometry data, Zinia Kwidama for providing AML samples, Tom Reuvekamp and Marisa Westers for fruitful data discussions (Amsterdam UMC location VUMC, the Netherlands). Zsolt Sebestyen (UMC Utrecht, the Netherlands) provided T-cell expansion feeders for generation of CAR T cells. Figures were created with BioRender.com.
Funding Statement
Funding: This work has been funded by a KIKA (329) program grant (to OH).
Data-sharing statement
Bulk RNA-sequencing data (count data) generated for this manuscript can be retrieved from the Gene Expression Omnibus (GSE270185). Requests for raw sequencing data should be addressed to the corresponding authors.
References
- 1.Subklewe M, Bücklein V, Sallman D, Daver N. Novel immunotherapies in the treatment of AML: is there hope? Hematology Am Soc Hematol Educ Program. 2023;2023(1):691-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koedijk JB, van der Werf I, Calkoen FG, et al. Paving the way for immunotherapy in pediatric acute myeloid leukemia: current knowledge and the way forward. Cancers (Basel). 2021;13(17):4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wijnen NE, Koedijk JB, Klein K, et al. Treating CD33-positive de novo acute myeloid leukemia in pediatric patients: focus on the clinical value of gemtuzumab ozogamicin. Onco Targets Ther. 2023;16:297-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vadakekolathu J, Rutella S. Escape from T-cell-targeting immunotherapies in acute myeloid leukemia. Blood. 2024;143(26):2689-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/ refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389. [DOI] [PubMed] [Google Scholar]
- 6.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kita K, Nakase K, Miwa H, et al. Phenotypical characteristics of acute myelocytic leukemia associated with the t(8;21)(q22;q22) chromosomal abnormality: frequent expression of immature B-cell antigen CD19 together with stem cell antigen CD34. Blood. 1992;80(2):470-477. [PubMed] [Google Scholar]
- 8.Plesa A, Labussière-Wallet H, Hayette S, Salles G, Thomas X, Sujobert P. Efficiency of blinatumomab in a t (8;21) myeloid leukemia expressing CD19. Haematologica. 2019;104(10):e487-e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danylesko I, Jacoby E, Yerushalmi R, et al. Remission of acute myeloid leukemia with t (8; 21) following CD19 CAR T-cells. Leukemia. 2020;34(7):1939-1942. [DOI] [PubMed] [Google Scholar]
- 10.Kellaway SG, Potluri S, Keane P, et al. Leukemic stem cells activate lineage inappropriate signalling pathways to promote their growth. Nat Commun. 2024;15(1):1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koedijk JB, van Beek TB, Vermeulen MA, et al. Case report: immune dysregulation associated with long-lasting regression of a (pre)leukemic clone. Front Immunol. 2023;14:1280885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman AM, Steen CB, Liu CL, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol. 2019;37(7):773-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang P, Gu S, Pan D, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24(10):1550-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dufva O, Pölönen P, Brück O, et al. Immunogenomic landscape of hematological malignancies. Cancer Cell. 2020;38(3):380-399.e13. [DOI] [PubMed] [Google Scholar]
- 15.Rutella S, Vadakekolathu J, Mazziotta F, et al. Immune dysfunction signatures predict outcomes and define checkpoint blockade-unresponsive microenvironments in acute myeloid leukemia. J Clin Invest. 2022;132(21):e159579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Bulk RNA-sequencing data (count data) generated for this manuscript can be retrieved from the Gene Expression Omnibus (GSE270185). Requests for raw sequencing data should be addressed to the corresponding authors.