Abstract
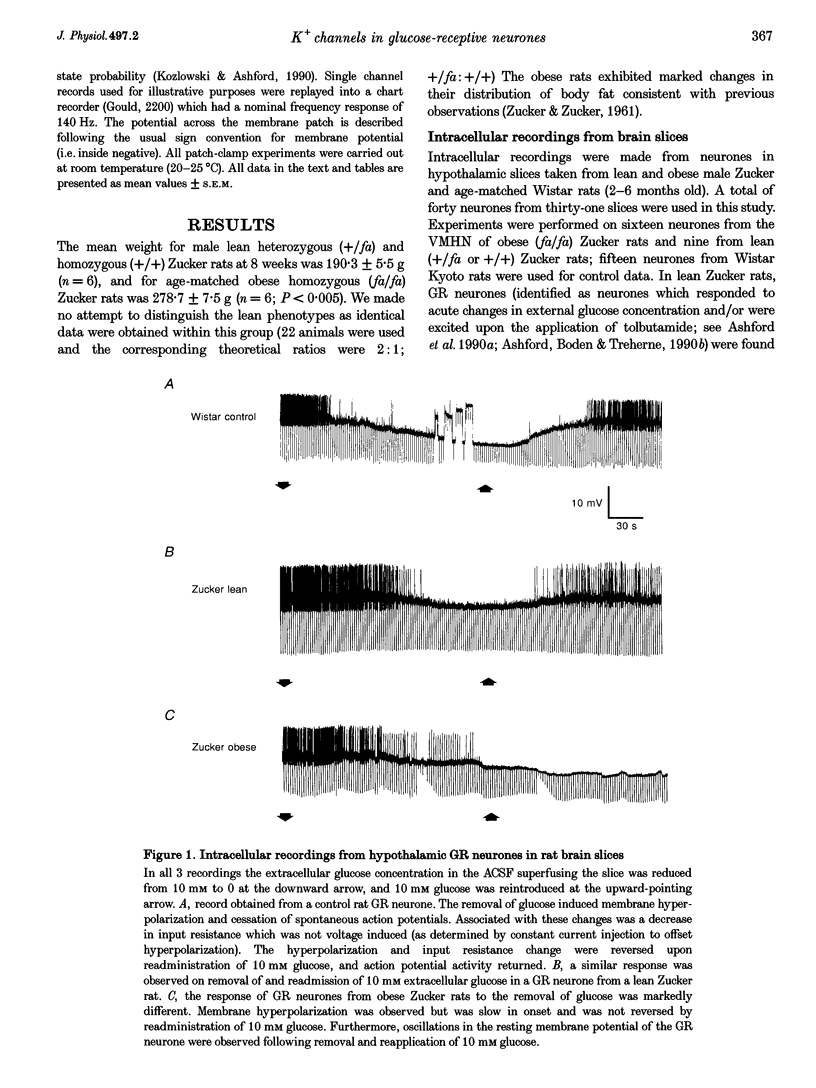
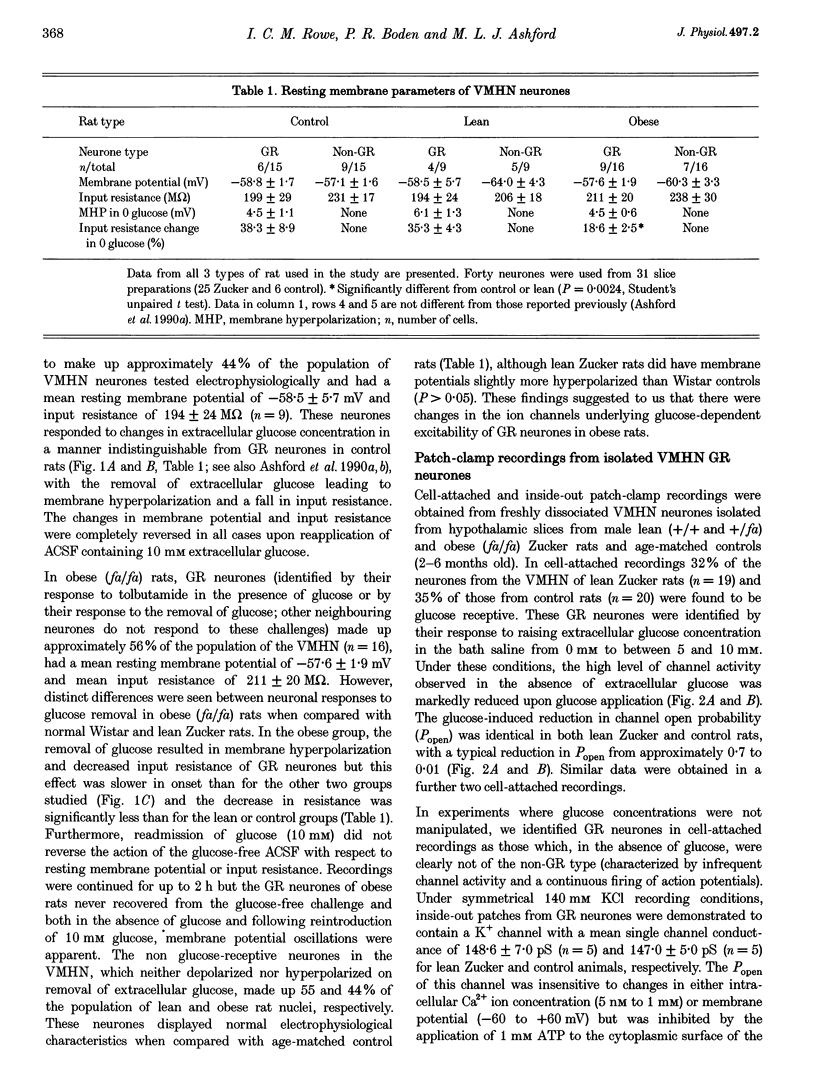
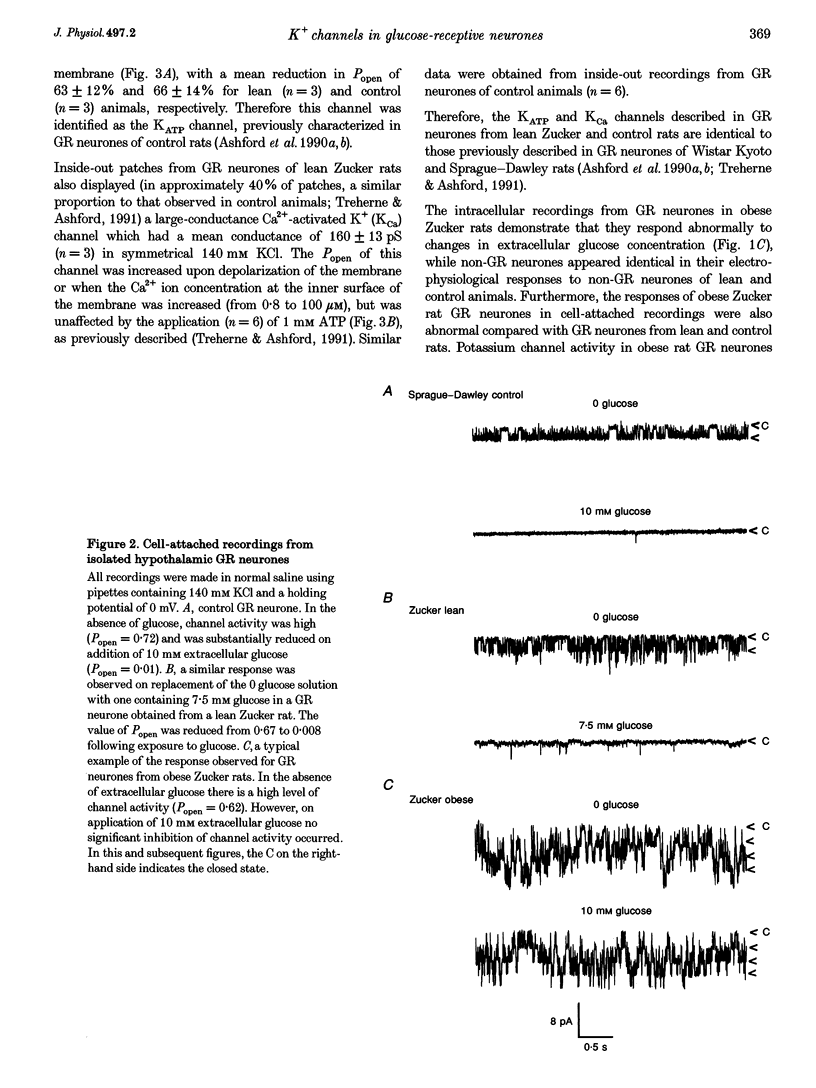
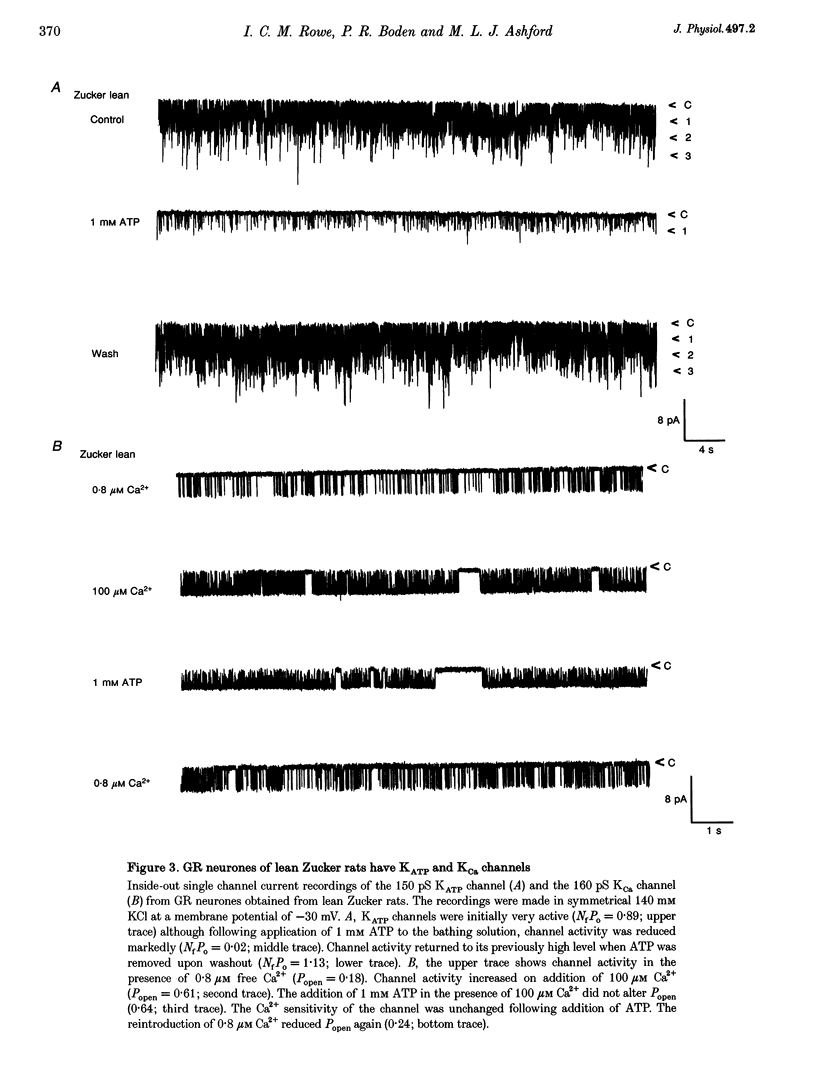
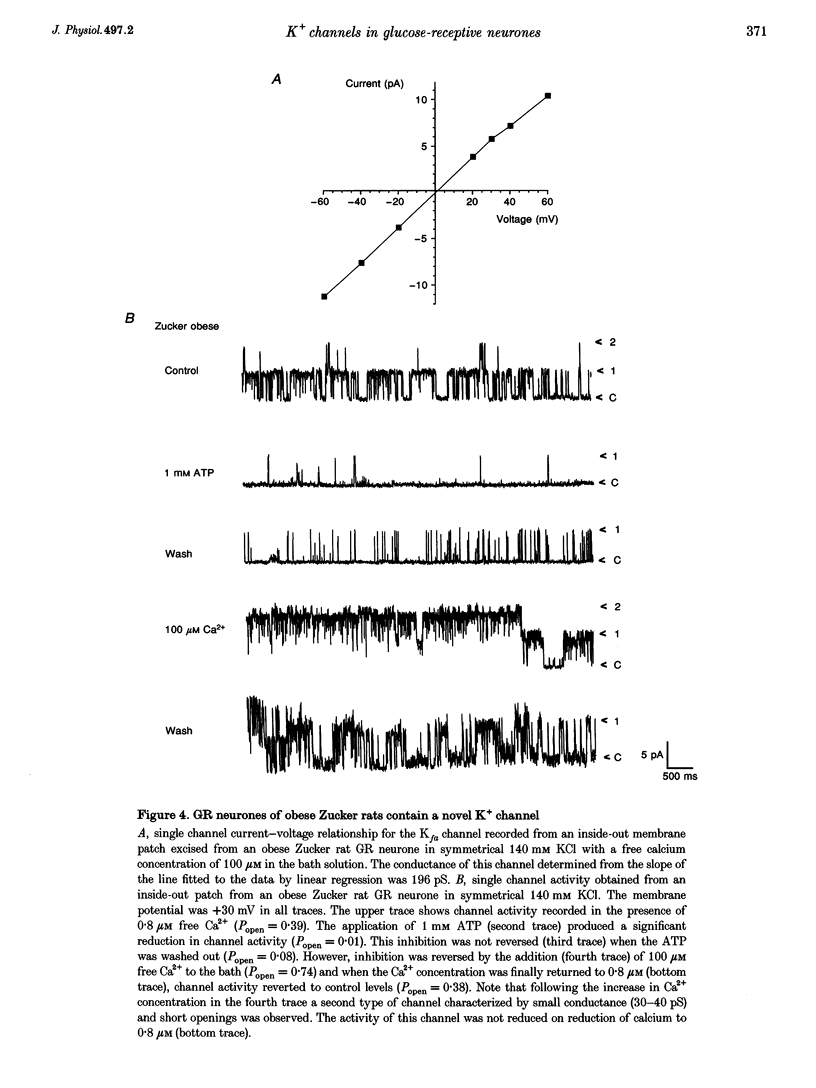
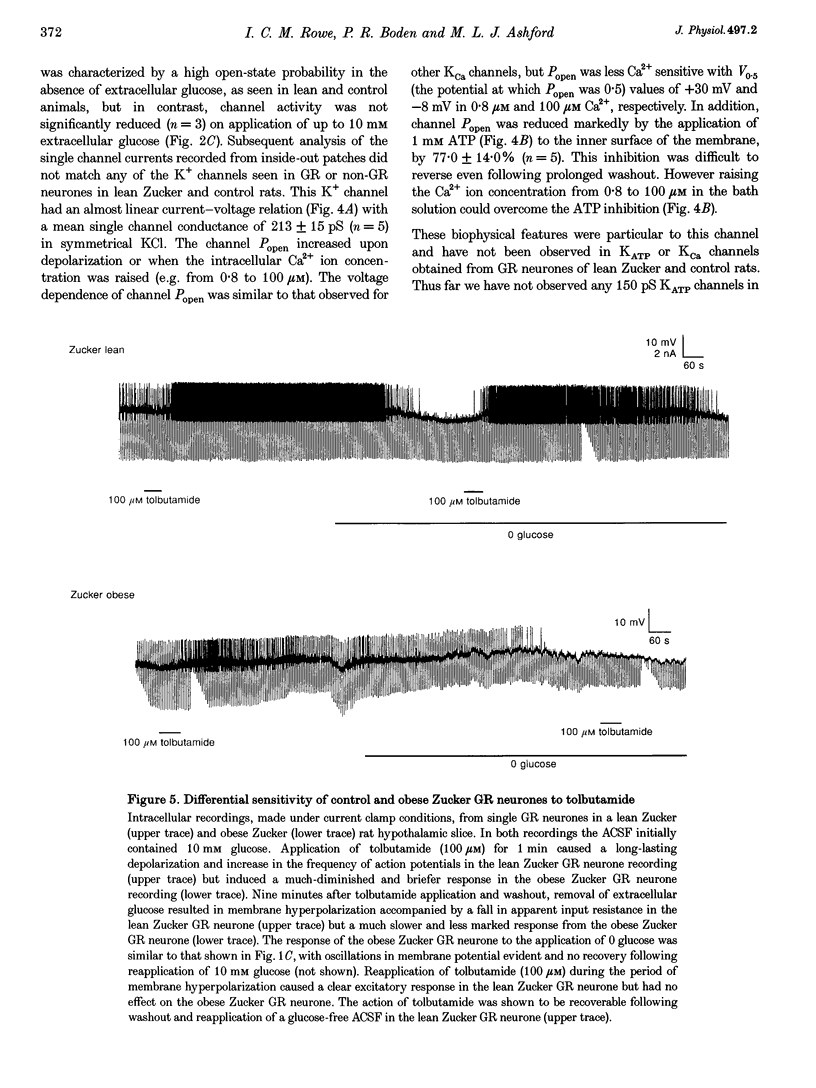
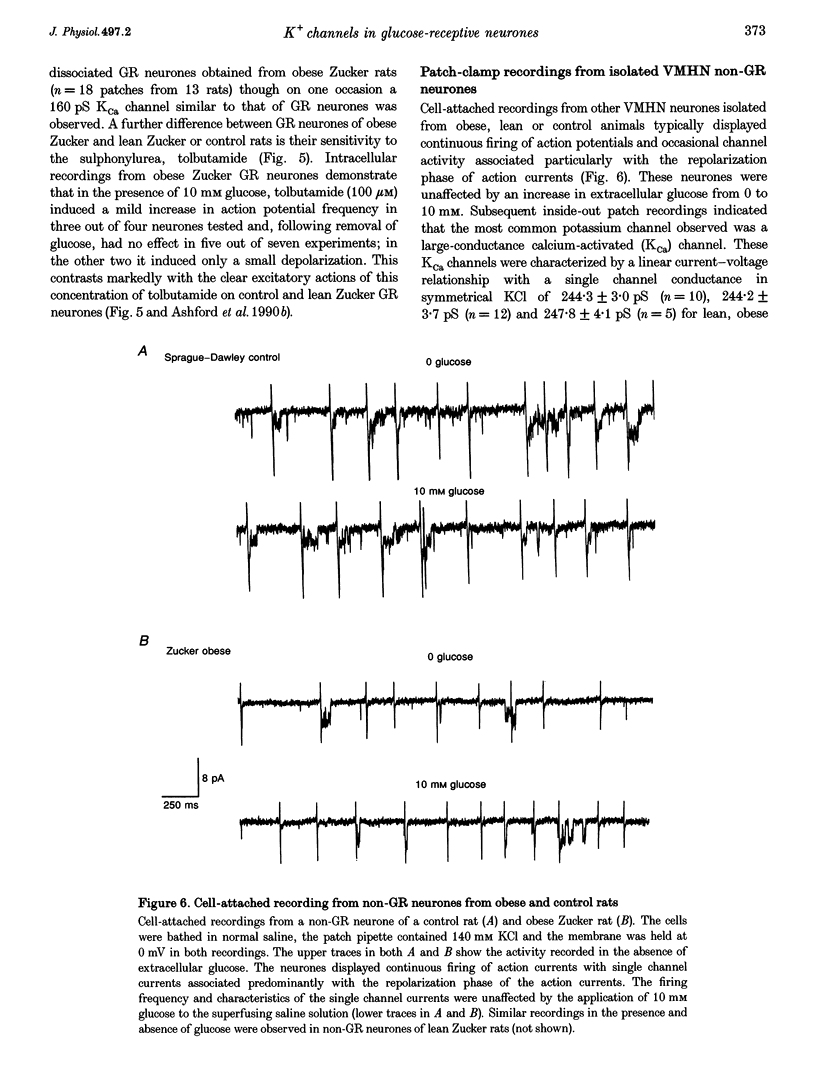
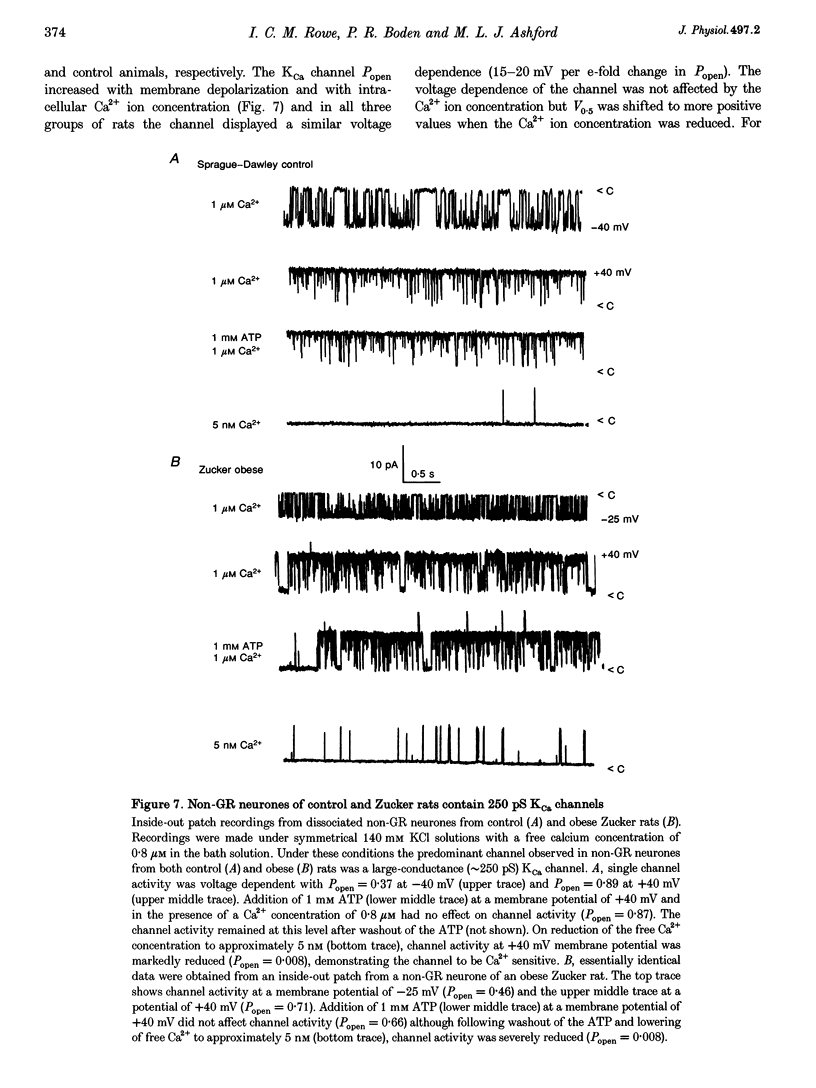
1. We have shown, using intracellular and cell-attached recordings, that glucose-receptive (GR) neurones of obese Zucker rats exhibit abnormal electrophysiological responses to changes in extracellular glucose concentration, whereas GR neurones of lean Zucker and control rats respond normally. 2. In inside-out recordings from obese rat GR neurones it was shown that the 150 pS ATP-sensitive K+ (KATP) and the 160 pS calcium-activated K+ (KCa) channels were absent, whereas both were present in GR neurones of lean Zucker and control rats. 3. The potassium channel most frequently observed in inside-out patches from obese GR neurones was characterized by a conductance of 213 pS, was activated by raising internal calcium and inhibited by application of internal ATP. This channel (which we have termed Kfa) was not observed in lean or control rat GR neurones. 4. Tolbutamide (100 microM) was found to induce no effect or to elicit a small depolarization of obese rat GR neurones in the absence of glucose, in contrast to its clear excitatory actions on control or lean Zucker GR neurones. 5. Intracellular, cell-attached and inside-out recordings from obese rat non-GR neurones showed that there was no alteration in their membrane properties or firing characteristics or in the characteristics of the large-conductance calcium-activated K+ channel (KCa) present in these neurones as compared with lean and control rats. 6. It is concluded that the Kfa channel is specific to GR neurones of obese Zucker rats and that the presence of this channel coupled with the absence of KATP and KCa channels results in the abnormal glucose-sensing response of these neurones.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Boden P. R., Treherne J. M. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990 Jan;415(4):479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Boden P. R., Treherne J. M. Tolbutamide excites rat glucoreceptive ventromedial hypothalamic neurones by indirect inhibition of ATP-K+ channels. Br J Pharmacol. 1990 Nov;101(3):531–540. doi: 10.1111/j.1476-5381.1990.tb14116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden P., Hill R. G. Effects of cholecystokinin and related peptides on neuronal activity in the ventromedial nucleus of the rat hypothalamus. Br J Pharmacol. 1988 May;94(1):246–252. doi: 10.1111/j.1476-5381.1988.tb11521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G. A., York D. A. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev. 1979 Jul;59(3):719–809. doi: 10.1152/physrev.1979.59.3.719. [DOI] [PubMed] [Google Scholar]
- Campfield L. A., Smith F. J., Guisez Y., Devos R., Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995 Jul 28;269(5223):546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Chung S. K., Reinhart P. H., Martin B. L., Brautigan D., Levitan I. B. Protein kinase activity closely associated with a reconstituted calcium-activated potassium channel. Science. 1991 Aug 2;253(5019):560–562. doi: 10.1126/science.1857986. [DOI] [PubMed] [Google Scholar]
- Funahashi T., Shimomura I., Hiraoka H., Arai T., Takahashi M., Nakamura T., Nozaki S., Yamashita S., Takemura K., Tokunaga K. Enhanced expression of rat obese (ob) gene in adipose tissues of ventromedial hypothalamus (VMH)-lesioned rats. Biochem Biophys Res Commun. 1995 Jun 15;211(2):469–475. doi: 10.1006/bbrc.1995.1837. [DOI] [PubMed] [Google Scholar]
- Guillaume-Gentil C., Rohner-Jeanrenaud F., Abramo F., Bestetti G. E., Rossi G. L., Jeanrenaud B. Abnormal regulation of the hypothalamo-pituitary-adrenal axis in the genetically obese fa/fa rat. Endocrinology. 1990 Apr;126(4):1873–1879. doi: 10.1210/endo-126-4-1873. [DOI] [PubMed] [Google Scholar]
- Halaas J. L., Gajiwala K. S., Maffei M., Cohen S. L., Chait B. T., Rabinowitz D., Lallone R. L., Burley S. K., Friedman J. M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995 Jul 28;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Shino A., Matsuo T., Iwatsuka H., Suzuoki Z. A new genetically obese-hyperglycemic rat (Wistar fatty). Diabetes. 1981 Dec;30(12):1045–1050. doi: 10.2337/diab.30.12.1045. [DOI] [PubMed] [Google Scholar]
- Ikeda H., West D. B., Pustek J. J., Figlewicz D. P., Greenwood M. R., Porte D., Jr, Woods S. C. Intraventricular insulin reduces food intake and body weight of lean but not obese Zucker rats. Appetite. 1986 Dec;7(4):381–386. doi: 10.1016/s0195-6663(86)80006-x. [DOI] [PubMed] [Google Scholar]
- Kozlowski R. Z., Ashford M. L. ATP-sensitive K(+)-channel run-down is Mg2+ dependent. Proc R Soc Lond B Biol Sci. 1990 Jun 22;240(1298):397–410. doi: 10.1098/rspb.1990.0044. [DOI] [PubMed] [Google Scholar]
- Lee G. H., Proenca R., Montez J. M., Carroll K. M., Darvishzadeh J. G., Lee J. I., Friedman J. M. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996 Feb 15;379(6566):632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Lee K., Rowe I. C., Ashford M. L. Characterization of an ATP-modulated large conductance Ca(2+)-activated K+ channel present in rat cortical neurones. J Physiol. 1995 Oct 15;488(Pt 2):319–337. doi: 10.1113/jphysiol.1995.sp020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M., Fei H., Lee G. H., Dani C., Leroy P., Zhang Y., Proenca R., Negrel R., Ailhaud G., Friedman J. M. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6957–6960. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzaki H., Hosoda K., Ogawa Y., Shigemoto M., Satoh N., Mori K., Tamura N., Nishi S., Yoshimasa Y., Yamori Y. Augmented expression of obese (ob) gene during the process of obesity in genetically obese-hyperglycemic Wistar fatty (fa/fa) rats. FEBS Lett. 1996 Jan 15;378(3):267–271. doi: 10.1016/0014-5793(95)01472-1. [DOI] [PubMed] [Google Scholar]
- Minami T., Oomura Y., Sugimori M. Electrophysiological properties and glucose responsiveness of guinea-pig ventromedial hypothalamic neurones in vitro. J Physiol. 1986 Nov;380:127–143. doi: 10.1113/jphysiol.1986.sp016276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley J. E., Levine A. S. The pharmacology of eating behavior. Annu Rev Pharmacol Toxicol. 1985;25:127–146. doi: 10.1146/annurev.pa.25.040185.001015. [DOI] [PubMed] [Google Scholar]
- Murakami T., Shima K. Cloning of rat obese cDNA and its expression in obese rats. Biochem Biophys Res Commun. 1995 Apr 26;209(3):944–952. doi: 10.1006/bbrc.1995.1589. [DOI] [PubMed] [Google Scholar]
- Niijima A. Nervous regulation of metabolism. Prog Neurobiol. 1989;33(2):135–147. doi: 10.1016/0301-0082(89)90037-3. [DOI] [PubMed] [Google Scholar]
- Ono T., Nishino H., Fukuda M., Sasaki K., Muramoto K., Oomura Y. Glucoresponsive neurons in rat ventromedial hypothalamic tissue slices in vitro. Brain Res. 1982 Jan 28;232(2):494–499. doi: 10.1016/0006-8993(82)90295-5. [DOI] [PubMed] [Google Scholar]
- Oomura Y., Ono T., Ooyama H., Wayner M. J. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969 Apr 19;222(5190):282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- Pelleymounter M. A., Cullen M. J., Baker M. B., Hecht R., Winters D., Boone T., Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995 Jul 28;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Phillips M. S., Liu Q., Hammond H. A., Dugan V., Hey P. J., Caskey C. J., Hess J. F. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996 May;13(1):18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Chung S., Martin B. L., Brautigan D. L., Levitan I. B. Modulation of calcium-activated potassium channels from rat brain by protein kinase A and phosphatase 2A. J Neurosci. 1991 Jun;11(6):1627–1635. doi: 10.1523/JNEUROSCI.11-06-01627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia L. A., Dembski M., Weng X., Deng N., Culpepper J., Devos R., Richards G. J., Campfield L. A., Clark F. T., Deeds J. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995 Dec 29;83(7):1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Truett G. E., Bahary N., Friedman J. M., Leibel R. L. Rat obesity gene fatty (fa) maps to chromosome 5: evidence for homology with the mouse gene diabetes (db). Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7806–7809. doi: 10.1073/pnas.88.17.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarjevski N., Doyle P., Jeanrenaud B. Muscle insulin resistance may not be a primary etiological factor in the genetically obese fa/fa rat. Endocrinology. 1992 Mar;130(3):1564–1570. doi: 10.1210/endo.130.3.1537306. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 1;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]


