Abstract
Introduction
In de novo metastatic hormone‐sensitive prostate cancer (mHSPC) treated with upfront intensification using androgen receptor signaling inhibitor or chemotherapy (Docetaxel), achieving a PSA nadir less than 0.2 ng/mL, indicative of superior survival in trials, may often be unattainable in real‐world settings. We explored the predictive value of the degree of PSA decline and time to PSA nadir (TTPN) on oncological outcomes.
Methods
A prospectively maintained database of consecutive prostate cancer cases in Hong Kong was accessed. Patients diagnosed with de novo mHSPC from 2016 to 2022 and treated with upfront intensification were included in this analysis. Landmark analysis on PSA kinetics at 6‐months following treatment intensification was performed. They were classified based on 1) TTPN (≥6 months vs. <6 months), and 2) a combined response (deep responders achieving both ≥95% PSA decline and TTPN ≥ 6 months vs. shallow responders). Multivariable regression analysis was employed to identify the effects of confounders.
Findings
A total of 131 patients were included in this analysis. Classifying patients by combined response best predicted survival outcomes. Deep responders had better progression‐free survival (HR = 0.56; 95%CI = 0.34–0.91; p = 0.019), overall survival (HR = 0.50; 95%CI = 0.26–0.97; p = 0.036), and cancer‐specific survival (HR = 0.43; 95%CI = 0.19–0.99; p = 0.042). Difference in overall survival remained significant after adjustment in multivariable regression analysis.
Conclusion
Our analysis demonstrates that alternative PSA targets can predict treatment response and survival outcomes in de novo mHSPC patients in a real‐world setting, providing valuable information for patient counselling and potentially guiding future trial design.
Keywords: androgen receptor signaling inhibitor, Asian patients, docetaxel, metastatic hormone‐sensitive prostate cancer, PSA kinetics, upfront intensified treatment
1. INTRODUCTION
Prostate cancer continues to pose a significant global health burden, profoundly affecting morbidity and mortality among men worldwide. 1 Recently, there has been an increase in the number of patients diagnosed with metastatic hormone‐sensitive prostate cancer (mHSPC), underscoring the necessity for effective treatment strategies. 2 The treatment landscape for mHSPC has rapidly evolved with the introduction of upfront intensification strategies that combine androgen deprivation therapy (ADT) with either androgen receptor signaling inhibitor (ARSIs) or chemotherapy. 3 Although these therapeutic advances have enhanced survival outcomes in clinical trials, there remains an urgent need for reliable prognostic markers to guide treatment decisions and patient counselling in real‐world clinical settings.
The European Association of Urology (EAU) guidelines highlight the importance of monitoring prostate‐specific antigen (PSA) in mHSPC patients undergoing systemic treatment. 4 These recommendations are still based on the findings from the SWOG 9346 study, conducted during the era of ADT monotherapy, which demonstrated that a PSA nadir of ≤4 ng/mL after 7 months of ADT was associated with improved survival outcomes. 5 Since then, multiple landmark trials involving upfront intensification have further underscored the prognostic value of PSA kinetics in mHSPC patients. 6 However, these findings may not be entirely reflective of the real‐world scenario, where patient or treatment characteristics can differ significantly from those in controlled clinical trials. The question of whether PSA kinetics retains its prognostic utility in the era of upfront intensified treatment remains unanswered.
In this study, we aimed to explore the predictive value of PSA response and time to PSA nadir in an Asian cohort of de novo mHSPC patients treated with upfront Docetaxel (DOC) or ARSI. We sought to provide insights into the real‐world application of PSA kinetics in predicting survival outcomes and optimising patient care in the context of mHSPC.
2. MATERIALS AND METHODS
2.1. Data acquisition
This analysis employed data sourced from the Hong Kong Prostate Cancer Study Group Database, a prospectively maintained database that recorded consecutive prostate cancer cases across three centres in Hong Kong. It was registered on Clinicaltrials. gov (NCT03344835). We specifically identified consecutive patients diagnosed Cases diagnosed from 2016 to 2022 with de novo metastatic hormone‐sensitive prostate cancer (mHSPC). The inclusion criteria were cases treated with upfront intensification, defined as the initiation of first‐line DOC or ARSI (Enzalutamide, Abiraterone, or Apalutamide) within 6 months of starting androgen deprivation therapy (ADT). Cases lacking comprehensive baseline or follow‐up data were excluded from the analysis.
2.2. Cohort information
Both baseline and follow‐up information were meticulously recorded. Baseline characteristics encompassed age, prostate‐specific antigen (PSA) levels before treatment initiation, Gleason scores from biopsy specimens, the choice of upfront intensification agent, and the presence of high‐volume or high‐risk disease. The definition of high‐volume disease was in line with the CHAARTED trial criteria, 7 which include the presence of visceral metastases or four or more bone lesions, with at least one outside of the vertebral bodies and pelvis. High‐risk disease classification followed the LATITUDE trial criteria, 8 which specify two out of three conditions: three or more sites of bone metastasis, any visceral metastasis, and ISUP grade 4 pathology or higher. Follow‐up data captured included subsequent PSA levels, progression to castration‐resistant prostate cancer (CRPC), and mortality.
2.3. Classification of cohort and outcomes of study
The cohort was stratified based on the biochemical response at the nadir level. PSA nadir was defined as the lowest PSA value recorded following the initiation of treatment, and the time to PSA nadir (TTPN) was documented in months. PSA response was quantified as the percentage reduction in PSA at nadir relative to the pretreatment level. The cohort was categorized at a landmark time point at 6 months post‐ADT according to (1) TTPN (time to PSA nadir of ≥6 months vs. <6 months); and (2) combined response (deep responders defined as achieving both ≥95% PSA decline and TTPN ≥ 6 months vs. shallow responders otherwise). It is known that by classifying a cohort at initial presentation with a factor that is presented subsequently at follow (in this analysis: TTPN), the analysis would be harmed by potential immortality time bias. Therefore, the approach of landmark analysis was adopted here. To scrutinise the effect of PSA decline (hence TTPN), any cases that did not make it to the 6 months would have to be eliminated in the subsequent analysis at the landmark time point. The primary outcome of the analysis is progression‐free survival (PFS) (as per the Prostate Cancer Working Group 2 criteria). Secondary outcomes include overall survival (OS), and cancer‐specific survival (CSS).
2.4. Statistical analysis
For statistical analysis, we adhered to established recommendations. 9 Categorical variables were presented using count and percentage, while continuous variables were reported as median with interquartile range or mean with standard deviation. The Chi‐square test and Fisher's exact test were used to compare categorical variables, while Student's T‐test and Mann–Whitey U test were applied to continuous variables. A two‐tailed p‐value of <0.05 was considered statistically significant. Landmark analysis at 6 months post‐ADT was performed. Kaplan–Meier analysis was employed to evaluate the primary outcomes, with group comparisons conducted using the log‐rank test. Multivariable Cox regression analysis was performed to identify factors influencing the outcomes. Factors that were demonstrated to affect disease outcomes were fitted into the model: age at diagnosis, PSA level before treatment, and choice of intensification agent. 10 The number of covariates was selected to prevent model overfitting in our multivariable regression analyses. 11 Kaplan‐Meier analysis would also be conducted to assess the impact of the type of intensification agent (ARSI vs. chemotherapy) on the outcomes. All statistical analyses were performed using SPSS version 25.0 (IBM) and R version 4.3.1.
3. RESULTS
3.1. PSA response level
After applying inclusion and exclusion criteria, a total of 131 cases were included in the study. The median follow‐up duration for the cohort was 38.2 months (IQR = 23.3–53.0 months). When categorized according to the level of PSA response, 98 patients (75.6%) achieved PSA95, while the remaining 33 patients (24.4%) did not. The two groups exhibited comparable baseline characteristics (Table 1a). The median PFS for the PSA95 and non‐PSA95 were 32.5 months versus 20.3 months respectively (HR = 0.56; p = 0.020). The median OS for the two groups was 68.8 months versus not reached (NR) (HR = 0.62; p = 0.149), and the CSS was 81.1 months versus NR (HR = 0.54; p = 0.105).
Table 1a.
Patient, disease and treatment characteristics stratified to PSA response level.
| Stratified to PSA response level | PSA95 | Non‐PSA95 | P value | ||
|---|---|---|---|---|---|
| N | %/SD | N | %/SD | ||
| Number of patients, % | 98 | 74.8% | 33 | 25.1% | |
| Mean age (years), SD | 68.7 | 8.2 | 68.2 | 5.6 | 0.739 |
| Median PSA prior treatment (ng/m), IQR | 211.0 | 136.9–285.2 | 313.0 | 252.7–373.3 | 0.683 |
| Gleason score ≥8, % | 85 | 86.% | 32 | 93.9% | 0.189 |
| High volume disease, % | 92 | 93.8% | 33 | 100% | 0.108 |
| High risk disease, % | 90 | 91.8% | 33 | 100% | 0.066 |
| Presence of visceral metastasis, % | 22 | 22.4% | 7 | 21.2% | 0.950 |
| Median time to PSA nadir (months), SD | 8.8 | 2.5–15.1 | 6.9 | 2.5–11.3 | 0.014 |
| Agents of upfront intensification, % | 0.019 | ||||
| Androgen receptor signaling inhibitor | 35 | 35.7% | 5 | 15.1% | |
| Chemotherapy | 63 | 64.3% | 28 | 84.9% | |
Note: PSA95 = PSA decline ≥95% at nadir from baseline.
Abbreviations: IQR, inter‐quartile range; PSA, prostate‐specific‐antigen; SD, standard deviation.
3.2. Time to PSA nadir
In total there were 123 cases that did not reach the endpoint of PFS at the 6‐month time‐point and were processed in the subsequent landmark analysis. They were cohort divided based on TTPN ≥ 6 months versus <6 months (Table 1b). There were 79 patients (64.2%) in the TTPN ≥ 6 months group and 44 patients (35.8%) in the <6 months group. A near statistical significant advantage was observed in the PFS, with the median PFS for the TTPN ≥ 6 months and <6 months groups being 26.6 months and 19.8 months from inception of the entire cohort, respectively (HR = 0.78) (Figure 1). The median OS was 75.1 months versus 61.8 months (HR = 0.69; p = 0.072), and the CSS was NR versus 61.8 months (HR = 0.57; p = 0.082).
Table 1b.
Patient, disease and treatment characteristics by TTPN and combined response marker, landmark analysis at 6 months.
| Time to PSA nadir | TTPN ≥ 6 months | TTPN < 6 months | P value | ||
|---|---|---|---|---|---|
| N | %/SD | N | %/SD | ||
| Number of patients, % | 79 | 64.2% | 44 | 35.8% | |
| Mean age (years), SD | 68.2 | 7.6 | 68.9 | 7.3 | 0.842 |
| Median PSA prior treatment (ng/m), IQR | 225.8 | 75.6–376.1 | 260.5 | 43.1–477.9 | 0.298 |
| Gleason score ≥8, % | 68 | 86.1% | 37 | 84.1% | 0.795 |
| High volume disease, % | 74 | 93.6% | 40 | 90.9% | 0.584 |
| High risk disease, % | 74 | 93.6% | 40 | 90.9% | 0.337 |
| Presence of visceral metastasis, % | 17 | 21.5% | 9 | 20.5% | 0.862 |
| Agents of upfront intensification, % | 0.244 | ||||
| Androgen receptor signaling inhibitor | 19 | 24.1% | 16 | 36.4% | |
| Chemotherapy | 60 | 75.9% | 28 | 63.6% | |
| Combined response | Deep responders | Shallow responders | P value | ||
|---|---|---|---|---|---|
| N | %/SD | N | %/SD | ||
| Number of patients, % | 64 | 53.8% | 55 | 46.2% | |
| Mean age (years), SD | 69.0 | 8.2 | 68.0 | 6.7 | 0.460 |
| Median PSA prior treatment (ng/m), IQR | 219.1 | 64.5–373.5 | 219.0 | 53.5–384.5 | 0.515 |
| Gleason score ≥8, % | 56 | 87.5% | 48 | 87.3% | 0.971 |
| High volume disease, % | 60 | 93.8% | 52 | 94.5% | 0.856 |
| High risk disease, % | 60 | 93.8% | 50 | 90.9% | 0.563 |
| Presence of visceral metastasis, % | 14 | 21.9% | 12 | 21.8% | 0.923 |
| Agents of upfront intensification, % | 0.490 | ||||
| Androgen receptor signaling inhibitor | 20 | 31.3% | 14 | 25.5% | |
| Chemotherapy | 44 | 68.7% | 41 | 54.5% | |
Abbreviations: IQR, inter‐quartile range; PSA, prostate‐specific‐antigen; SD, standard deviation; TTPN, time to PSA nadir.
Figure 1.
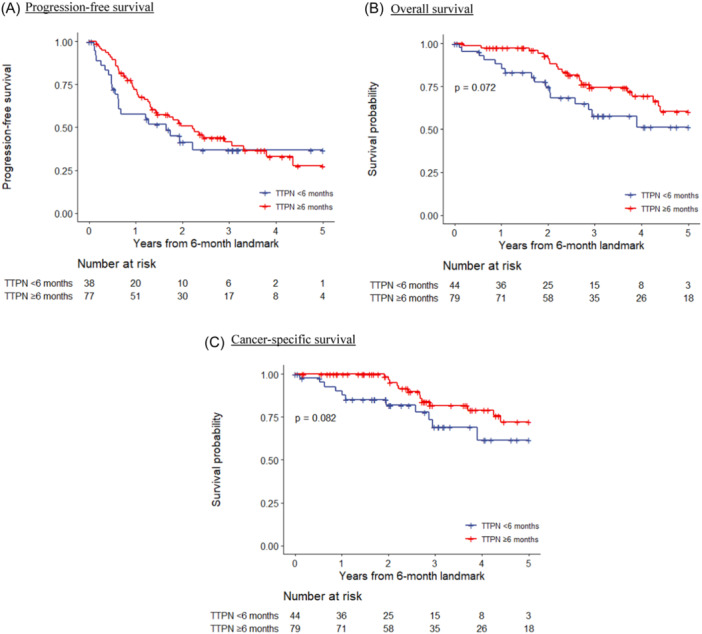
Kaplan–Meier survival curves on oncological outcomes grouped according to TTPN, landmark analysis at 6 months. (A) Progression‐free survival. (B) Overall survival. (C) Cancer‐specific survival. TTPN, time to PSA nadir. [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Combined response
Finally the remaining cohort was further stratified according to a combined marker: achieving both PSA95 and TTPN ≥ 6 months (deep response group) versus the rest (shallow response group), which included those with non‐PSA95 or TTPN < 6 months. A total of 64 patients (53.8%) were classified into the deep response group, and 55 patients (46.2%) into the shallow response group. The characteristics of the two groups were comparable. The median PSA for the two groups were comparable. A majority of cases in both groups had a Gleason score of at least 8 (87.5% in deep responders vs 87.3% in shallow responders, p = 0.971). Most of the cohort was classified as having high‐volume (93.8% vs 94.5%, p = 0.856) and high‐risk disease (93.8% vs 90.9%, p = 0.563). A similar proportion of patients in each group received an ARSI or DOC (p = 0.490) (Table 1b).
Advantages of the deep response group were noted in all of the three survival outcomes with statistical significance. Kaplan–Meier survival analyses revealed that the median PFS for the deep responders was 42.7 months compared to 20.3 months for the shallow responders (HR = 0.56; p = 0.019). The median OS for the two groups was 72.1 months versus 67.8 months (HR = 0.50; p = 0.036), and the CSS was NR versus 67.8 months (HR = 0.43; p = 0.042) (Figure 2).
Figure 2.
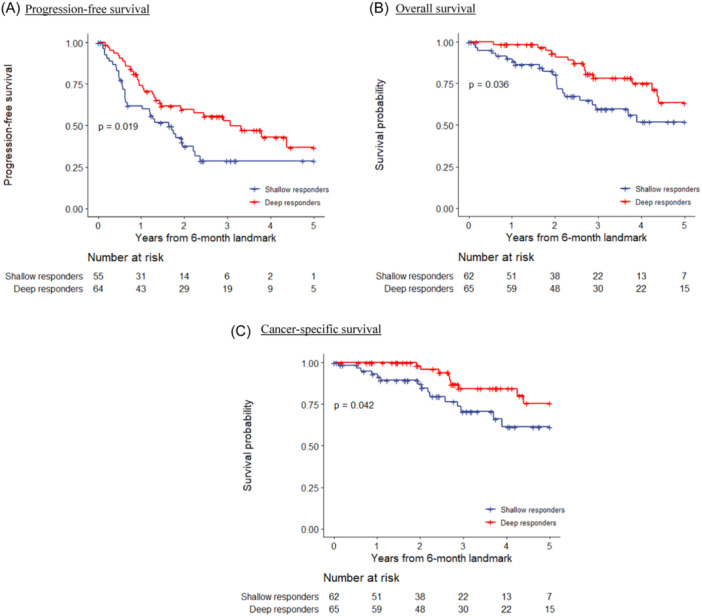
Kaplan‐Meier survival curves on oncological outcomes according to combined response, landmark analysis at 6 months. (A) Progression‐free survival. (B) Overall survival. (C) Cancer‐specific survival. [Color figure can be viewed at wileyonlinelibrary.com]
Univariate cox regression analysis identified PSA response, baseline PSA prior treatment and choice of intensification agent (favouring ARSI over DOCE) were predictors of PFS. PSA response subsequently loss its statistical significance in multivariate analysis for PFS. In the analysis for OS, PSA response was the only significant predictor factor, in both univariate and multivariate analysis, favoring deep response group. In the analysis for CSS, PSA response was a statistical significant predictor in univariate analysis, and the only near‐significant factor in multivariate analysis (Table 2). Subgroup sensitivity analyses evaluating the effect of the intensification agent were also performed, with results depicted in the Kaplan‐Meier curves in Figure 3. Visual inspection of the curves confirmed the predictive power of segregating the cohort into deep and shallow responders, across both the ARSI and chemotherapy subgroups.
Table 2.
Univariate and multivariable Cox regression analysis on factors associated with outcomes, landmark analysis at 6 months.
| Progression free survival | Univariate analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Deep PSA responders | 0.56 | 0.34 | 0.91 | 0.020 | 0.62 | 0.37 | 1.04 | 0.071 |
| Age at diagnosis | 0.98 | 0.95 | 1.01 | 0.2 | 1.00 | 0.95 | 1.04 | 0.9 |
| Gleason score ≥ 8 | 1.33 | 0.60 | 2.91 | 0.5 | 1.07 | 0.48 | 2.41 | 0.9 |
| Baseline PSA (log) | 1.22 | 1.04 | 1.43 | 0.014 | 1.20 | 1.02 | 1.41 | 0.032 |
| Intensification agent | 0.49 | 0.26 | 0.91 | 0.024 | 0.51 | 0.25 | 1.06 | 0.072 |
| Visceral metastasis | 1.02 | 0.88 | 1.16 | 0.7 | 1.01 | 0.85 | 1.17 | 0.9 |
| Overall survival | Univariate analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Deep PSA responders | 0.50 | 0.26 | 0.97 | 0.040 | 0.47 | 0.24 | 0.92 | 0.027 |
| Age at diagnosis | 0.99 | 0.95 | 1.04 | 0.7 | 0.98 | 0.93 | 1.03 | 0.5 |
| Gleason score ≥ 8 | 1.79 | 0.55 | 5.85 | 0.3 | 1.82 | 0.54 | 6.13 | 0.3 |
| Baseline PSA (log) | 1.05 | 0.86 | 1.28 | 0.6 | 1.03 | 0.85 | 1.24 | 0.8 |
| Intensification agent | 1.32 | 0.66 | 2.63 | 0.4 | 1.83 | 0.82 | 4.07 | 0.14 |
| Visceral metastasis | 0.96 | 0.79 | 1.13 | 0.8 | 0.99 | 0.82 | 1.16 | 0.8 |
| Cancer‐specific survival | Univariate analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Deep PSA responders | 0.43 | 0.19 | 0.99 | 0.048 | 0.44 | 0.19 | 1.03 | 0.059 |
| Age at diagnosis | 1.0 | 0.94 | 1.05 | 0.9 | 0.99 | 0.93 | 1.06 | 0.8 |
| Gleason score ≥ 8 | 1.76 | 0.41 | 7.51 | 0.4 | 2.10 | 0.47 | 9.45 | 0.3 |
| Baseline PSA (log) | 1.19 | 0.92 | 1.55 | 0.2 | 1.16 | 0.90 | 1.48 | 0.3 |
| Intensification agent | 0.43 | 0.19 | 0.99 | 0.048 | 1.83 | 0.68 | 4.96 | 0.2 |
| Visceral metastasis | 0.88 | 0.72 | 1.04 | 0.5 | 0.97 | 0.89 | 1.05 | 0.8 |
Note: Intensification agent (Chemotherapy as reference).
Abbreviations: 95%CI, 95% confidence interval; HR, hazard ratio; PSA, prostate‐specific‐antigen.
Figure 3.
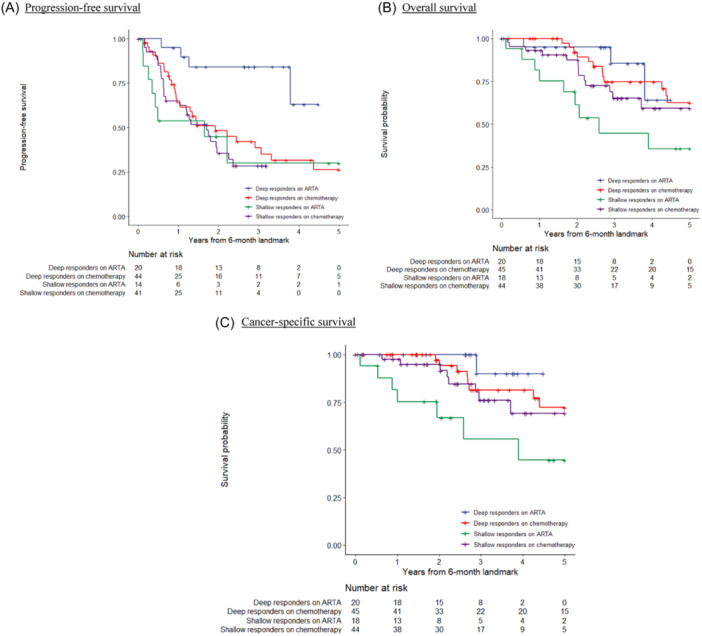
Kaplan–Meier survival curves on oncological outcomes according to intensification agent and combined response, landmark analysis at 6 months. (A) Progression‐free survival. (B) Overall survival. (C) Cancer‐specific survival. [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In this study, we scrutinised the implications of PSA kinetics on survival outcomes for de novo mHSPC patients undergoing upfront intensification in a real‐world setting. From a cohort predominantly characterized by high‐risk and high‐volume disease, we observed that both the degree of PSA decline and the time to PSA nadir significantly contributed to predicting treatment response. Combining these markers provided superior statistical power in delineating survival outcomes. Out of the three methodologies that classified our cohort based on PSA kinetics, a combined approach was shown superior by being able to predict all of PFS, OS and CSS. Notably, the deep response group was associated with better outcomes in terms of OS after adjusting for confounders through multivariable regression analysis, and a near‐statistical advantage in the multivariate analysis for PFS and CSS.
There is a growing body of evidence supporting the monitoring of PSA response following intensified ADT. Much of this evidence, demonstrating a correlation between PSA decline and survival outcomes, has predominantly emanated from prospective trials. Notably, in the CHAARTED trial, 6 patients achieving a PSA value ≤ 0.2 ng/mL at 7 months following ADT combined with DOC exhibited better OS compared to those whose PSA levels exceeded 4 ng/mL. Furthermore, a post‐hoc analysis of the LATITUDE study indicated that a PSA decline exceeding 90% (PSA90) was associated with a significantly reduced risk of death (RR = 0.12) in high‐risk mHSPC patients treated with abiraterone in addition to ADT. 12
More recent trials have corroborated these findings. The ARASENS trial reported that among the 48.7% of patients treated with ADT+Darolutamide, 13 those achieving undetectable PSA levels at 24 and 36 weeks saw improved overall survival rates. Similarly, the TITAN trial found that achieving PSA90, or a PSA level ≤0.2 ng/mL, was associated with improved survival outcomes, including OS, radiographic PFS, and time to CRPC. 14 A recent analysis of the ENZAMET trial, as reported at ASCO 2024, echoed these results, further substantiating the prognostic value of PSA metrics in this patient population. 15
These findings highlight the potential of PSA kinetics as a robust prognostic tool in managing mHSPC, particularly when employing upfront intensification strategies. However, it is important to note that these data were derived from well‐designed prospective trials on a large scale with stringent follow‐up protocols. Such conditions could not be entirely replicable in the real‐world setting. A systematic review in 2023 by Dokins et al. 16 detailed the challenges that hinder the utilisation of upfront intensification in everyday clinical practice. Factors such as financial barriers, geographic access, educational level, racial differences, and whether patient care is directed by a urologist or oncologist, all contribute to the underutilisation of these therapies.
With these considerations in mind, we assert that the importance of reporting PSA kinetics outcomes from a real‐world database is paramount. In actual clinical settings, case inclusion is often more diverse than in controlled clinical trials. Patients present a broad spectrum of initial PSA levels, unlike the uniform cohorts typically assembled for clinical trials. Achieving a significantly low PSA value within a predetermined timeframe can be clinically challenging. For instance, only 63% of patients in the TITAN trial 14 achieved a PSA < 0.2 ng/mL, and merely 18% reached an ultralow PSA nadir of ≤0.02 ng/mL at 3 months—a subgroup associated with the best survival outcomes. From our current analysis, a mojority of the cohort reached a PSA95 at any point following treatment. Remarkably, half of our patients met the criteria of deep responders, an event associated with better outcomes. This more achievable PSA cutoff could be applicable to a broader segment of mHSPC patients. Allowing a PSA nadir to be reached at any time rather than within a fixed period following treatment would also make this marker a more flexible and robust tool in clinical practice.
As we witness continual surge of evidence in trial data, real‐world evidence of PSA kinetics on mHSPC with intensified treatment was also growing. In the current analysis, the ARSI group that yielded shallow response was especially associated with inferior survival outcomes, much of it appearing to be in line of available literatures. Kafka et al. 17 studied 42 patients receiving upfront ARSI or DOC and reported that a PSA nadir of ≤0.05 ng/mL was associated with better OS. In 2023, Lopez‐Abad et al. 18 retrospectively analysed a cohort of 193 patients treated with ADT + Apalutamide and found that a PSA nadir cutoff of ≤0.2 ng/mL distinguished patients with better and worse OS. More recently in 2024, Gebrael et al. 19 analysed 205 intensified mHSPC patients (progressive or de novo disease) and reported that achieving a PSA nadir of ≤0.2 ng/mL at any time during treatment was associated with improved PFS and OS. Also in 2024, Wenzel et al. investigated 238 German mHSPC patients on upfront intensification and reported that a more stringent cutoff of PSA decline ≥99% (PSA99) yielded a significant difference in OS. 20 Overall, our study aligns with these existing literatures, demonstrating that even a more relaxed PSA95 threshold can effectively subclassify mHSPC patients with varying survival outlooks.
Aside from PSA response level, the relationship of TTPN with oncological outcomes has historically been reported in cohorts of mHSPC treated with ADT monotherapy. From real world experience, a profound, adequate response was usually found to take more than 6 or 12 months. Teoh et al. [10] from a 419‐patient cohort reported that both OS and PFS extended with increasing TTPN, noting an advantage for those with TTPN ≥ 6 months. Tomioka et al. also reported TTPN of ≥6 months as a positive prognostic indicator from their 286‐patient ADT monotherapy cohort. 21 In the era of upfront combination therapy, fewer studies have reported the effects of TTPN. Fascinatingly, in trial patients of combinatorial ARSI, a rapid—rather than delayed—PSA drop to nadir within 3 months was found to lead to survival advantages. 22 One may be baffled why seemingly contradicting results were observed in real world studies. This could potentially be explained by the anticipated degree and nadir of PSA drop following intensified treatment. PSA decline to ultra‐low levels of <0.02 ng/mL was found to be associated with even better outcomes. Attaining such level would take considerable time of more than 6 months, as illustrated in the TITAN trial. 14 Therefore, we postulate that an extended TTPN may serve actually as a surrogate marker of ultra‐low or durable response in the real‐world setting. In a retrospective review of real‐word data, Wenzel et al. noted a shorter time to CRPC in mHSPC patients treated with upfront ARSI, DOC, or triplet therapy who had a TTPN of more than 6 or 12 months, 20 echoing with our hypothesis. Considering the predictive effect of both PSA95 and TTPN, our proposed combined response marker—with deep responders achieving both PSA95 and TTPN ≥ 6 months—correlated significantly with long‐term survival figures in the analysis. This marker proved to be a viable predictor in patients treated with both ADT + DOC and ADT + ARSI. Adopting a landmark analysis at 6 months following treatment intensification, it could potentially serve as an interim prognosticating marker to facilitate progress monitoring. A significant portion of patients were able to achieve a status of deep response, underscoring its clinical applicability. The dichotomous cutoff also made it a practical and straightforward tool for estimating treatment response in everyday clinical settings.
Lastly, it is important to acknowledge the limitations of this study. Given its retrospective nature, the results should be interpreted with caution. The population of the cohort was unavoidably heterogenous, constituting of patients treated with DOC and ARSI. Despite effort in subgroup sensitivity analysis, their effect to the outcomes could not be fully neglected. Meanwhile, as we attempted to explore the effect of TTPN, there was a methodological need to reclassify patients at 6 months from initiation of treatment, thus unavoidably some cases were lost to further analysis. Additionally, further subgroup analyses were not feasible due to the limited patient population, which may restrict the generalizability of our findings.
5. CONCLUSION
This study identifies a PSA response of ≥95% decline and a time to PSA nadir of ≥6 months as favorable indicators in mHSPC patients treated with upfront docetaxel or androgen receptor signaling inhibitor. These markers effectively stratify patients by treatment response, enhancing personalized treatment plans. While further research is needed to validate these findings, our results highlight the practical application of PSA kinetics in real‐world clinical settings, offering applicable insights for optimizing mHSPC management.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Wong CH‐M, Ko IC‐H, Leung DK‐W, et al. Deep PSA response and extended time‐to‐nadir as robust predictors of survival in Asian patients with de novo metastatic hormone‐sensitive prostate cancer receiving upfront intensified treatment. Prostate. 2025;85:30‐39. 10.1002/pros.24797
DATA AVAILABILITY STATEMENT
The data involved in the study could be made available from the corresponding author upon request.
REFERENCES
- 1. Pernar CH, Ebot EM, Wilson KM, Mucci LA. The epidemiology of prostate cancer. Cold Spring Harbor Perspect Med. 2018;8(12):a030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7‐33. [DOI] [PubMed] [Google Scholar]
- 3. Cornford P, van den Bergh RCN, Briers E, et al. EAU‐EANM‐ESTRO‐ESUR‐SIOG guidelines on prostate cancer. part II‐2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79(2):263‐282. [DOI] [PubMed] [Google Scholar]
- 4. Mottet N, van den Bergh RCN, Briers E, et al. EAU‐EANM‐ESTRO‐ESUR‐SIOG guidelines on prostate cancer‐2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243‐262. [DOI] [PubMed] [Google Scholar]
- 5. Hussain M, Tangen CM, Higano C, et al. Absolute prostate‐specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from southwest oncology group trial 9346 (INT‐0162). J Clin Oncol. 2006;24(24):3984‐3990. [DOI] [PubMed] [Google Scholar]
- 6. Harshman LC, Chen YH, Liu G, et al. Seven‐month prostate‐specific antigen is prognostic in metastatic hormone‐sensitive prostate cancer treated with androgen deprivation with or without docetaxel. J Clin Oncol. 2018;36(4):376‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone‐sensitive prostate cancer. N Engl J Med. 2015;373(8):737‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration‐sensitive prostate cancer. N Engl J Med. 2017;377(4):352‐360. [DOI] [PubMed] [Google Scholar]
- 9. Assel M, Sjoberg D, Elders A, et al. Guidelines for reporting of statistics for clinical research in urology. Eur Urol. 2019;75(3):358‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beyer K, Moris L, Lardas M, et al. Diagnostic and prognostic factors in patients with prostate cancer: a systematic review. BMJ Open. 2022;12(4):e058267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faber NM, Rajkó R. How to avoid over‐fitting in multivariate calibration—the conventional validation approach and an alternative. Anal Chim Acta. 2007;595(1‐2):98‐106. [DOI] [PubMed] [Google Scholar]
- 12. Matsubara N, Chi KN, Özgüroğlu M, et al. Correlation of prostate‐specific antigen kinetics with overall survival and radiological progression‐free survival in metastatic castration‐sensitive prostate cancer treated with abiraterone acetate plus prednisone or placebos added to androgen deprivation therapy: post hoc analysis of phase 3 LATITUDE study. Eur Urol. 2020;77(4):494‐500. [DOI] [PubMed] [Google Scholar]
- 13. Saad F, Hussain MHA, Tombal B, et al. Deep and durable prostate‐specific antigen response to darolutamide with androgen deprivation therapy and docetaxel, and association with clinical outcomes for patients with high‐ or low‐volume metastatic hormone‐sensitive prostate cancer: analyses of the randomized phase 3 ARASENS study. Eur Urol . 2024;86(4):329‐339. 10.1016/j.eururo.2024.03.036 [DOI] [PubMed] [Google Scholar]
- 14. Merseburger AS, Agarwal N, Bhaumik A, et al. Apalutamide plus androgen deprivation therapy in clinical subgroups of patients with metastatic castration‐sensitive prostate cancer: a subgroup analysis of the randomised clinical TITAN study. Eur J Cancer. 2023;193:113290. [DOI] [PubMed] [Google Scholar]
- 15. Mc Laughlin RA, Thomas H, Davis ID, et al. Prognostic implications of PSA levels at 7 months in metastatic hormone‐sensitive prostate cancer treated with enzalutamide: landmark analysis of ENZAMET (ANZUP 1304). J Clin Oncol. 2024;42(16_suppl):5079. [Google Scholar]
- 16. Dodkins J, Nossiter J, Cook A, et al. Does research from clinical trials in metastatic hormone‐sensitive prostate cancer treatment translate into access to treatments for patients in the “real world”? A Systematic Review. Eur Urol Oncol. 7, 2024:14‐24(1). [DOI] [PubMed] [Google Scholar]
- 17. Kafka M, Burtscher T, Fritz J, et al. Real‐world comparison of docetaxel versus new hormonal agents in combination with androgen‐deprivation therapy in metastatic hormone‐sensitive prostate cancer descrying PSA nadir </= 0.05 ng/ml as marker for treatment response. World J Urol. 2023;41(8):2043‐2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. López‐Abad A, Ramírez Backhaus M, Server Gómez G, et al. Real‐world prostate‐specific antigen reduction and survival outcomes of metastatic hormone‐sensitive prostate cancer patients treated with apalutamide: an observational, retrospective, and multicentre study. Prostate Int. 2024;12(1):20‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gebrael G, Sayegh N, Thomas VM, et al. Survival outcomes of real world patients with metastatic hormone‐sensitive prostate cancer who do not achieve optimal PSA response with intensified androgen deprivation therapy with docetaxel or androgen receptor pathway inhibitors. Prostate Cancer Prostatic Dis. 2024;27(2):279‐282. [DOI] [PubMed] [Google Scholar]
- 20. Wenzel M, Hoeh B, Hurst F, et al. Impact of PSA nadir, PSA response and time to PSA nadir on overall survival in real‐world setting of metastatic hormone‐sensitive prostate cancer patients. Prostate. 2024;84(13):1189‐1197. 10.1002/pros.24754 [DOI] [PubMed] [Google Scholar]
- 21. Tomioka A, Tanaka N, Yoshikawa M, et al. Nadir PSA level and time to nadir PSA are prognostic factors in patients with metastatic prostate cancer. BMC Urol. 2014;14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Small EJ, Chi KN, Chowdhury S, et al. Post Hoc analysis of rapid and deep prostate‐specific antigen decline and patient‐reported health‐related quality of life in SPARTAN and TITAN patients with advanced prostate cancer. Eur Urol Oncol. 2024;7(4):844‐852. 10.1016/j.euo.2023.11.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data involved in the study could be made available from the corresponding author upon request.


