Abstract
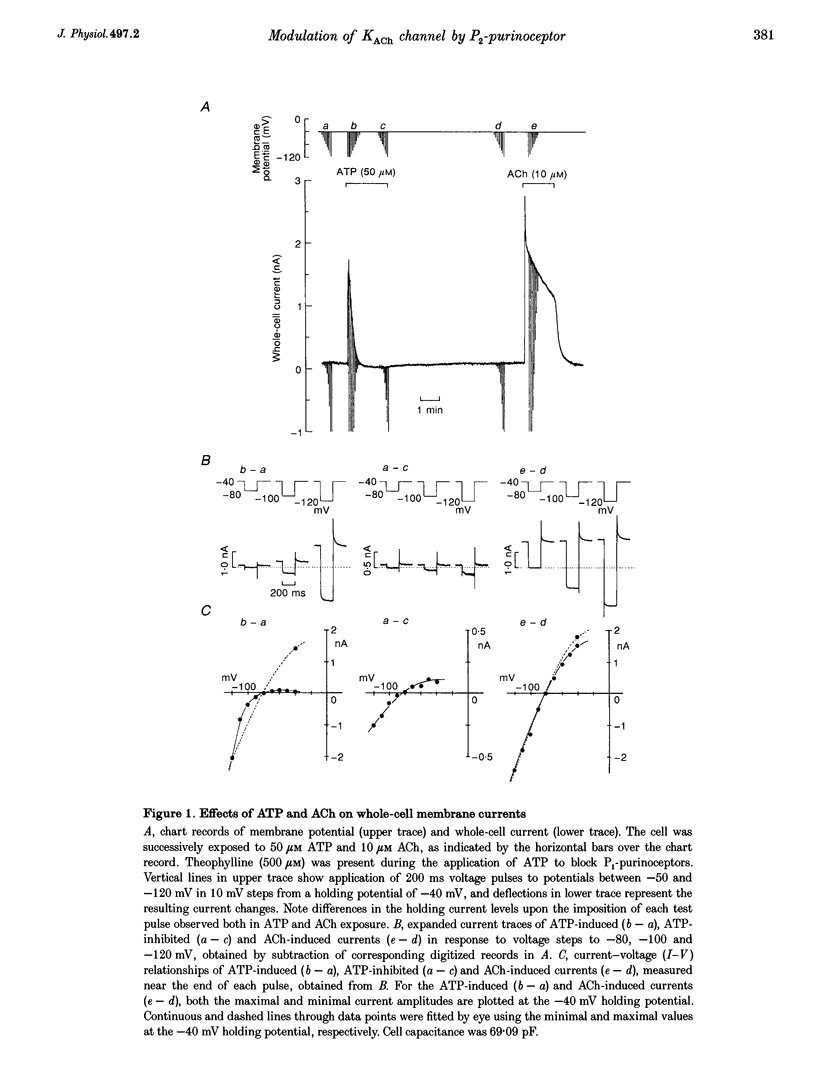
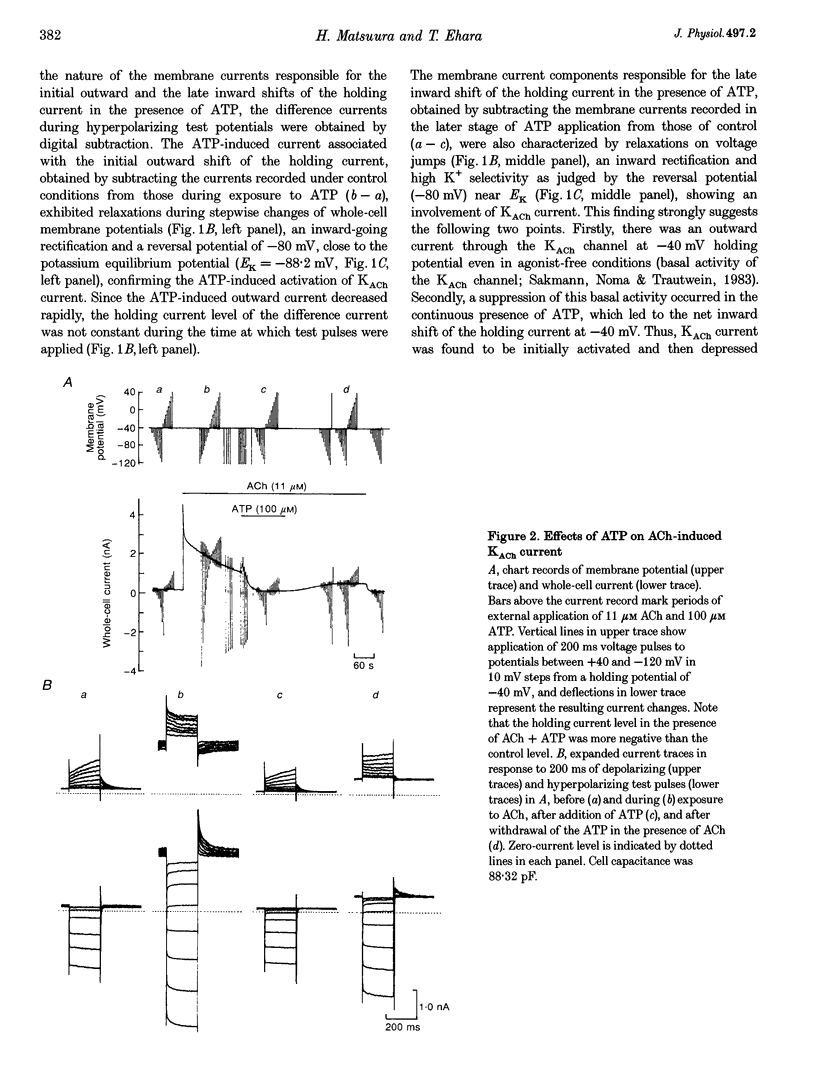
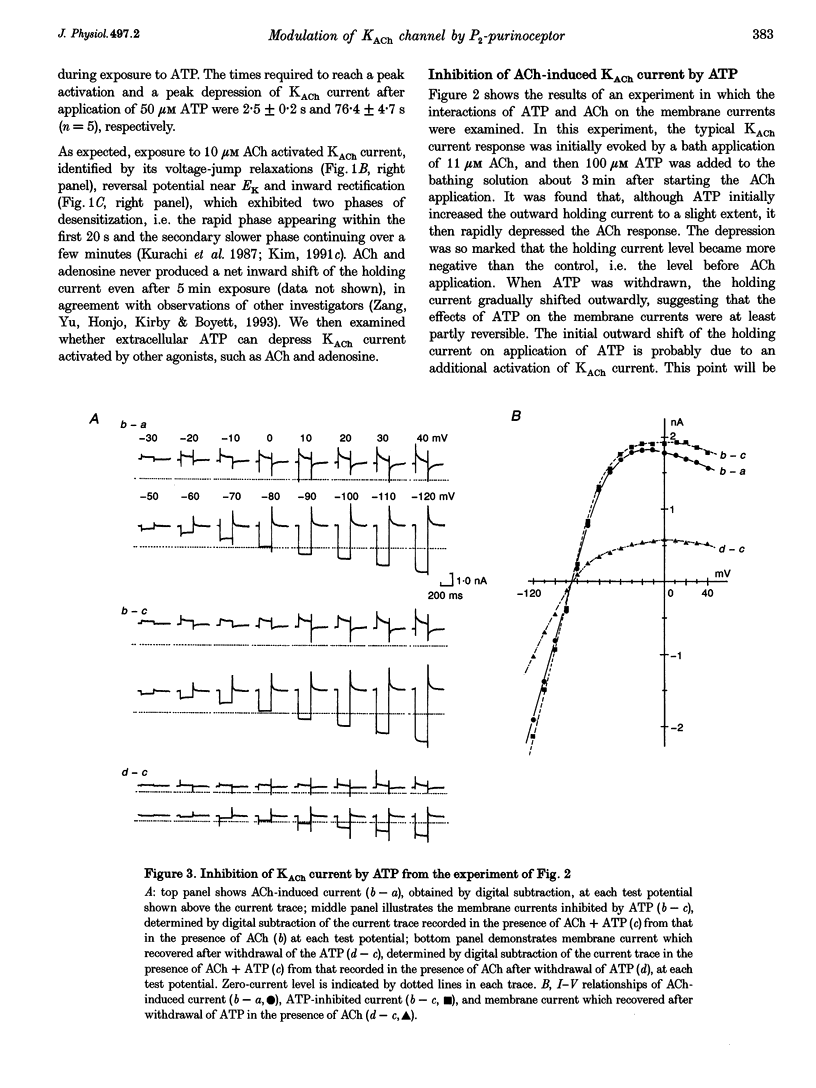
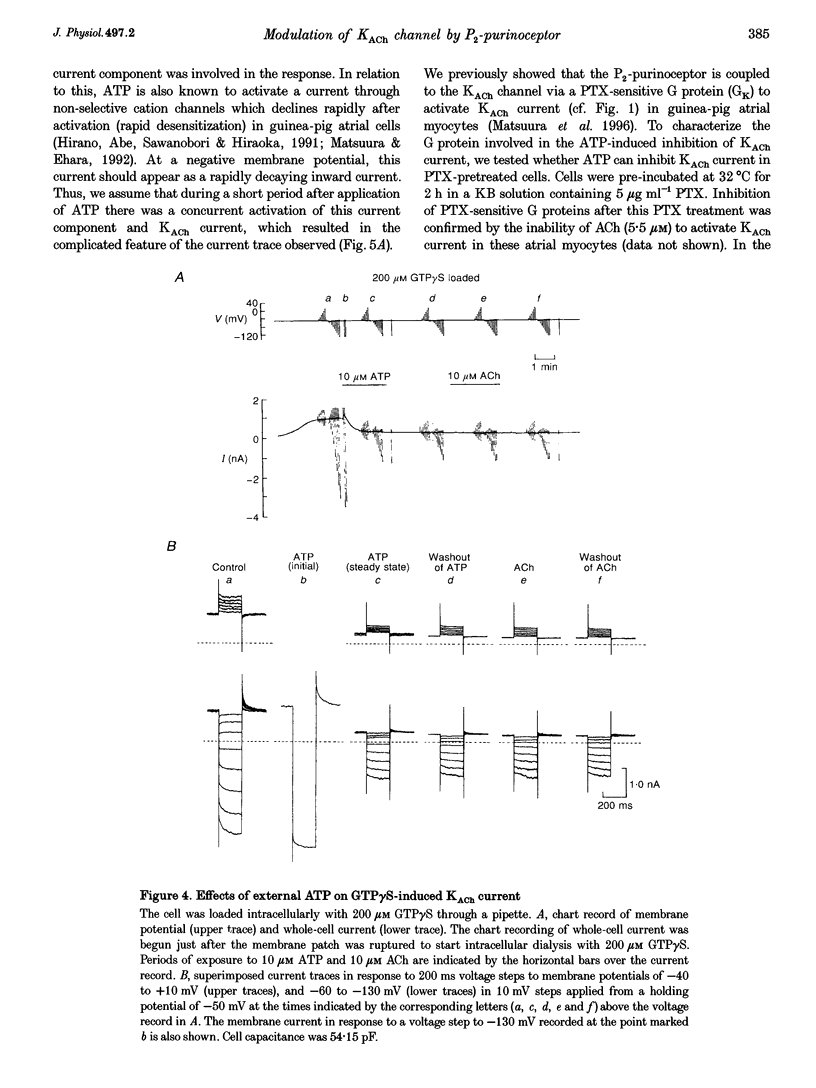
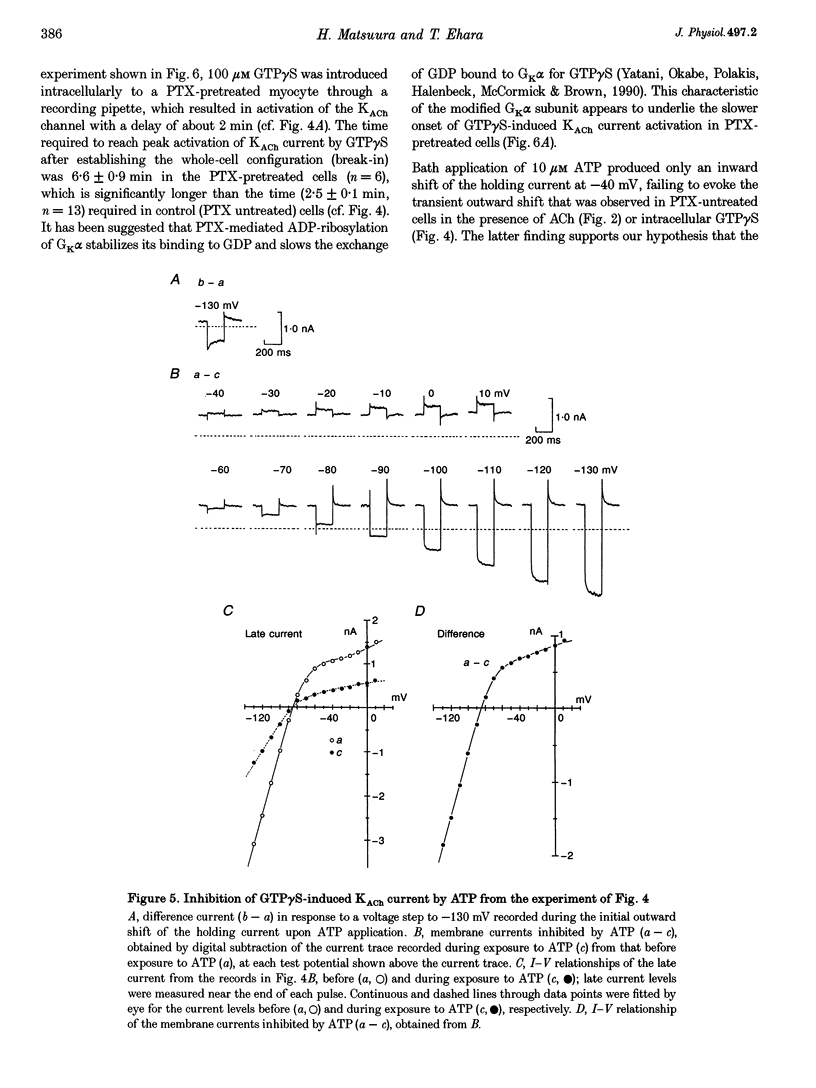
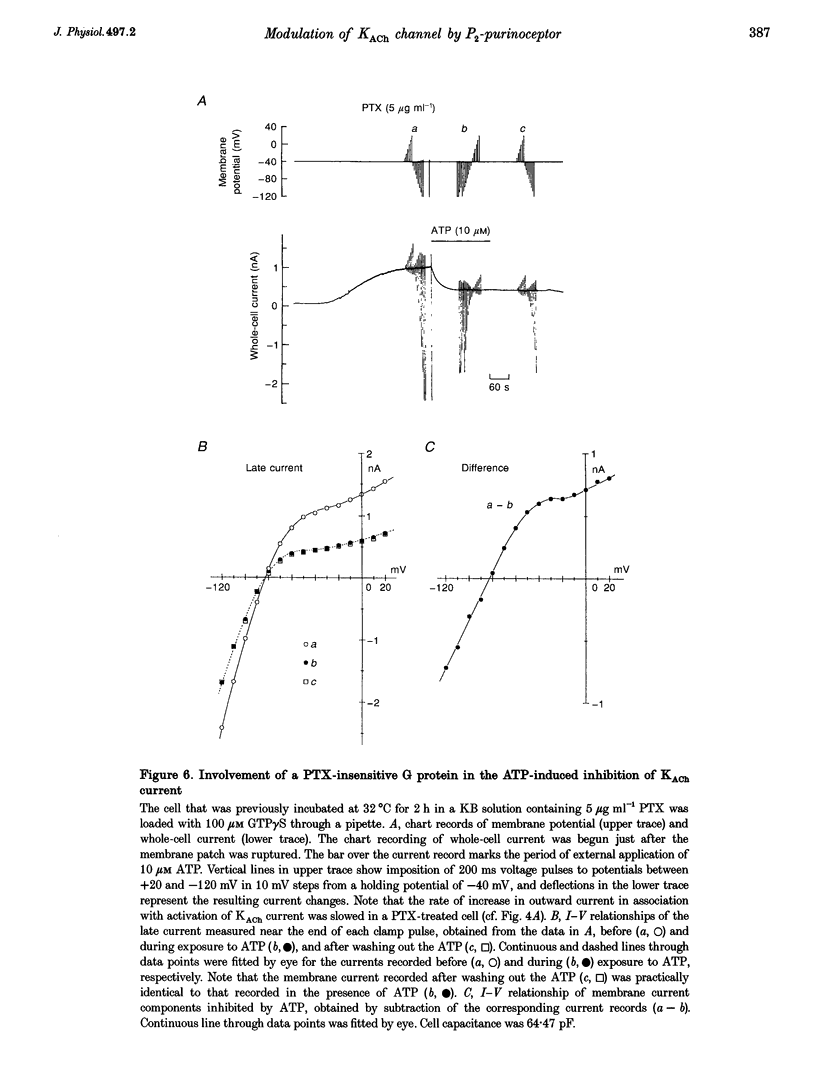
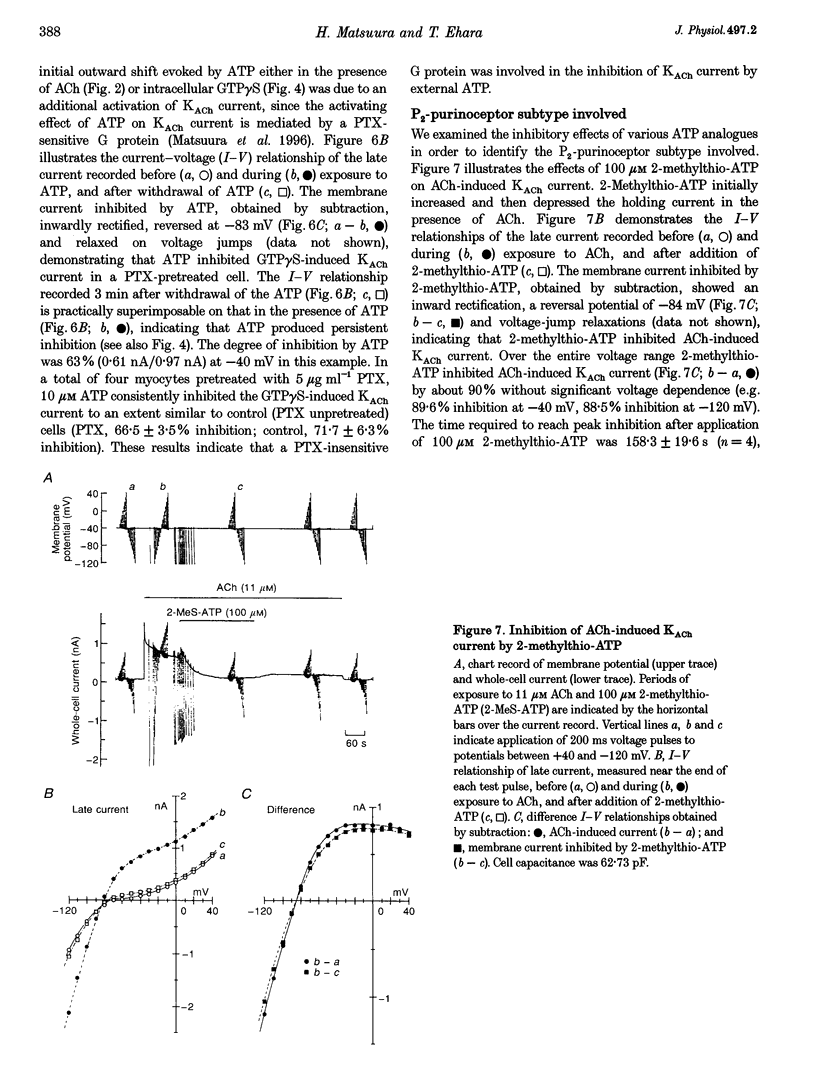
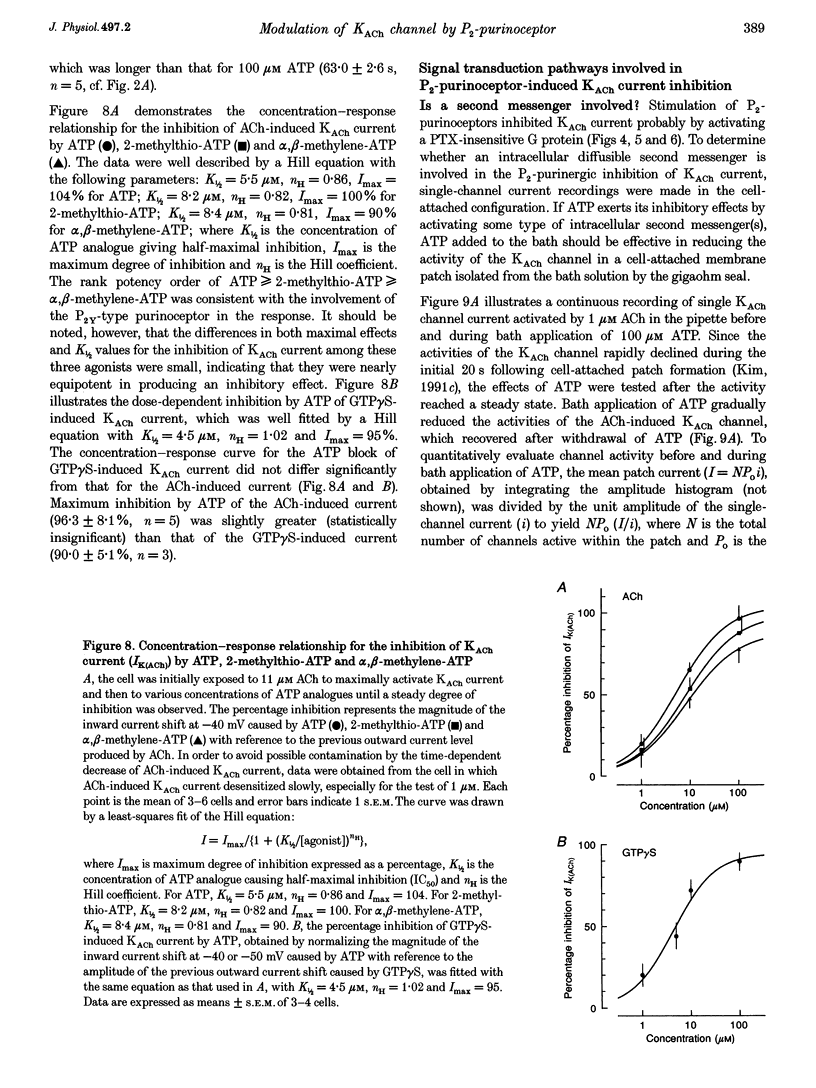
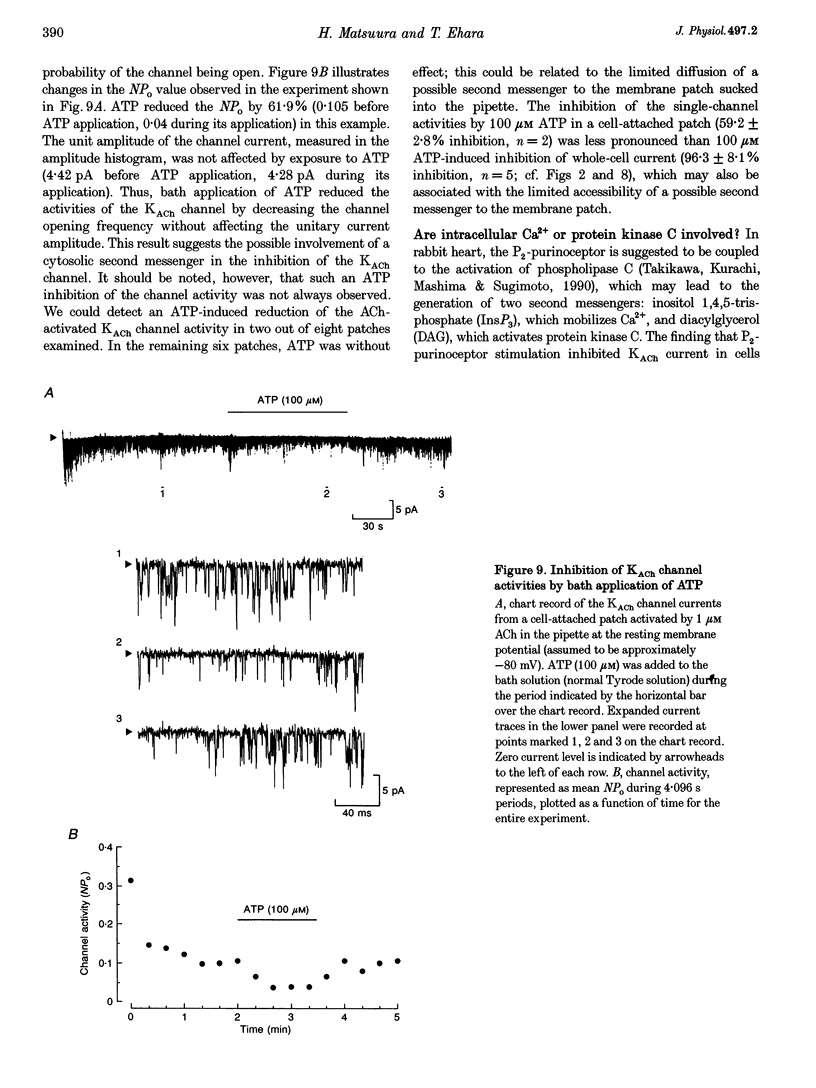
1. Activation of muscarinic K+ (KACh) channels by P2-purinergic agonists, such as ATP, decreases monotonically in the continued presence of agonist. We investigated the mechanisms underlying this process of decline in guinea-pig atrial myocytes using the patch-clamp technique. 2. External ATP reversibly depressed the acetylcholine (ACh, 5.5-11 microM)-induced KACh current in a concentration-dependent manner with a half-maximal inhibitory concentration (IC50) of 5.4 microM. 3. External ATP irreversibly reduced guanosine-5'-O-(3-thiotriphosphate) (GTP gamma S)-induced KACh current both in control and pertussis toxin (PTX)-pretreated cells, suggesting (i) that the ATP-induced inhibition of KACh current occurred at some step(s) downstream from the activation of the PTX-sensitive G protein, GK, and (ii) that a PTX-insensitive G protein was involved in the signal transduction pathway. 4. The potency order of ATP analogues in reducing KACh current was ATP > or = 2-methylthio-ATP > or = alpha, beta-methylene-ATP, indicating involvement of a P2Y-type purinoceptor. 5. In the cell-attached patch recording, ATP (100 microM) applied to the bath solution reduced the activity of the KACh channels activated by ACh in the pipette, in two out of eight experiments, suggesting the possible involvement of cytosolic second messengers in the inhibition of KACh channels. 6. The ATP-induced reduction of KACh current was not affected by a protein kinase C inhibitor, 1-(5-isoquinolinesulphonyl)-2-methylpiperazine dihydrochloride (H-7), suggesting that this response was not mediated by the activation of protein kinase C. 7. These results demonstrate that, in addition to the membrane-delimited activation through GK, external ATP causes an inhibition of the KACh channel probably by activating a PTX-insensitive G protein and cytosolic second messenger(s), which may underlie the monotonic decrease of the ATP-activated KACh current.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belardinelli L., Shryock J., West G. A., Clemo H. F., DiMarco J. P., Berne R. M. Effects of adenosine and adenine nucleotides on the atrioventricular node of isolated guinea pig hearts. Circulation. 1984 Dec;70(6):1083–1091. doi: 10.1161/01.cir.70.6.1083. [DOI] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purines and cotransmitters in adrenergic and cholinergic neurones. Prog Brain Res. 1986;68:193–203. doi: 10.1016/s0079-6123(08)60239-3. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Mubagwa K. Desensitization of the acetylcholine-induced increase of potassium conductance in rabbit cardiac Purkinje fibres. J Physiol. 1986 Feb;371:239–255. doi: 10.1113/jphysiol.1986.sp015971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. G., Forrester T. Appearance of adenosine triphosphate in the coronary sinus effluent from isolated working rat heart in response to hypoxia. J Physiol. 1981 Mar;312:143–158. doi: 10.1113/jphysiol.1981.sp013621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Abe S., Sawanobori T., Hiraoka M. External ATP-induced changes in [Ca2+]i and membrane currents in mammalian atrial myocytes. Am J Physiol. 1991 Apr;260(4 Pt 1):C673–C680. doi: 10.1152/ajpcell.1991.260.4.C673. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Horie M., Nairn A. C., Gadsby D. C. Role of GTP-binding proteins in the regulation of mammalian cardiac chloride conductance. J Gen Physiol. 1992 Apr;99(4):465–489. doi: 10.1085/jgp.99.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblad J., Heilbronn E. P2-purinoceptor-stimulated phosphoinositide turnover in chick myotubes. Calcium mobilization and the role of guanyl nucleotide-binding proteins. FEBS Lett. 1988 Aug 1;235(1-2):133–136. doi: 10.1016/0014-5793(88)81248-1. [DOI] [PubMed] [Google Scholar]
- Irving H. R., Exton J. H. Phosphatidylcholine breakdown in rat liver plasma membranes. Roles of guanine nucleotides and P2-purinergic agonists. J Biol Chem. 1987 Mar 15;262(8):3440–3443. [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Gehring U., Gaugler B., Pfeuffer T., Schultz G. Occurrence of an inhibitory guanine nucleotide-binding regulatory component of the adenylate cyclase system in cyc- variants of S49 lymphoma cells. Eur J Biochem. 1983 Feb 15;130(3):605–611. doi: 10.1111/j.1432-1033.1983.tb07192.x. [DOI] [PubMed] [Google Scholar]
- Kim D. Calcitonin-gene-related peptide activates the muscarinic-gated K+ current in atrial cells. Pflugers Arch. 1991 May;418(4):338–345. doi: 10.1007/BF00550871. [DOI] [PubMed] [Google Scholar]
- Kim D. Endothelin activation of an inwardly rectifying K+ current in atrial cells. Circ Res. 1991 Jul;69(1):250–255. doi: 10.1161/01.res.69.1.250. [DOI] [PubMed] [Google Scholar]
- Kim D. Modulation of acetylcholine-activated K+ channel function in rat atrial cells by phosphorylation. J Physiol. 1991 Jun;437:133–155. doi: 10.1113/jphysiol.1991.sp018588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi Y. G protein regulation of cardiac muscarinic potassium channel. Am J Physiol. 1995 Oct;269(4 Pt 1):C821–C830. doi: 10.1152/ajpcell.1995.269.4.C821. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Short-term desensitization of muscarinic K+ channel current in isolated atrial myocytes and possible role of GTP-binding proteins. Pflugers Arch. 1987 Oct;410(3):227–233. doi: 10.1007/BF00580270. [DOI] [PubMed] [Google Scholar]
- Kwatra M. M., Hosey M. M. Phosphorylation of the cardiac muscarinic receptor in intact chick heart and its regulation by a muscarinic agonist. J Biol Chem. 1986 Sep 25;261(27):12429–12432. [PubMed] [Google Scholar]
- Lewis D. L., Clapham D. E. Somatostatin activates an inwardly rectifying K+ channel in neonatal rat atrial cells. Pflugers Arch. 1989 Aug;414(4):492–494. doi: 10.1007/BF00585062. [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Ehara T. Activation of chloride current by purinergic stimulation in guinea pig heart cells. Circ Res. 1992 Apr;70(4):851–855. doi: 10.1161/01.res.70.4.851. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Sakaguchi M., Tsuruhara Y., Ehara T. Activation of the muscarinic K+ channel by P2-purinoceptors via pertussis toxin-sensitive G proteins in guinea-pig atrial cells. J Physiol. 1996 Feb 1;490(Pt 3):659–671. doi: 10.1113/jphysiol.1996.sp021175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieder W., Yang Q. F., Trautwein W. The time course of the muscarinic response to ionophoretic acetylcholine application to the S-A node of the rabbit heart. Pflugers Arch. 1981 Mar;389(3):283–291. doi: 10.1007/BF00584791. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Pirotton S., Erneux C., Boeynaems J. M. Dual role of GTP-binding proteins in the control of endothelial prostacyclin. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1113–1120. doi: 10.1016/s0006-291x(87)80185-7. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y., Campbell D. L., Strauss H. C. Modulation of L-type Ca2+ current by extracellular ATP in ferret isolated right ventricular myocytes. J Physiol. 1993 Nov;471:295–317. doi: 10.1113/jphysiol.1993.sp019902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Takano K., Stanfield P. R., Nakajima S., Nakajima Y. Protein kinase C-mediated inhibition of an inward rectifier potassium channel by substance P in nucleus basalis neurons. Neuron. 1995 May;14(5):999–1008. doi: 10.1016/0896-6273(95)90338-0. [DOI] [PubMed] [Google Scholar]
- Takikawa R., Kurachi Y., Mashima S., Sugimoto T. Adenosine-5'-triphosphate-induced sinus tachycardia mediated by prostaglandin synthesis via phospholipase C in the rabbit heart. Pflugers Arch. 1990 Sep;417(1):13–20. doi: 10.1007/BF00370763. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Webb T. E., Simon J., Krishek B. J., Bateson A. N., Smart T. G., King B. F., Burnstock G., Barnard E. A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993 Jun 14;324(2):219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- Yatani A., Brown A. M. Rapid beta-adrenergic modulation of cardiac calcium channel currents by a fast G protein pathway. Science. 1989 Jul 7;245(4913):71–74. doi: 10.1126/science.2544999. [DOI] [PubMed] [Google Scholar]
- Yatani A., Okabe K., Polakis P., Halenbeck R., McCormick F., Brown A. M. ras p21 and GAP inhibit coupling of muscarinic receptors to atrial K+ channels. Cell. 1990 Jun 1;61(5):769–776. doi: 10.1016/0092-8674(90)90187-j. [DOI] [PubMed] [Google Scholar]
- Zang W. J., Yu X. J., Honjo H., Kirby M. S., Boyett M. R. On the role of G protein activation and phosphorylation in desensitization to acetylcholine in guinea-pig atrial cells. J Physiol. 1993 May;464:649–679. doi: 10.1113/jphysiol.1993.sp019656. [DOI] [PMC free article] [PubMed] [Google Scholar]


