Abstract
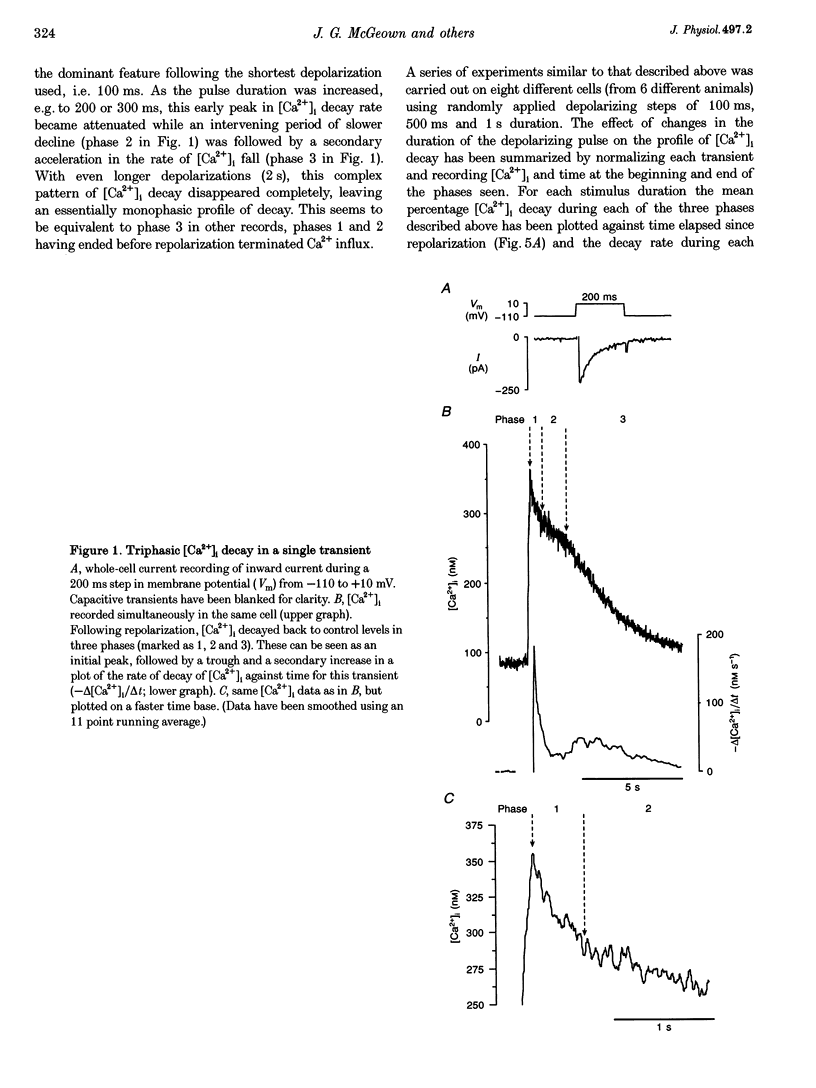
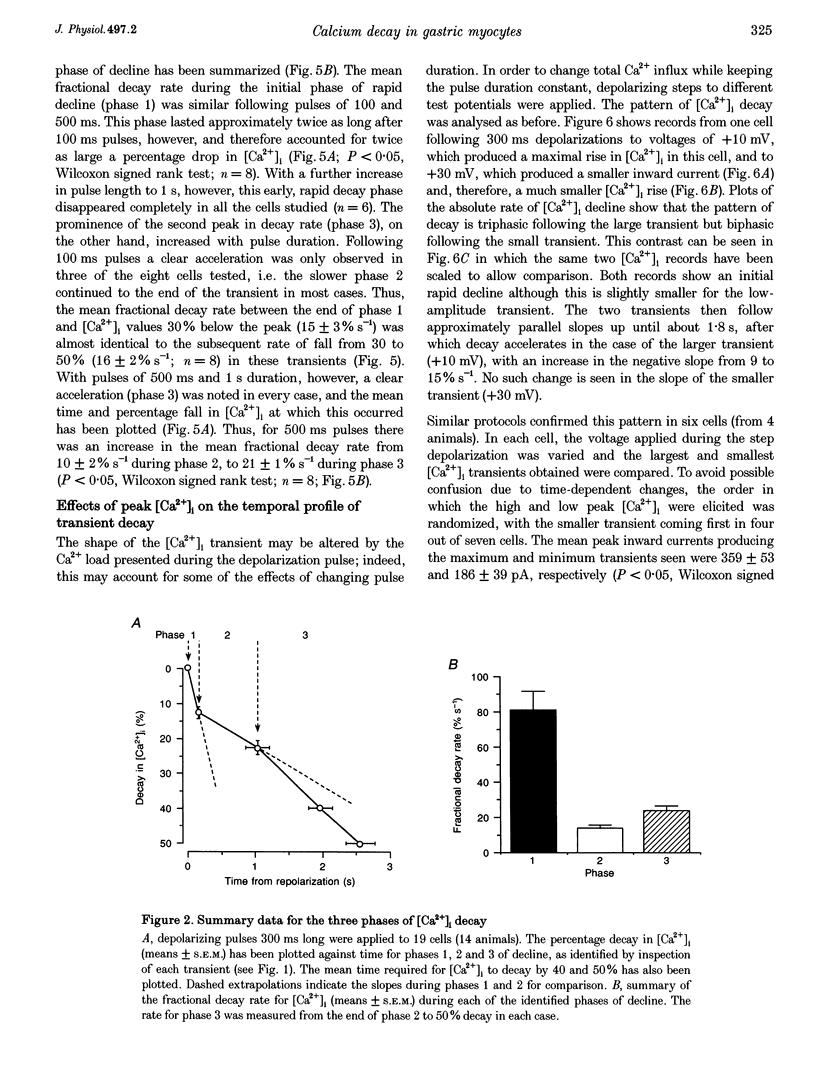
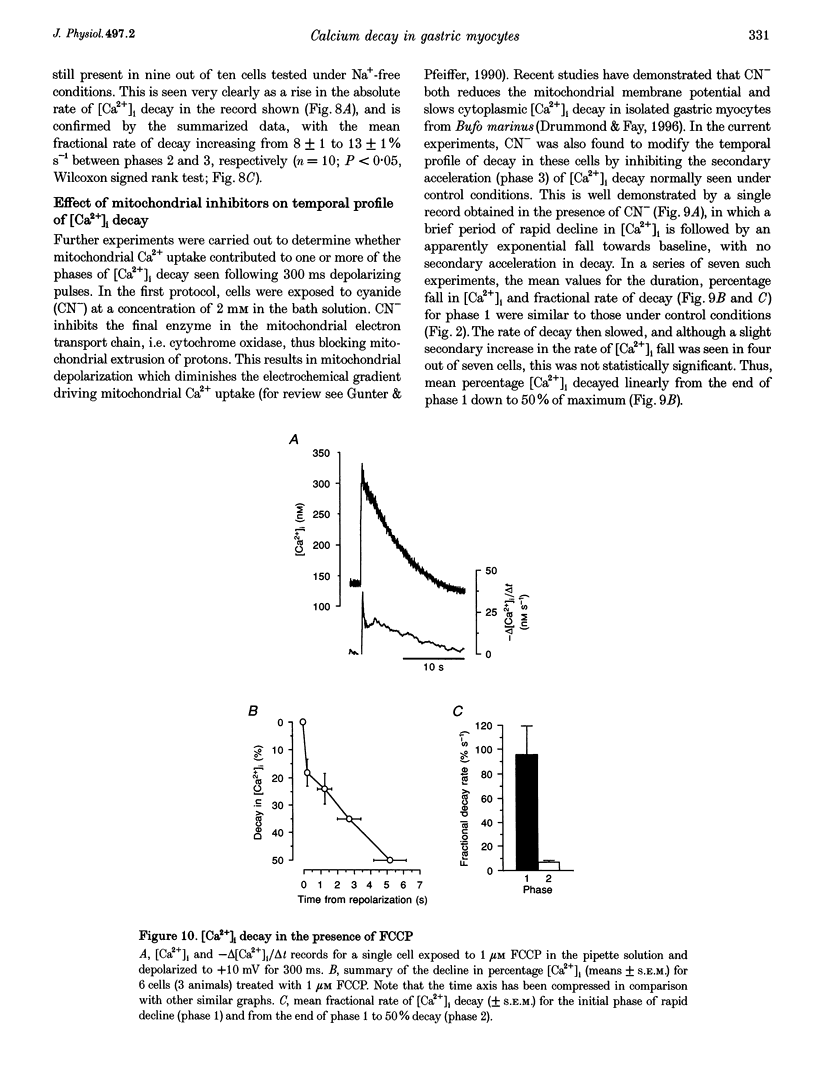
1. Decay in intracellular calcium concentration ([Ca2+]i) was recorded following step depolarizations in voltage clamped gastric myocytes from Bufo marinus. 2. Depolarizations (300 ms) to +10 mV were followed by three phases of [Ca2+]i decay with repolarization to both -110 and -50 mV. The decline was initially rapid (mean fractional decay rate = 81 +/- 11%s-1 at -110 mV), then slowed (decay rate = 14 +/- 2%s-1) and finally accelerated again (decay rate = 24 +/- 3%s-1; n = 19). 3. The initial phase of rapid decay became shorter as the length of the depolarizing pulse increased but was unaffected by changes in pulse voltage. 4. The delayed acceleration in [Ca2+]i decay was no longer seen when the duration of the depolarizing pulses was reduced to 100 ms, but was clearly evident following 500 ms pulses. This phase was abolished when the depolarizing voltage was altered to minimize the rise in [Ca2+]i. 5. Ryanodine and caffeine had no effect on the temporal profile of [Ca2+]i decay. 6. Removal of extracellular Na+ decreased the decay rate during all three phases at -110 mV, but this effect was particularly marked for the initial rapid phase of decay, the rate of which was reduced by 75%. A delayed increase in decay rate was still seen. 7. Inhibition of mitochondrial Ca2+ uptake with cyanide, carbonyl cyanide p-trifluoromethoxy-phenylhydrazone or Ruthenium Red had no effect on the initial rate of [Ca2+]i decay but blocked the delayed acceleration. 8. These results are discussed in terms of a model in which rapid influx of Ca2+ produces a high subsarcolemmal [Ca2+], favouring rapid Ca2+ removal by near-membrane mechanisms, particularly Na(+)-Ca2+ exchange. Mitochondrial Ca2+ removal produces a delayed increase in [Ca2+]i decay if the global [Ca2+]i is raised high enough for long enough.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Klee C. B. Purification and characterization of smooth muscle myosin light chain kinase. J Biol Chem. 1981 Jul 25;256(14):7501–7509. [PubMed] [Google Scholar]
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Becker P. L., Singer J. J., Walsh J. V., Jr, Fay F. S. Regulation of calcium concentration in voltage-clamped smooth muscle cells. Science. 1989 Apr 14;244(4901):211–214. doi: 10.1126/science.2704996. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991 Jan;71(1):129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- Chen Q., Cannell M., van Breemen C. The superficial buffer barrier in vascular smooth muscle. Can J Physiol Pharmacol. 1992 Apr;70(4):509–514. doi: 10.1139/y92-066. [DOI] [PubMed] [Google Scholar]
- Daniel E. E., van Breemen C., Schilling W. P., Kwan C. Y. Regulation of vascular tone: cross-talk between sarcoplasmic reticulum and plasmalemma. Can J Physiol Pharmacol. 1995 May;73(5):551–557. doi: 10.1139/y95-070. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ as a second messenger within mitochondria of the heart and other tissues. Annu Rev Physiol. 1990;52:451–466. doi: 10.1146/annurev.ph.52.030190.002315. [DOI] [PubMed] [Google Scholar]
- Drummond R. M., Fay F. S. Mitochondria contribute to Ca2+ removal in smooth muscle cells. Pflugers Arch. 1996 Feb;431(4):473–482. doi: 10.1007/BF02191893. [DOI] [PubMed] [Google Scholar]
- Etter E. F., Kuhn M. A., Fay F. S. Detection of changes in near-membrane Ca2+ concentration using a novel membrane-associated Ca2+ indicator. J Biol Chem. 1994 Apr 1;269(13):10141–10149. [PubMed] [Google Scholar]
- Etter E. F., Minta A., Poenie M., Fay F. S. Near-membrane [Ca2+] transients resolved using the Ca2+ indicator FFP18. Proc Natl Acad Sci U S A. 1996 May 28;93(11):5368–5373. doi: 10.1073/pnas.93.11.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay F. S., Hoffmann R., Leclair S., Merriam P. Preparation of individual smooth muscle cells from the stomach of Bufo marinus. Methods Enzymol. 1982;85(Pt B):284–292. doi: 10.1016/0076-6879(82)85027-1. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Efficacy of peak Ca2+ currents (ICa) as trigger of sarcoplasmic reticulum Ca2+ release in myocytes from the guinea-pig coronary artery. J Physiol. 1995 Apr 15;484(Pt 2):287–306. doi: 10.1113/jphysiol.1995.sp020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich V. Y., Isenberg G. Dissociation of subsarcolemmal from global cytosolic [Ca2+] in myocytes from guinea-pig coronary artery. J Physiol. 1996 Jan 15;490(Pt 2):305–318. doi: 10.1113/jphysiol.1996.sp021145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gurney A. M., Tsien R. Y., Lester H. A. Activation of a potassium current by rapid photochemically generated step increases of intracellular calcium in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 May;84(10):3496–3500. doi: 10.1073/pnas.84.10.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D. W., Nicoll D. A., Philipson K. D. Charge movement during Na+ translocation by native and cloned cardiac Na+/Ca2+ exchanger. Nature. 1991 Aug 22;352(6337):715–718. doi: 10.1038/352715a0. [DOI] [PubMed] [Google Scholar]
- Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989 Aug;94(2):363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargacin G. J. Calcium signaling in restricted diffusion spaces. Biophys J. 1994 Jul;67(1):262–272. doi: 10.1016/S0006-3495(94)80477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargacin G., Fay F. S. Ca2+ movement in smooth muscle cells studied with one- and two-dimensional diffusion models. Biophys J. 1991 Nov;60(5):1088–1100. doi: 10.1016/S0006-3495(91)82145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Shirokov R. E. Deactivation kinetics of different components of calcium inward current in the membrane of mice sensory neurones. J Physiol. 1989 Feb;409:343–355. doi: 10.1113/jphysiol.1989.sp017501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlib M. A., Kihara M., Farrell C., Dage R. C. The Na+-Ca2+ exchange system in vascular smooth muscle cell membrane vesicles isolated from cultured cells and from tissue is similar. Biochim Biophys Acta. 1988 Mar 22;939(1):173–177. doi: 10.1016/0005-2736(88)90060-0. [DOI] [PubMed] [Google Scholar]
- McCarron J. G., McGeown J. G., Reardon S., Ikebe M., Fay F. S., Walsh J. V., Jr Calcium-dependent enhancement of calcium current in smooth muscle by calmodulin-dependent protein kinase II. Nature. 1992 May 7;357(6373):74–77. doi: 10.1038/357074a0. [DOI] [PubMed] [Google Scholar]
- McCarron J. G., Walsh J. V., Jr, Fay F. S. Sodium/calcium exchange regulates cytoplasmic calcium in smooth muscle. Pflugers Arch. 1994 Feb;426(3-4):199–205. doi: 10.1007/BF00374772. [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Droogmans G., Himpens B., Casteels R. Calcium ion homeostasis in smooth muscle. Pharmacol Ther. 1992 Nov;56(2):191–231. doi: 10.1016/0163-7258(92)90017-t. [DOI] [PubMed] [Google Scholar]
- Moore E. D., Etter E. F., Philipson K. D., Carrington W. A., Fogarty K. E., Lifshitz L. M., Fay F. S. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993 Oct 14;365(6447):657–660. doi: 10.1038/365657a0. [DOI] [PubMed] [Google Scholar]
- Morel N., Godfraind T. Sodium/calcium exchange in smooth-muscle microsomal fractions. Biochem J. 1984 Mar 1;218(2):421–427. doi: 10.1042/bj2180421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold C. M., Van Riper D. A., Chen X. L. Focal [Ca2+]i increases detected by aequorin but not by fura-2 in histamine- and caffeine-stimulated swine carotid artery. J Physiol. 1995 Nov 1;488(Pt 3):549–564. doi: 10.1113/jphysiol.1995.sp020989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C. S., Vasington F. D., Carafoli E. The effect of ruthenium red on the uptake and release of Ca 2+ by mitochondria. Biochem Biophys Res Commun. 1973 Feb 5;50(3):846–852. doi: 10.1016/0006-291x(73)91322-3. [DOI] [PubMed] [Google Scholar]
- Scheid C. R., Fay F. S. Transmembrane 45Ca fluxes in isolated smooth muscle cells: basal Ca2+ fluxes. Am J Physiol. 1984 May;246(5 Pt 1):C422–C430. doi: 10.1152/ajpcell.1984.246.5.C422. [DOI] [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V., Jr Characterization of calcium-activated potassium channels in single smooth muscle cells using the patch-clamp technique. Pflugers Arch. 1987 Feb;408(2):98–111. doi: 10.1007/BF00581337. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P. Excitation-contraction coupling and the ultrastructure of smooth muscle. Circ Res. 1985 Oct;57(4):497–507. doi: 10.1161/01.res.57.4.497. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L., Sturek M. Spontaneous sarcoplasmic reticulum calcium release and extrusion from bovine, not porcine, coronary artery smooth muscle. J Physiol. 1992;451:49–78. doi: 10.1113/jphysiol.1992.sp019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]
- Yagi S., Becker P. L., Fay F. S. Relationship between force and Ca2+ concentration in smooth muscle as revealed by measurements on single cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4109–4113. doi: 10.1073/pnas.85.11.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]


