Abstract
BACKGROUND:
Assistive technology is often incorporated into rehabilitation and support for those impacted by upper limb impairments. When powered, these devices provide additional force to the joints of users with muscle weakness. Actuated devices allow dynamic movement compared to splints, therefore improving the ability to complete activities of daily living. However, these devices are not often prescribed and are underrepresented in research and clinical settings.
OBJECTIVE:
This review examined the existing literature on devices developed to support hand and wrist functionality in daily activities. Focusing on active, powered, and actuated devices, to gain a clearer understanding of the current limitations in their design and prescription.
METHODOLOGY:
The scoping review was conducted using the PRISMA-ScR guidelines. A systematic search was done on MEDLINE, EMBASE, Scopus, Web of Science, and NHS the Knowledge Network from inception to May 2023. Articles were included if the device was portable; supported the hands and wrist actively using an actuator; and could be used for assistive living during or post-rehabilitation period.
FINDINGS:
A total of 135 studies were included in the analysis of which 34 were clinical trials. The design and control methods of 121 devices were analyzed. Electrical stimulation and direct mechanical transmission were popular actuation methods. Electromyography (EMG) and joint movement detection were highly used control methods to translate user intentions to device actuation. A total of 226 validation methods were reported, of which 44% were clinically validated. Studies were often not conducted in operational environments with 69% at technology readiness levels ≤ 6, indicating that further development and testing is required.
CONCLUSION:
The existing literature on hand and wrist exoskeletons presents large variations in validation methods and technical requirements for user-specific characteristics. This suggests a need for well-defined testing protocols and refined reporting of device designs. This would improve the significance of clinical outcomes and new assistive technology.
Keywords: Upper Limbs, Exoskeletons, Assistive Devices, Wearable Devices, Design, Actuators, Outcome Measures, Systematic Review, Daily Activities, Wrist, Electromyography, Hand
INTRODUCTION
Upper limb impairment, resulting from a range of factors such as injury, neurological disorders, diseases, conditions, and general comorbidities, can have a profound and detrimental impact on an individual's overall quality of life.1 This impairment often leads to significant limitations in physical activity2,3 and can contribute to mental health challenges, due to the loss of independence and functionality.4 Symptoms such as muscle weakness, reduced muscle control, neurological issues, and prehension difficulties vary in severity and permanence.
Due to this variability, a one-size-fits-all approach is inadequate. Tailored rehabilitation programs and assistive interventions must be designed to accommodate the specific requirements of individuals, enabling them to perform activities of daily living (ADLs) more effectively and improving their overall well-being.
Spasticity, muscle weakness and prehension difficulties affect the upper limb differently. Spasticity is defined as velocity-dependent resistance,5 due to this muscle contracture, impaired control of voluntary hand-opening tasks and activities is seen.4 In contrast, muscle weakness affects hand-closing tasks such as grasping utensils and opening doors. The hands are the only prehensile organ in the human body.6 Prehension is required for feedback during tasks and coordination, therefore reduced prehension disrupts the balance between power and precision requirements of dexterous tasks.7 When a person receives no feedback during functional tasks, they may be unable to gauge if they have optimal hand orientation or enough strength to hold an item. Hand and wrist impairments of all types target a person’s ability to perform ADLs.
In addition to performing ADLs, biopsychosocial factors are also impacted by hand impairment.8 The biopsychosocial model is a concept which allows for the classification of factors which may contribute to any individual’s mental and physical health.9–11 The psychological impact of hand impairment can be presented as distress, depression, and low self-efficacy. Persons with hand impairments have also shown a reduction of measures determining quality of life.2 Sense of freedom, belonging and security are major social factors affected by having upper limb impairment.8 These people may also have reduced independence and may rely on family, caregivers, and allied health professionals for support. The biopsychosocial factors mentioned introduce a global burden on resources, cost, time and availability of support.12–14 Fortunately, assistive technology may reduce that burden while also attaining sustainable development goals for the future ageing population affected by these impairments.15,16
To facilitate upper limb functional tasks, interventions such as rehabilitation and assistive technology may be provided. The objective of assistive technology is to ensure safety, and accessibility, promote independence and improve quality of life. To achieve these objectives, devices must be tailored to the user’s requirements. For users who require augmented strength and functionality to perform tasks, a powered and actuated device would be appropriate. Examples of active devices include exoskeletons and exosuits.17 The introduction of actuators makes the device active, compared to passive devices that use elastics, levers and springs to support user motion such as dynamic orthosis. These devices function by applying force from an actuator on segments of the upper limb. Actuators are devices which convert energy to motion; this energy may be electric such as DC motors. Depending on the position and power of the force applied to the upper limb, the device can assist in various functional tasks.
The evolution of upper limb assistive devices has had rapid advancements in technology. It has grown in popularity within the commercial sector as workplace health and safety systems, and as stationary end-effector devices within physical rehabilitation settings.18 Despite the advantages of using these devices,16,19,20 the National Service Framework for Long-Term Conditions and Clinical Commissioning Groups (national to the United Kingdom) have minimal to absent policies for using these motorized devices.21 The rationale behind this regulatory stance is uncertain. However, global reports on assistive technology have postulated several factors for the general lack of prescription of assistive devices including limited-service provision, inadequate products, market shortcomings, governance and funding constraints, as well as sociodemographic barriers.16 These factors may apply to actuated devices, but these reports16,21 do not focus on actuated devices.
Furthermore, literature reviewing the upper limb exoskeletons rarely discusses the hands and wrist segments,18 and of those which have, there is a lack of breadth on clinical utility and outcome measures.17,20,22,23 Based on the gaps in global reports and review literature, a study summarizing actuated devices would be appropriate.
This scoping review aimed to explore the research question: What is known about active actuated and assistive devices for the hands and wrist? The secondary objectives include: 1) Defining the intended populations of these devices, 2) Abstracting an overview of the device design: including modes of actuation, user intention methods and force transmission methods, 3) Summarize and categorize validation strategies used in the study of these devices.
METHODOLOGY
A scoping review summarizing the breadth of existing literature concerning active, actuated (powered and motorized) and assistive (provides support during functional tasks) devices designed for the hands and wrists was conducted. The scoping review offers a methodological approach to survey the evidence, key concepts, and analyze knowledge gaps.24–27 This may illuminate potential rationales for the underrepresentation of hand and wrist assistive devices in literature. It may also ascertain if the barriers outlined in the global report on assistive technology16 apply to actuated devices.
A scoping review was chosen as it maps out the extent of existing research on a broad topic. For this study, it is active assistive devices for hand and wrist actuation. Scoping reviews have more inclusive eligibility criteria compared to systematic reviews. This encourages the use of larger sources of literature, more time-effective analysis, and provides evidence for future systematic reviews.
This scoping review follows the Preferred Reporting Items for Systematic Reviews and meta-analysis extension for Scoping Reviews (PRISMA-ScR).28 This extension is an update from the PRISMA guidelines which is a validated systematic approach for evidence syntheses.24 The search criteria for the database were structured according to Population, Concept, Context (PCC) framework.29 The population was defined as individuals experiencing hand and/or wrist impairment. The concepts focused on devices with active actuation and power. The context encompassed devices which assist ADLs during and post-rehabilitation. The definition of post-rehabilitation in this study refers to the phase of recovery and support that follows an initial rehabilitation program.
Database Search
Five databases were searched from inception to the date of search (May 25th, 2023). The databases selected were MEDLINE (Ovid), EMBASE (Ovid), Scopus, Web of Science, and NHS the Knowledge Network. No limitations or filters were applied to the results during the systematic database search. These databases were chosen based on their optimal combination, and collectively satisfy the minimum requirement of databases necessary to ensure adequate and efficient coverage of studies.30,31 Search terms were combined with Boolean logic ((Hand OR hands OR extremity) AND (Wrist OR wrists OR carpus) AND (Device OR devices OR assistive devices OR actuated devices OR powered devices OR exoskeleton OR glove OR dynamic) AND (Functional OR function OR assist OR assistive OR assistance OR aid OR aiding OR support)).
Database search results were imported to Endnote v20 in an RIS file format. Duplicates and retractions were removed using Endnote v20 software.
Selection Criteria
Two screening processes were used: The first examined titles and abstracts for all papers on Microsoft Excel 2018 Version 2409. The inclusion criteria were “Is this an active, actuated, and assistive device for the hands and wrist?”. Papers were marked “include”, “exclude”, “duplicate” and “maybe”. The process of tagging studies was conducted by 2 reviewers with 86.9% agreement, and any disagreements were resolved with consensus. All studies tagged as duplicates were checked to ensure a version was kept within the dataset. The second screening process examined the full paper against the inclusion criteria shown in Table 1. The studies were tagged with include or exclude using these criteria.
Table 1:
Exclusions Criteria for Second Screening.
| Decision Tag | Exclusion Criteria | Additional Notes |
|---|---|---|
| Reason 1: | Is the device mobile? | Devices grounded to static tables are excluded, but devices mounted to wheelchairs are included as it is mobile. |
| Reason 2: | Does the device actively support hand and/or wrist movement? | Devices which immobilize joints are excluded. Devices which support the wrist in a static position and do not support hand movement are also excluded. |
| Reason 3: | Is this a complete system? | A complete system must include hardware and software. |
| Reason 4: | Does the device support ADLs? | If the hand and wrist are put in a static position, it can be assumed ADLs are not being completed and therefore excluded. Devices which train the hand/wrist for ADLs are included. |
| Reason 5: | Is the study primary research and not a review? | Excludes all reviews; examples include systematic, scoping, narrative, and state-of-the-art reviews. |
| Miscellaneous: | Access to full paper in English | Excludes research posters, published abstracts, and conference abstracts. Excludes papers not provided with English translation. |
Data Extraction
A total of 24 data items were charted independently by researcher AG. The full list of data charting items collected, and their definitions can be found in Table 2. Records from the same research group were considered individually if the devices described were mechanically different from each other, whereas articles regarding different iterations of the same device were grouped with the latest prototype iteration considered. For records using the same device, the most representative across all papers was chosen.
Table 2:
List of all data items collected, and their definitions.
| Data Item | Definition |
|---|---|
| Title | Title of the article as found in the database. |
| Reference ID | Reference number linked to list of all referenced in the dataset. |
| Author | List of all authors. |
| Year | The year the article was published. |
| Country of Study | The country of study is either given based on the institution or location of the clinic of the affiliated author. |
| Study Type | The study type was defined by the publisher. Options cited include articles, research papers, case reports, letters, and pilot studies. |
| Method | Methodology of the study. |
| Sum of Participants | The sum of the participants in the study. |
| Male | The sum of male participants (when provided). |
| Female | The sum of female participants (when provided). |
| Age Range | Based on the participants, the youngest to oldest participants make the age range. |
| Target Population | The intended population/user group for the device. |
| Grouped Target Population | To reduce variations in a target population, the grouped target was separated into 22 subgroups with 13 unique groups that were often combined:
|
| Study Population | The condition of the participants in the study. |
| Device | Name of the device if provided. |
| Weight of Device (g) | Weight of the device on the upper limb unless specified otherwise. |
| DoF (Degree of Freedom) | DoF of the entire device refers to the number of independent ways the mechanical transmission can move joints in the hand/wrist. |
| Mechanical Transmission | Method of applying active force from an actuator to the joint of the user. |
| Grouped Mechanical Transmission | To reduce variations in mechanical transmissions, sub-classes were Grouped into 6:
|
| Hand/Wrist | Is the device aimed to support the hand, the wrist, or the hand and wrist together? |
| User Intent/Detection Methods | The user intent/detection methods are how the device is controlled. The user will actively trigger the device, this can be by using a joystick, by contracting muscles, and many more. |
| Outcome Measures | All outcome measures and outcome measurement tools that were used in the study. |
| Technology Readiness Level (TRL) | The TRL was assigned according to the TRL definition provided by the HORIZON 2020 - Work Program 2014–2015 defined in Table 4. The TRL is a scale used to measure how developed and ready a technology is for practical use. |
| Outcome Measure Field | The outcome measures were separated into clinical, technical, or clinical and technical measures. Clinical outcomes focus on the patient’s health and quality of life, technical outcomes focus on the functionality and performance of the device. Classification of outcome measures was aided by the WHO ICF Model.11 |
The data charting items provided a comprehensive summary of participants demographic features, interventions, validations, and technology readiness levels (TRLs) of the included studies. Participants demographics include country, the sum of participants, gender, age, and patient conditions (if applicable). The intervention comprises device name, weight, degree of freedom (DoF), mechanical transmission, user intent/detection methods, and limb segment the device supports. The synthesis of validation includes both clinical outcome measures and non-clinical. TRLs were also part of the data extraction and can be analyzed against all data items to investigate potential trends in technological advancements.
RESULTS
Overview
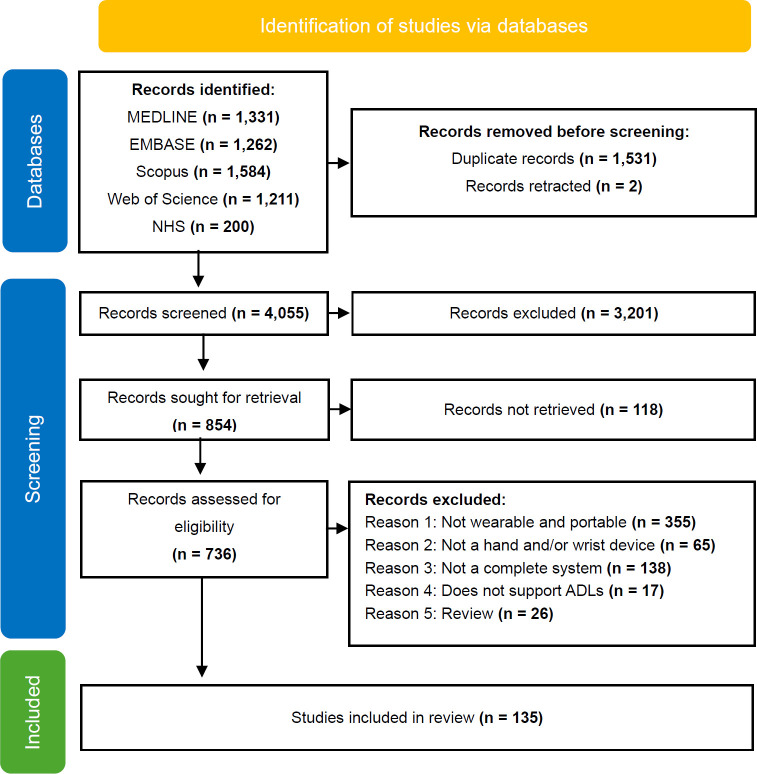
A total of 5,588 records were identified from the initial database search conducted in May 2023, of which 135 studies were included in the scoping review dataset.32 The selected studies were published between 1995 to 2023 (m = 2016, SD = 6.64), with 54% (73/135) studies published in the last 5 years. The selection process is provided in Figure 1. Two publications were identified and retracted using EndNote software.
Figure 1:
PRISMA Flowchart of database search, inspired by PRISMA2020.39
The most popular methodology used an experimental design (25%, 34/135), followed by feasibility studies (21%, 28/135). Clinical methodologies, such as RCTs (Randomized Control Trials, n = 12) and single group trials (n = 12), made up 34% (46/135) of the dataset.
Thirty-one countries contributed to the field of hand and wrist exoskeletons. Of which, the USA (20%, 27/135), China (16%, 21/135), Japan (12%, 16/135), Italy (8%, 11/135) and South Korea (6%, 8/135) produced the highest number of studies.
Following the World Development Indicators for income classification,33 3 studies were completed in Low-Middle Income Economies,34–36 31 in Upper-Middle Income and 101 in High Income. The correlation (r) between the number of studies published per country and the sum of participants was foreseeably high (r=0.867). An outlier to this trend is one study from Russia by Abramovich et al,37 which included 96 participants. This was also the second largest sum of participants in one study, with the largest sum of participants in a study conducted by Takebayashi et al with 115 participants.38
Participants
The sum of participants within the dataset totaled 1310. Of the 1310 participants (female: male 39%:61%), 46% (597/1310) had upper limb impairment due to stroke, 28% (371/1310) have been affected by Spinal Cord Injury (SCI) in the form of tetraplegia, hemiparesis, or hemiplegia, and 11% (140/1310) were considered healthy. The least reported conditions for support included persons with Cerebral palsy40 with 19 participants, upper limb tremors34,41 with 20 participants, Parkinson's disease36 with 10 participants, and support post-burns42 with 20 participants.
Of the 39 studies which recruited healthy participants solely, two devices43,44 were intended for human augmentation in healthy user groups. Age of participants ranged from 12–83 years old: two studies45,46 included a device for non-adults.
Intervention
In all, 121 devices were presented within the studies. A summary of the devices is presented in Table 3. Devices were categorized by their Weight (g), Degree of Freedom (DoF), Power Transmission, Mechanical Transmission, segment of support (Hand and or Wrist) and User intent. Of the target support joint, 37% (45/121) of devices supported hand actuation, 36% (44/121) supported both the hand and wrist and 26% (32/121) supported wrist actuation only.
Table 3:
Summary of devices analyzed.
| Device name | Reference | Weight of Device on Arm (g) | DoF | Power Transmission | Mechanical Transmission | Hand/Wrist | User Intent |
|---|---|---|---|---|---|---|---|
| 2-channel portable battery47operated FES system | 47 | – | 3 | Electrical stimulation | Muscle contraction | Hand and wrist | EMG signal |
| 3-CRP | 48 | 2700 | 3 | DC motors | Direct | Hand and wrist | Concurrent movement |
| 4-DOF wheelchair exoskeleton and Carbon hand | 49 | 4000 | 4 | Maxon DC motor | Cable and gear | Hand and wrist | Joint position and tactile |
| A5 hand function training system | 42 | – | 6 | Linear actuator | Bar linkage | Hand and wrist | Muscle torque |
| Anthropomimetic upper limb assistive device | 35 | – | 12 | DC motors | Pulley | Hand and wrist | Manual selection |
| Armeo Power II | 50 | 205000* | 7 | Motors | Gears | Wrist | Joint torque |
| Attention-controlled wrist rehabilitation method | 51 | 415 | 2 | Linear actuator | Push-pull cable | Wrist | EEG signal |
| BOTAS | 52 | – | 6 | Electrical stimulation | Direct | Hand and wrist | EMG signal and EEG signal |
| BRIDGE EMPATIA | 53 | – | 5 | Stepper motor | Bar linkage | Wrist | Manual selection (joystick) |
| DiaDENS-PKM | 54 | 350 | – | Electrical stimulation | Muscle contraction | Wrist | EMG signal |
| Distributed FES and Assessment System | 55 | – | 2 | Electrical stimulation | Muscle contraction | Hand and wrist | Concurrent EMG signal and finger angle |
| DTF Splint | 56 | – | 1 | Pneumatic actuator | Pneumatic | Hand | Manual selection |
| DTSaM Orthosis | 57 | – | 2 | Pneumatic actuator | Pneumatic | Wrist | Joint angle |
| DULEX-II | 58 | 504 | 3 | pneumatic and linear actuator | Pneumatic | Hand and wrist | concurrent EMG |
| Electrical stimulation | 59 | – | – | Electrical stimulation | Muscle contraction | Wrist | Manual selection |
| Electromechanical orthosis and MyoSystem BrI system | 60 | – | 2 | DC motors | Pulley | Hand and wrist | EMG |
| EMG-driven exoneuromusculoskeleton | 61 | 368 | – | Pneumatic actuator | Pneumatic | Hand | Muscle torque |
| EMG-driven NMES-robotic arm | 62 | – | – | DC servo motors | Direct | Wrist | EMG signal |
| EMG-Driven NMES-Robotic Hand | 63 | – | 4 | Linear actuator | Bar linkage | Hand | EMG |
| EMG-driven WH-ENMS | 64 | – | 5 | Pneumatic actuator | Pneumatic | Hand and wrist | EMG |
| Emotiv EPOC and Rehastim | 65 | – | – | Electrical stimulation | Muscle contraction | Hand and wrist | EEG signal |
| Empi FOCUS | 66 | – | – | Electrical stimulation | Muscle contraction | Hand and wrist | Manual selection |
| EMS 400 and Ultraflex | 40 | – | 2 | Eletrical stimulation | Muscle contraction | Wrist | Manual selection |
| Energy-efficient wrist exoskeleton | 67 | – | 1 | Pneumatic actuator | Pneumatic | Wrist | Joint angle |
| ETS-MARSE | 68 | 7072 | 7 | Brushless DC motors | Gears | Wrist | Muscle torque |
| eWrist | 69 | 556 | 1 | Brushless DC motors | Gears | Wrist | Joint angle and EMG signal |
| ExoFinger | 70 | – | 2 | DC servo motors | Bar linkage | Hand | EMG signal, Finger temperature and Joint angle |
| EXOTIC upper limb exoskeleton and ITCI and Carbon hand | 71 | 6000 | 4 | Maxon DC motor | Cable and gear | Hand and wrist | Manual tongue |
| Exo-Wrist | 72 | 1003 | 2 | Rotary encoder | Pulley | Wrist | Muscle torque |
| EXTEND exoskeleton | 73 | 105 | 3 | Linear actuator | Bowden cable | Hand | Manual selection |
| Fesia grasp Device | 74 | 91 | 8 | Electrical stimulation | Muscle contraction | Hand and wrist | EMG signal |
| FESMATE CE1230 | 75 | – | – | Electrical stimulation | Muscle contraction | Hand and wrist | EMG |
| FESMED 4050 device | 76 | 200 | – | Electrical stimulation | Muscle contraction | Hand and wrist | Manual selection |
| Five-digit 3D printed battery-powered and force augmenting orthotic exoskeleton | 77 | – | – | Linear actuator | Cable | Hand | Muscle torque |
| Five-fingered exoskeleton hand | 78,79 | 2000 | 3 | DC motors | Bar linkage | Hand and wrist | EMG and wrist joint angle |
| Flexohand | 80 | 280 | 6 | DC servo motors | Bowden cable | Hand | Manual selection |
| Foot-controlled hand/forearm exoskeleton | 81 | – | 4 | DC servo motors | Pulley | Hand and wrist | Manual Foot selection |
| GBBAs | 82 | 95 | 3 | Pneumatic actuator | Pneumatic | Hand | Joint angle and muscle torque |
| Gloreha lite glove | 83 | 80 | 5 | Pneumatic actuator | Pneumatic | Hand | Manualselection |
| Glove-based assistive device | 84 | – | 2 | Pneumatic actuator | Pneumatic | Wrist | Wrist movement |
| GraspyGlove | 85 | 340 | 4 | Maxon DC motor | Push-pull cable | Hand | Sensor proximity |
| Hand assistive device | 86 | – | 1 | Linear actuator | Bowden cable | Hand | Muscle torque (index) |
| Hand exoskeleton | 87 | 114 | 3 | DC motors | Bowden cable | Hand | Joint angle and muscle torque |
| Hand exoskeleton system HES | 88 | 350 | 2 | DC servo motors | Bar linkage | Hand | Manual hand |
| Hand function rehabilitation robot | 89 | 450 | 2 | Linear actuator | Bar linkage | Hand | Manual hand (touch screen) |
| Hand/Wrist exoskeleton | 90 | 1815 | 7 | DC Torque motor | Bar linkage | Hand | EMG and joint motion |
| HANDS therapy | 91,92 | – | – | Electrical stimulation | Muscle contraction | Hand and wrist | EMG signal |
| Hybrid system | 93 | 402 | 5 | Linear actuator | Bar linkage | Hand | EMG signal and EEG signal |
| Hybrid-driven compliant hand exoskeleton | 94 | 147 | – | DC Torque motor | Cable | Hand | Finger torque |
| Implanted sensor-controlled microstimulator system | 95 | – | – | Electrical stimulation | Muscle contraction | Hand and wrist | EMG signal |
| INTFES | 96 | 170 | – | Electrical stimulation | Muscle contraction | Hand and wrist | EMG signal |
| intracortical MEA-BCI-FES | 97 | – | – | Electrical stimulation | Muscle contraction | Hand and wrist | EEG signal (Implant) |
| IOTA | 98 | 230 | 2 | DC servo motors | Cable | Hand | Manual hand |
| Layer jamming-based soft Tremor Suppression Glove | 34 | 30 | 6 | DC servo motors | Hydraulic | Hand | Tremor |
| MAH system | 99 | 580 | 6 | DC servo motors | Supernumerary | Hand | Wrist angle |
| MAHI Exo-II | 100,101 | 340 | 4 | DC motors | Bar linkage | Wrist | Manual selection |
| MeCFES | 102 | – | 2 | Electrical stimulation | Muscle contraction | Hand | EMG wrist |
| MeFES | 103 | – | – | Electrical stimulation | Muscle contraction | Hand and wrist | EMG signal |
| Mirror hand HS 001 | 104 | 800 | 5 | Motors | Bar linkage | Hand | Mirrored motion |
| Mirror-image motion device with an exoskeleton | 105 | 1800 | 3 | Brushless DC motors | Cable | Wrist | Mirroredmotion |
| Motor orthotic device | 106 | – | 1 | Ultrasonic motor | Gears | Wrist | EMG signal |
| MWDO | 107 | 330 | 2 | DC motors | Bar linkage | Hand and wrist | Wrist torque |
| Myoelectric control | 108 | – | 2 | Electrical stimulation | Muscle contraction | Wrist | EMG |
| MyoPro | 109–112 | 1814 | 2 | Motors | Direct | Hand and wrist | EMG signal |
| NESM and 5-DOF wrist-hand exoskeleton | 113 | – | 9 | DC motors | Bar linkage | Hand and wrist | Joint position |
| NESS handmaster system | 114–116 | – | – | Electrical stimulation | Muscle contraction | Hand | Manualselection |
| Neuro-orthosis | 117,118 | – | 2 | Electrical stimulation | Muscle contraction | Wrist | Joint angle |
| NMES-robot arm | 119 | 895 | 2 | DC Torque motor | Muscle contraction and direct | Wrist | EMG signal and NMES signal |
| Odstock 2-channel Programmable Stimulator | 120 | 200 | – | Electrical stimulation | Muscle contraction | Hand and wrist | EMG signal |
| Paediatric hand exoskeleton PEXO | 45 | 107 | 1 | Linear actuator | Cable | Hand and wrist | Manual hand OR hands-free voice control based on keyword detection |
| Pinch assistant | 121 | 580 | 5 | DC servo motors | Pulley | Hand | Index andthumb torque |
| Pinotti portable robotic exoskeleton PPRE | 122 | 1600 | 2 | DC motors | Gears | Hand and wrist | Manual hand |
| PneuGlove | 123 | – | 2 | Pneumatic actuator | Pneumatic | Hand | Joint angle |
| Pneumatic-controlled finger extension system | 43 | 2000* | 1 | Pneumatic actuator | Pneumatic | Hand | EEG signal |
| Power augmentation soft glove | 124 | 120 | 4 | Pneumatic actuator | McKibben | Joint torque (index) | |
| Power-assisted FES | 125 | – | 3 | Electrical stimulation | Muscle contraction | Hand and wrist | EMG signal |
| REHA 2030 | 126 | – | 1 | DC motors | Bar linkage | Wrist | Wrist angle and velocity |
| ReIn-hand system (Empi 300 and EMG collection unit) | 127,128 | 227 | – | Electrical stimulation | Muscle contraction | Hand and wrist | EMG signal |
| RELab tenoexo | 129,130 | 148 | 3 | Maxon DC motor | Bowden cable | Hand | Finger torqueand bend |
| ReoGo-J | 38 | 79000* | 3 | Motors | Direct | Wrist | Manual selection |
| Rope-driven flexible robot | 131 | – | – | Linear actuator | Pulley | Hand | Manual selection (touch screen) |
| RUPERT IV | 132,133 | – | 5 | Pneumatic actuator | Pneumatic | Wrist | Joint positionand tactile |
| SaeboFlex and BMR Neurotech electrical stimulator unit | 134 | 1587 | 5 | Electrical stimulation | Muscle contraction | Hand and wrist | Muscle torque |
| SaeboMAS and accelerometer-triggered FES | 135 | – | – | Electrical stimulation | Muscle contraction | Hand and wrist | Joint position |
| SCRIPT Active orthosis SAO-i3 | 136 | – | 3 | DC motors | Bar linkage | Hand and wrist | Joint angle |
| SCRIPT1 Project | 137 | – | – | Elastic torque | Pulley | Hand and wrist | Wrist motion and muscle torque |
| SEM Glove | 138 | 700 | 3 | Brushless DC motors | Bowden cable | Hand | Fingertip tactile |
| Semi-soft assistive glove SAG | 139 | – | 2 | DC motors | Cable | Hand | Wrist motion and EMG |
| SETS system | 41 | 255 | 3 | Flexible semiactive actuator | Direct | Wrist | Tremor |
| SMA muscle | 140 | 300 | 2 | SMA | Hydraulic | Wrist | Manual selection |
| SNU Exo-glove | 141 | – | 3 | Brushless DC motors | Cable | Hand | Joint velocityand joint tensile |
| Soft glove | 142 | 237 | 6 | Pneumatic actuator | Pneumatic | Hand andwrist | Manual selection |
| Soft modular elbow-wrist rehabilitation exoskeleton driven by PAMs | 143 | – | 2 | Pneumatic actuator | Pneumatic | Wrist | Joint position |
| Soft robotic rehabilitation glove | 144 | – | – | Pneumatic actuator | Pneumatic | Hand | Manualselection |
| Soft sixth finger | 145,146 | 140 | 1 | DC servo motors | Supernumerary | Hand | EMG |
| SoftHand X system | 147 | 500 | – | Maxon DC motor | Supernumerary | Wrist | Joint angle (finger) |
| SR Fingers | 148 | 750 | 6 | DC servo motors | Supernumerary | Hand | Hand position |
| SSVEP-BCI controlled soft robotic glove rehabilitation system | 149 | – | 2 | Pneumatic actuator | Pneumatic | Hand | EEG signal |
| Super stim ZZAEV906 | 46 | – | 3 | Electrical stimulation | Muscle contraction | Hand and wrist | EMG signal |
| Supernumerary robotic finger SRF | 44 | 650 | 6 | DC servo motors | Supernumerary | Hand | Joint angle |
| TCAMs-Exo | 150 | 135 | 2 | DC motors | Artificial muscle | Wrist | EMG and wrist joint angle |
| tDCS | 151 | – | – | Electrical stimulation | Muscle contraction | Hand and wrist | EMG signal |
| TDS-HM the hand mentor and tongue drive system | 152 | – | 2 | Pneumatic actuator | Pneumatic | Hand andwrist | Tongue position |
| TENS Stimulator N604 | 153 | – | – | Electrical stimulation | Muscle contraction | Hand and wrist | EMG |
| T-GRIP exoskeleton | 154 | 50 | 1 | Linear actuator | Bar linkage | Hand | Joint angle (wrist) |
| The Bionic glove | 155 | – | – | Electrical stimulation | Muscle contraction | Hand | Wrist position |
| The Hand exoskeleton | 156 | 1800 | 15 | Linear actuator | Push-pull cable | Hand | Mirrored motion |
| TIGER | 157,158 | 420 | 2 | Brushless DC motors | Bar linkage | Hand andwrist | Manual hand (touch screen) |
| Upper limb rehabilitation robot | 159 | – | 6 | DC Motors | Gears | Wrist | Manual selection |
| Utah microelectrode array and NMES | 160 | – | 6 | Electrical stimulation | Muscle contraction | Hand and wrist | EEG signal |
| WDFHO | 161 | – | 1 | Linear actuator | Gears | Hand | Joint angle (wrist) |
| Wearable glove with incorporated compliant mechanical transmission | 162 | – | 2 | Pneumatic actuator | Pneumatic | Hand | Manual selection (touch screen) |
| Wearable mechanism to suppress axial vibration | 36 | 268 | 3 | DC motors | Direct | Wrist | Tremor |
| WearME Glove | 163 | 500 | 3 | Brushless DC motors | Pulley | Hand and wrist | Joint angle |
| W-EXOS | 164 | 1900 | 3 | DC motors | Gears | Wrist | Muscle torque and EMG signal |
| WHOs | 165 | – | 1 | Motors | Bar linkage | Hand | Joint angle (wrist) |
| Wireless distributed FES system | 166 | 45 | – | Electrical stimulation | Muscle contraction | Hand | EMG and joint movement |
| Wireless wearable device | 167 | – | 2 | Electrical stimulation | Muscle contraction | Hand and wrist | Joint position and movement (wrist) |
| Wrist exoskeleton | 168 | 288 | 2 | Linear actuator | Push-pull cable | Wrist | Manual selection |
| Wrist exoskeleton | 169 | 728 | 1 | DC motors | Gears | Wrist | Mirrored motion |
| X-Glove | 170 | – | 5 | Linear actuator | Cable | Hand | Manual selection |
Recordings on the weight of the device were poor in the literature with only 52% (63/121) mentioning weight. Weight spanned from 30g (Layer jamming-based soft Tremor Suppression Glove34) to 205kg (Armeo Power II50). From the limited reported data, there were indications that the weight of the device on the upper limb was reduced each year on average. The degrees of freedom (DoF) were reported in 62% (84/135) of studies and tended to be low, with many devices actuating one (11%, 13/121) or two DoF (24%, 29/121). Assistive devices which actuated 1 DoF had the lowest weight on average at 285g, followed by 6 DoF at 422g. Devices with higher levels of DoF tended to be designed for the hands: Average hand device DoF was 3.6, whereas wrist devices were 2.8 DoF.
The categories of mechanical transmission described in Table 2, were inspired by Bos et al structured overview of dynamic hand orthoses.17 However, this study included muscle contraction and supernumerary devices. This improves the inclusivity of unconventional actuation methods; muscle contraction due to electrical stimulation acts as an internally applied active force, and supernumerary devices use indirect mechanical force to attain ADLs. Muscle contraction (26%, 31/121), bar linkage (15%, 18/121) and pneumatic devices (15%, 18/121) were among the most popular mechanical transmission methods across all applications.
To apply the active force, a command signal must be sent to a control unit. This command signal was charted as the “user intent” defined in Table 2, results are shown in Table 3. The user’s intention to control the device was detected predominantly with Electromyography (EMG) (30%, 36/121) and users’ joint movement (30%, 37/121). The placement of electrodes for EMG varied widely and most EMG intention methods were combined with muscle contraction to actuate the upper limb (61%, 22/36), this is the foundation of Functional Electrical Stimulation (FES).171 Other user intention methods include detecting a force applied by the joint typically the fingertips, by manually selecting how and when the actuator moves using a touchscreen or joystick, and EEG systems such as the Emotiv.65
Outcome measures
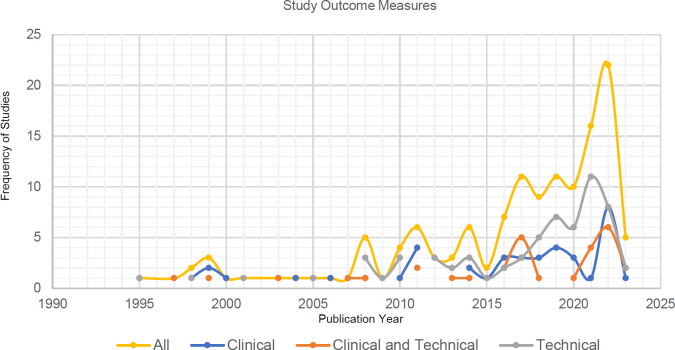
A total of 226 unique outcome measures were extracted from the 765 tests completed in the data set. From the 226 outcome measures extracted, 100 were considered clinical tools using the WHO-ICF Model of functional outcomes alongside additional validated sources.10,11,172,173 Therefore, 126 outcome measures were considered technical or non-clinical. A dip in the number of clinical-based outcome measures used was found in 2020. While testing of devices on patients had decreased, the past 10 years have seen an exponential increase in research publications on upper limb devices seen in Figure 2.
Figure 2:
Distribution of studies based on the field of outcome measures.
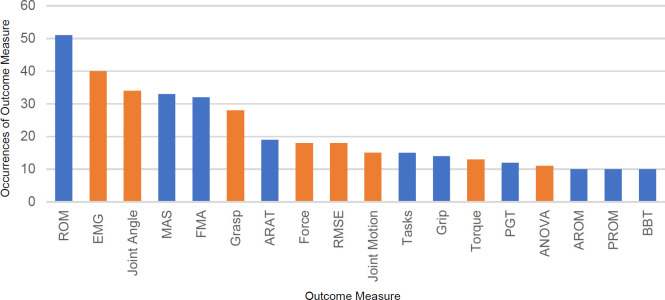
The frequency of outcome measures repeated between studies tended to be low (8%, 18/226). The majority of outcome measures appeared in less than 10 studies (92%, 208/226); Figure 3 presents the outcome measures most regularly used (outcome measures used in ≥10 studies).
Figure 3:
Count of outcome measures. Blue indicates clinical outcomes; orange indicates technical outcomes. The outcome measures in order: ROM (Range of Motion), EMG, Joint angle, MAS (Motor Assessment Scale), FMA (Fugl-Meyer Assessment), Grasp, ARAT (Action Research Arm Test), Force, RMSE (Root Mean Square Error), Joint motion, Tasks, Grip, Torque, PGT (Pinch Grip Test), ANOVA (Analysis of variance), AROM (Active ROM), PROM (Passive ROM), BBT (Box and Blocks Test).
The clinical outcome measures trended towards observational ordinal scales inspecting mobility (MAS, FMA, and BBT) and movement functions (ROM, ARAT and functional tasks). The technical and non-clinical outcome measures were either statistical analysis methods (RMSE, ANOVA and kinematic analysis) or usability tests (EMG, joint angle, and grasp force of device). Patient-reported outcome measures such as the Motor Activity Log (MAL), ABILHAND, Disabilities of the Arm, Shoulder, and Hand Scale (DASH), and quickDASH were often under-utilized with 4% use out of all tests extracted (30/765).
Factors such as introducing variations and modifications in a validation method caused 65% (148/226) of the outcome measurement tools to only be present in the dataset once. Another factor increasing the number of unique outcome measures extracted is the use of condition-specific outcome measures such as the Stroke Impact Scale.38,110,134,152 These tailored methods are useful tools to benchmark a person’s functionality within a set population173–176 but make validation across different cohorts difficult as it may not be an appropriate outcome measure for all.
Technology Readiness Levels
TRL 1 (proof of concept studies) and TRL 2 (software prototype studies) were not present due to our inclusion criteria provided in Table 1. The distribution of all TRL extracted can be found in Table 4, which also includes the definitions used in the data extraction.
Table 4:
TRL Definitions defined by the HORIZON 2020 - Work Programme 2014-2015 and the scoping review abstraction of the HORIZON 2020 definitions.
| TRL | Definition | Scoping Review E[]planation | Count |
|---|---|---|---|
| 1 | Basic principle observed | The idea has been formulated, proof of concept only | 0 |
| 2 | Technology concept formulated | A software prototype has been made and tested virtually | 0 |
| 3 | Experimental proof of concept | Analytical studies and feasibility studies. The device must be built | 17 |
| 4 | Technology validated in the lab | The device has been tested on non-human or one healthy case study for validation. | 26 |
| 5 | Technology validated in the relevant environment validation. | The device has been tested on healthy participants | 21 |
| 6 | Technology demonstrated in the relevant environment | The device has been tested on a target population in a clinical setting (ISO Standard not complete) | 29 |
| 7 | System prototype demonstration in an operational environment | The device has been tested for its intended purpose in an operational environment (outside of the clinic and lab) ISO Standards should be complete | 14 |
| 8 | System complete and qualified | The device is ready to be commercialized and has been validated | 1 |
| 9 | Actual system is proven in an operational environment | The device is available in the market | 27 |
Overall, TRL 6 (21%, 28/135), TRL 9 (20%, 27/135) and TRL 4 (19%, 26/135) were the most prominent advancement levels. FES (63%, 17/27), EMG (44%, 12/27) and devices made to support people with cardiovascular diseases (74%, 20/27) made up most of the technological advancements of TRL 9. Non-electrical stimulation devices at TRL 9 included the MyoPro,109–112 which uses an EMG threshold for control and has been commercialized since 2006, the SEM Glove,138 ReoGo-J,38 and Armeo Power II.50 Of these, SEM Glove, ReoGo-J, and Armeo Power II were the only TRL 9 devices that did not include FES or EMG.
Trends in TRL and demographics were also noticed; as the number of participants increases, the TRL level improves: case studies (1 participant) were an exemption to this trend. High-income countries also conducted studies at higher TRL and there has been a steady development in TRL in device testing over the years.
Devices in category TRL 3 were proof of concept (Table 4), therefore these studies use analytical or feasibility methodologies. These methods focus on the validation of the device and include only healthy participants. Of these TRL 3 devices, 47% (8/17) used cable conduit mechanisms, and 29% (5/17) used pneumatic actuation. These devices tended to be designed for supporting the hands (47%, 8/17) and had on average between 2-3 DoF. Various user detection methods were charted, but manual control of the device was quite frequent in both TRL 3 and 9.
DISCUSSION
This study provides an overview of 135 research papers focused on actuated assistive devices for the hand and wrist. A notable result was the scarcity of rigorous clinical methodologies, with 34% (46/135) of studies involving clinical trials, of which 12 studies conducted RCTs. From these studies, 121 unique devices were analyzed to scope their intended user populations, design features, validation strategies, and TRLs. Most of the devices were designed for individuals with upper limb impairment due to stroke 46% (597/1310), and a significant proportion of devices had low DoF, particularly for wrist devices at an average of 2.8 DoF. Regarding the design, the devices predominantly utilized EMG (30%, 36/121) which tended to be in combination with muscle contraction via electrical stimulation (FES). Along with EMG, user interfaces such as buttons, joysticks, and touch screens were used to detect user intentions. The study categorized a total of 226 unique clinical and technical outcome measures. The validation methods predominantly relied on statistical analyses for technical outcomes, while clinical assessments were often observational. There was a lack of consistency across studies, with many outcome measures used only once (65%, 148/226). Objective or patient-reported outcomes were less frequently employed.
Most of the studies were conducted in high to upper-middle-income economies (90%, 28/31). Although the need for assistive technology in low-income countries is high, there may be a lack of awareness and access to actuated devices, contributing to fewer studies conducted in these economies.15,16 Low-income economies must often import medical equipment,177 therefore these actuated devices must achieve high TRL to be considered for ordering and prescription. Yet, these devices have not met TRL >6 requirements (76%, 92/121). To fulfil TRL >6, the device must meet the ISO standards, and regulatory requirements (such as CE marking) before distribution in the market or testing in operational environments (Table 4). These conditions provide insurance for device quality, safety and efficiency.178 A few factors which may contribute to these devices not surpassing TRL 6 include overcoming the dynamic and rapidly developing policies to meet regulatory requirements for testing,179 a lack of streamlined clinical tests and validation processes for these devices,180,181 and the effects of COVID-19 on reduced face-to-face research.182–184
To validate these devices, 226 outcome measurement tools were charted. Classification of validation methods showed that 44% (99/226) of the outcome measurement tools were considered clinical; ROM, MAS and FMA were the most used for clinical trials whereas EMG, joint angles and device grasp force were conducted in technical studies (Figure 2). Since many of the devices were designed for stroke rehabilitation (46%, 597/1310), the outcome measures recorded show a strong correlation with existing literature on upper limb outcome measures in stroke recovery.173 Patient-reported outcome tests were implemented 4% of the time (30/765). This value is considerably low as these outcomes are invaluable to validate the use of the assistive device.8,173,185 Patient-reported outcomes also provide valuable psychometric properties to the evidence base185 and are an important part of upper limb assessment. It should be noted that comorbidities were not often reported, and outcome measures were not standardized, therefore inter-comparability of devices and populations was limited.
The lack of inter-comparability was also noticed in the inconsistency in reporting device specifications. DoF and weight of the device were not reported routinely (62% and 52% respectively), with some studies quoting their device as “lightweight” without reference to their objective weight. A slight trend toward reducing the weight of upper limb devices over the years was observed, but there is insufficient statistical evidence to support this claim. Many devices were designed with low (1 or 2) DoF and varied greatly in weight from 33 g to 205 kg. The variation in weight was due to differences in reporting weight, some studies report weight on the upper limb, while others report weight of the full system. The implication of these differing reporting styles makes synthesizing findings difficult for decision-making and provides barriers to further research as the evidence base lacks standardized measures and methods. To improve inter-comparability, frameworks for development can be implemented,186,187 alongside robust and systematic testing using a large cohort.187,188
In line with the works of Zhu et al, the field of soft wearable robotics has experienced rapid growth189 as demonstrated by the increasing number of fluidic transmission actuators identified in the study. These fluidic actuators, which include pneumatic and hydraulic, are typically lighter (averaging 234 g on the arm) and provide multidirectional force due to their flexible design.190 Previous studies have predicted the rise of soft robotics,187 which may continue to improve for use as an actuated assistive device. In addition to fluidic transmission actuators, supernumerary devices (n = 5) have shown potential for human augmentation.191 However, due to their state-of-the-art nature, the availability of real-world applications and longitudinal evidence supporting their effectiveness is limited.191,192 As these novel actuators continue to advance, future assistive devices should integrate them to improve weight and multifunctionality.
To detect a user’s intention, EMG (30%, 36/121) and joint movement (30%, 37/121) sensors were regularly implemented. EMG control methods, which include surface electrodes, implanted wires, and probes,193 have a long history of use. However, they are not suitable for all individuals with hand and wrist impairment194 and may encounter system failures outside of testing settings.187 EMG and joint movement sensors are limited by muscle activation threshold requirements, making them inadequate for addressing the full spectrum of people with upper limb impairment. The prescription of these devices would not be appropriate. Consequently, alternative user intention systems were explored including tongue-based interfaces,71,152 hands-free voice control,44 and foot-based interfaces.81 These systems are not limited by upper limb muscle threshold, yet they did not attain TRL >6. Alongside the requirements for attaining TRL >6, design factors may contribute to why these devices are not suitable for operating in a real-world context. Wearable sensing and control technology includes various elements which were not abstracted such as cost, consumption and battery lifespan, these may all affect useability.187,195 A systematic analysis of control systems which do not require upper limb muscle activation may be appropriate to validate the use of these underrepresented systems.
Limitations
The results of a scoping review are often quite broad; a synthesis of the conclusions will require additional resources to be used in policymaking. In addition, scoping reviews rarely include critical appraisal of included studies; therefore, the reliability of findings may be skewed. Despite this, a scoping review addresses the exploratory nature of upper limb devices compared to other methodologies.
In addition, as with many studies, the design of this study is subject to limitations. These concerned the selection of studies, definitions of terms during screening and the exclusion of data charting items. Due to time constraints, this study did not screen all forms of grey literature such as market reports, patents or working papers, and the keyword selection may have excluded appropriate studies. In addition, 118 studies were not retrieved (Figure 1) due to restricted access to certain relevant research papers. This limitation arose primarily due to paywalls and institutional access restrictions. This introduced selection bias and may have hindered the scope and number of devices investigated with higher technological readiness levels. During the screening process, the reviewers ultimately agreed on a consensus with 86.9% accuracy, but the definition of portable was defined as easily moveable by healthy users. This meant results on the weight of the device had large variability. This limitation was somewhat mitigated by recording the device's weight on the arm, although some studies only reported the total weight of the device. This study did not chart how the device interacts with the user’s joint-segment, such as enabling voluntary hand-opening or supporting wrist flexion. This data charting item would have provided more context for the device's functions.
CONCLUSION
Active, actuated assistive devices offer promising solutions to improve functionality and quality of life for individuals with hand impairments. This study reviews 135 studies covering 121 devices, providing insights into actuated devices for hand and wrist support in ADLs. Innovation in actuation systems and control methods is evident, yet many devices have not advanced beyond TRL 7, highlighting the gap between research and market-ready products. EMG and FES systems dominate the field but may not be suitable for users with limited muscle activation, showing the need for alternative approaches such as tongue interfaces and voice control systems.
Key barriers to prescription included insufficient real-world evidence, concentration of development in high- and middle-income countries, lack of standardized reporting, and the absence of accepted clinical validation processes. To overcome these challenges, it is essential to establish standards for device design, testing, and reporting (e.g., weight, degrees of freedom), develop comprehensive outcome measures combining objective methods with patient-reported experiences, and improve the accessibility of devices in low-income countries.
The field of hand and wrist exoskeletons shows increased popularity in the innovation of control systems and actuators. Addressing these challenges and implementing standardized frameworks will help improve the prescription of these devices. As technology advances, tailored solutions for individuals with varying levels of hand functionality are becoming increasingly feasible, offering significant benefits to those with upper limb impairments. Overall, there is promise and growth in the field of hand and wrist exoskeletons.
DECLARATION OF CONFLICTING INTERESTS
The authors declare no conflict of interest.
AUTHORS’ CONTRIBUTION
Angel Galbert: Study conception and design, data collection, analysis and interpretation of results, draft manuscript preparation, and manuscript revision.
Arjan Buis: Supervision, study conception and design, and manuscript revision.
Both authors have read and approved the final version of the manuscript.
SOURCES OF SUPPORT
ESPRC doctoral training grant (EP/S02249X/).
ACKNOWLEDGEMENTS
We would like to acknowledge the funding and support from the University of Strathclyde and the UK Engineering and Physical Sciences Research Council (EP/S02249X).
Glossary
List of Abbreviations
- ADL
Activities of Daily Living
- AHA
Assisting hand assessment
- AHP
Allied Health Professional
- AMEA
Absolute Mean Error Analysis
- ANCOVA
Analysis of Covariance
- ANOVA
Analysis of Variance
- AOU
Amount of Use
- ARAT
Action Research Arm Test
- ARI
Active Resistance Index
- AROM
Active Range of Motion
- ASIA
American Spinal Injury Association (ASIA) Impairmen Scale
- BBT
Box and Block Test
- BI
Barthel Index
- BMRC
British Medical Research Council Scale
- CGI
Clinical Global Impression
- Chedocke
Chedocke McMaster Hand Portion
- CMC
Coefficient of Multiple Correlation
- CMSA
Chedocke-McMaster Stroke Assessment
- COPM
Canadian Occupational Performance Measure
- CTS
Carpal Tunnel Syndrome
- CUE-T
Capabilities of upper extremity test
- CVA
Cerebral Vascular Accident
- DMD
Duchenne Muscular Dystrophy
- DOF
Degree of Freedom
- Donn/Doff
Putting on and Removing Task
- D-QUEST
Dutch-Quebec User Evaluation of Satisfaction with Assistive Technology
- DTM
Dart throwing motion
- DTSaM
Dynamic Traction Splint by Artificial Muscle
- EEG
Electroencephalogram
- EMG
Electromyography
- FAT
The Frenchay Arm Test
- FEA
Finite Element Analysis
- FES
Functional Electrical Stimulation
- FIM
Function Independence Measurement
- FMA
Fugl-Meyer Assessment
- fMRI
Functional magnetic resonance imaging
- GAIN
Global Appraisal of Individual Needs
- GRASSP
Graded Redefined Assessment of Strength, Sensibility and Prehension
- GRT
Grasp and Release test
- IBEP
Integral Value of a Bioelectric Potential
- IMU
Inertial measurement units
- IOTA
Isolated orthosis for thumb actuation
- IRQ
Interquartile range
- JTHFT
Jebson Taylor Hand Function Test
- KINARM
Kinesiological Instrument for Normal and Altered Reaching Movement
- LGMD
Limb Girdle Muscular Dystrophies
- MAL
Motor Activity Log
- MANOVA
multivariate analysis of variance
- MAPR
Multi-Attribute Preference Response
- mARAT
Modified Action Research Arm Test
- MARP
Mean arrest period ratio
- MAS
Modified Ashworth Score
- MAV
Mean Absolute Value
- MDC
Minimal detectable change
- MES
Mean Error Squared
- MMSE
Mini-Mental State Examination
- MMT
Manual Muscle Testing
- Movement ABC
Movement Assessment Battery for Children
- MPF
Mean Power Frequency
- mPPT
modified Purdue Pegboard Test
- MVC
Maximum Voluntary Contraction
- NASA-TLX
The NASA Task Load Index
- NHPT
Nine Hole Peg Test
- NIHSS
National Institute of Health Stroke Scale
- NSA
Nottingham Sensory Assessment
- PCGI-I
Patient Clinical Impressions-Improvements
- PIADS
Psychosocial Impact of Assistive Devices Scale
- PRISMA-ScR
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis Extension for Scoping Reviews
- PROM
Passion range of motion
- PRS
Pain Assessment Rating Scale
- PUL
Performance of the Upper Limb scale
- QIF-SF
Quadriplegia Index of Function-Short Form
- QoL
Quality of Life
- QOM
Quality of movement scale
- QUEST
Quebec User Evaluation of Satisfaction with Assistive Technology
- QuickDAS H
Quick Disabilities of the Arm, Shoulder, and Hand Questionnaire
- RCT
Randomized Control Trial
- RMA
Rivemead Motor Assessment
- RMSD
Root means square difference
- RMSE
Root means square error
- RMSED
Root Mean Standard Error of Deviation
- ROM
Range of motion
- RTLX
Raw NASA-Task Load Index
- SAL
Spectral arc length
- SCIM-SR
Spinal Cord Independence Measure–Self-Report
- sEMG
Surface Electromyography
- SEPs
Somatosensory evoked potentials
- SIAS
Stroke Impairment Assessment Set
- SIS
Stroke Impact Scale
- SUS
System Usability Scale
- SWMT
Semmes-Weinstein monofilament test
- TAM
Total Active Motion
- TBI
Traumatic Brain Injury
- TLT
Thumb localizing test
- TRI-HFT
Toronto Rehabilitation Institute Hand Function Test
- TRL
Technology Readiness Level
- UDQ
Use of Device Questionnaire
- USE
the Usefulness-Satisfaction-and-Ease-of-use-Questionnaire
- VAS
Visual Analog Pain Assessment Scale
- WHO-ICF
World Health Organisation - The International Classification of Functioning, Disability and Health
- Wilcoxon Test
The Wilcoxon signed-rank test
- WMFT
Wolf Motor Function Test
REFERENCES
- 1.Chen K-L, Tseng M-H, Shieh J-Y, Lu L, Huang C-Y.. Determinants of quality of life in children with cerebral palsy: A comprehensive biopsychosocial approach. Res Dev Disabil. 2014; 35(2):520–528. DOI: 10.1016/j.ridd.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 2.Mercier L, Audet T, Hébert R, Rochette A, Dubois M-F.. Impact of motor, cognitive, and perceptual disorders on ability to perform activities of daily living after stroke. Stroke. 2001; 32(11):2602–2608. DOI: 10.1161/hs1101.098154 [DOI] [PubMed] [Google Scholar]
- 3.Rondinelli RD, Dunn W, Hassanein KM, Keesling CA, Meredith SC, Schulz TL, et al. A simulation of hand impairments: Effects on upper extremity function and implications toward medical impairment rating and disability determination. Arch Phys Med Rehabil. 1997;78(12):1358–1363. DOI: 10.1016/S0003-9993(97)90310-5 [DOI] [PubMed] [Google Scholar]
- 4.Welmer A-K, Von Arbin M, Widén Holmqvist L, Sommerfeld DK.. Spasticity and its association with functioning and health-related quality of life 18 months after stroke. Cerebrovasc Dis. 2006;21(4):247–253. DOI: 10.1159/000091222 [DOI] [PubMed] [Google Scholar]
- 5.Sheean G. The pathophysiology of spasticity. Eur J Neurol. 2002; 9:3–9. DOI: 10.1046/j.1468-1331.2002.0090s1003.x [DOI] [PubMed] [Google Scholar]
- 6.Jones LA, Lederman SJ.. Human hand function. New York: Oxford University Press. 2006. DOI: 10.1093/acprof:oso/9780195173154.001.0001 [DOI] [Google Scholar]
- 7.Iberall T. Human Prehension and Dexterous Robot Hands. Int J Robot Res. 1997;16(3):285–299. DOI: 10.1177/027836499701600302 [DOI] [Google Scholar]
- 8.McKee PR, Rivard A.. Biopsychosocial Approach to Orthotic Intervention. J Hand Ther. 2011; 24(2):155–163. DOI: 10.1016/j.jht.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Lugg W. The biopsychosocial model – history, controversy and Engel. Australas Psychiatry. 2022; 30(1):55–59. DOI: 10.1177/10398562211037333 [DOI] [PubMed] [Google Scholar]
- 10.Üstün TB, Chatterji S, Bickenbach J, Kostanjsek N, Schneider M.. The international classification of functioning, disability and health: A new tool for understanding disability and health. Disabil Rehabil. 2003;25(11-12):565–271. DOI: 10.1080/0963828031000137063 [DOI] [PubMed] [Google Scholar]
- 11.International classification of functioning, disability and health (ICF) [Internet]. World Health Organization. 2001; [cited 2024, August 12]. Available from: http://www.who.int/classifications/icf/en/ [Google Scholar]
- 12.Briggs AM, Woolf AD, Dreinhöfer K, Homb N, Hoy DG, Kopansky-Giles D, et al. Reducing the global burden of musculoskeletal conditions. Bull World Health Organ. 2018; 96(5):366–368]. DOI: 10.2471/blt.17.204891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackerman IN, Buchbinder R, March L.. Global burden of disease study 2019: An opportunity to understand the growing prevalence and impact of hip, knee, hand and other osteoarthritis in Australia. Intern Med J. 2023; 53(10):1875–1882. DOI: 10.1111/imj.15933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson LS, Sarkies M, Brown T, O’Brien L.. Direct, indirect and intangible costs of acute hand and wrist injuries: A systematic review. Injury. 2016; 47(12):2614–2626. DOI: 10.1016/j.injury.2016.09.041 [DOI] [PubMed] [Google Scholar]
- 15.Tangcharoensathien V, Witthayapipopsakul W, Viriyathorn S, Patcharanarumol W.. Improving access to assistive technologies: challenges and solutions in low- and middle-income countries [Internet]. World Health Organization. 2018; [cited 2024, August 12]. Available from: https://iris.who.int/handle/10665/329578 [DOI] [PubMed] [Google Scholar]
- 16.Global report on assistive technology [Internet]. World Health Organization and the United Nations Children’s Fund (UNICEF). 2022; [cited 2024, August 12]. Available from: https://iris.who.int/bitstream/handle/10665/354357/9789240049451-eng.pdf?sequence=1 [Google Scholar]
- 17.Bos RA, Haarman CJW, Stortelder T, Nizamis K, Herder JL, Stienen AHA, et al. A structured overview of trends and technologies used in dynamic hand orthoses. J Neuroeng Rehabil. 2016;13(1):62. DOI: 10.1186/s12984-016-0168-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopura R, Kiguchi K, Bandara D.. A brief review on upper extremity robotic exoskeleton systems. 6th Int Conf Ind Inform Syst. 2011: 346–351. DOI: 10.1109/ICIINFS.2011.6038092 [DOI]
- 19.Eraifej J, Clark W, France B, Desando S, Moore D.. Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: A systematic review and meta-analysis. Syst Rev. 2017; 6:1–21. DOI: 10.1186/s13643-017-0435-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Looze MP, Bosch T, Krause F, Stadler KS, O’sullivan LW.. Exoskeletons for industrial application and their potential effects on physical work load. Ergonomics. 2016; 59(5):671–681. DOI: 10.1080/00140139.2015.1081988 [DOI] [PubMed] [Google Scholar]
- 21.National service framework: Long-term conditions [Internet] Department of Health and Social Care, Gov.UK. 2005;1–106. [cited 2024, August 12]. Available from: https://www.gov.uk/government/publications/quality-standards-for-supporting-people-with-long-term-conditions
- 22.Pan M, Yuan C, Liang X, Dong T, Liu T, Zhang J, et al. Soft actuators and robotic devices for rehabilitation and assistance. Adv Intell Syst. 2022; 4(4). DOI: 10.1002/aisy.202100140. [DOI] [Google Scholar]
- 23.Desplenter T, Zhou Y, Edmonds BP, Lidka M, Goldman A, Trejos AL.. Rehabilitative and assistive wearable mechatronic upper-limb devices: A review. J Rehabil Assist Technol Eng. 2020; 7. DOI: 10.1177/2055668320917870 [DOI] [PMC free article] [PubMed]
- 24.Peters MD, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI evidence synthesis. 2020; 18(10):2119–2126. DOI: 10.11124/JBIES-20-00167 [DOI] [PubMed] [Google Scholar]
- 25.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E.. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018; 18(1):143. DOI: 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aromataris E, Lockwood C, Porritt K, Pilla B, Jordan Z, editors. JBI Manual for Evidence Synthesis. JBI; 2024. Available from: https://synthesismanual.jbi.global [Google Scholar]
- 27.Grant MJ, Booth A.. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf Libr J. 2009; 26(2):91–108. DOI: 10.1111/j.1471-1842.2009.00848.x [DOI] [PubMed] [Google Scholar]
- 28.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018; 169(7):467–473. DOI: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 29.Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB.. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015; 13(3):141–146. DOI: 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.5 (updated August 2024). Cochrane, 2024. Available from www.training.cochrane.org/handbook [Google Scholar]
- 31.Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH.. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst Rev. 2017; 6(1):245. DOI: 10.1186/s13643-017-0644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galbert, A, Buis, A.. Data for “Active, actuated, and assistive: A scoping review of exoskeletons for the hands and wrists”. University of Strathclyde. AAA_Dataset(.xlsx). 10.15129/b79e6928-1b33-432a-9671-24b6b9b5691a. Available from: https://pureportal.strath.ac.uk/en/datasets/data-for-active-actuated-and-assistive-a-scoping-review-of-exoske [Google Scholar]
- 33.World Development Indicators [Internet]. World Bank Group. 2023; [cited 2024, August 12]. Available from: https://datacatalog.worldbank.org/search/dataset/0037712/World-Development-Indicators [Google Scholar]
- 34.Wanasinghe AT, Awantha WVI, Kavindya AGP, Kulasekera AL, Chathuranga DS, Senanayake B.. A layer jamming soft glove for hand tremor suppression. IEEE Trans Neural Syst Rehabil Eng. 2021;29:2684–2694. DOI: 10.1109/TNSRE.2021.3135497 [DOI] [PubMed] [Google Scholar]
- 35.Chandrasekhar V, Vazhayil V, Rao M.. Design of a portable anthropomimetic upper limb rehabilitation device for patients suffering from neuromuscular disability. Conf Proc IEEE Eng Med Biol Soc. 2020;4708–4712. DOI: 10.1109/EMBC44109.2020.9176399 [DOI] [PubMed]
- 36.Phan Van H, Ngo HQT.. Developing an assisting device to reduce the vibration on the hands of elders. Appl Sci. 2021; 11(11):502. DOI: 10.3390/app11115026 [DOI] [Google Scholar]
- 37.Abramovich SG, Drobyshev VA, Pyatova AE, Yumashev AV, Koneva ES.. Comprehensive use of dynamic electrical neurostimulation and botulinum toxin therapy in patients with post-stroke spasticity. J Stroke Cerebrovasc Dis. 2020;29(11):105189. DOI: 10.1016/j.jstrokecerebrovasdis.2020.105189 [DOI] [PubMed] [Google Scholar]
- 38.Takebayashi T, Takahashi K, Amano S, Gosho M, Sakai M, Hashimoto K, et al. Robot-assisted training as self-training for upper-limb hemiplegia in chronic stroke: a randomized controlled trial. Stroke. 2022;53(7):2182–2191. DOI: 10.1161/STROKEAHA.121.037260 [DOI] [PubMed] [Google Scholar]
- 39.Haddaway NR, Page MJ, Pritchard CC, McGuinness LA.. PRISMA2020: An R package and shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev. 2022;18:e1230. DOI: 10.1002/cl2.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheker LR, Chesher SP, Ramirez S.. Neuromuscular electrical stimulation and dynamic bracing as a treatment for upper-extremity spasticity in children with cerebral palsy. J Hand Surg Br. 1999;24(2):226–232. DOI: 10.1054/jhsb.1998.0002 [DOI] [PubMed] [Google Scholar]
- 41.Zahedi A, Zhang B, Yi A, Zhang D.. A soft exoskeleton for tremor suppression equipped with flexible semiactive actuator. Soft Robotics. 2021; 8(4):432–447. DOI: 10.1089/soro.2019.0194 [DOI] [PubMed] [Google Scholar]
- 42.Xu J, Xue Y, Yu Z, Zhao D, Li X, Fan J, et al. Effect of exoskeleton manipulator on hand function rehabilitation for postburn patients. Disabil Rehabil. 2022;45(24):1–8. DOI: 10.1080/09638288.2022.2143577 [DOI] [PubMed] [Google Scholar]
- 43.Al-Fahaam H, Davis S, Nefti-Meziani S, Theodoridis T.. Novel soft bending actuator-based power augmentation hand exoskeleton controlled by human intention. Intell Serv Robot. 2018;11(3):247–268. DOI: 10.1007/s11370-018-0250-4 [DOI] [Google Scholar]
- 44.Ariyanto M, Ismail R, Setiawan JD, Arifin Z.. Development of low cost supernumerary robotic fingers as an assistive device. 4th Int Conf Electr Eng Comput Sci Informatics (EECSI). 2017: 533–538. DOI: 10.1109/EECSI.2017.8239172 [DOI]
- 45.Dittli J, Vasileiou C, Asanovski H, Lieber J, Lin JB, Meyer-Heim A, et al. Design of a compliant, stabilizing wrist mechanism for a pediatric hand exoskeleton. IEEE Int Conf Rehabil Rob. 2022;1–6. DOI: 10.1109/ICORR55369.2022.9896550 [DOI] [PubMed]
- 46.Metzler MJ, Cole L, Kirton A.. A case of neuromuscular electrical stimulation for childhood stroke hyperkinesis: A brief report. Dev Neurorehabil. 2020;23(6):407–411. DOI: 10.1080/17518423.2020.1773956 [DOI] [PubMed] [Google Scholar]
- 47.Saxena S, Nikolic S, Popovic D.. An EMG-controlled grasping system for tetraplegics. J Rehabil Res Dev. 1995;32(1):17–24. [PubMed] [Google Scholar]
- 48.Ertas IH, Patoglu V.. A multi-functional rehabilitation device to assist forearm/wrist and grasp therapies. International conference on haptics: Generating and perceiving tangible sensations, EuroHaptics 2010; Amsterdam; Netherlands. DOI: 10.1007/978-3-642-14075-4_41 [DOI] [Google Scholar]
- 49.Gull MA, Thoegersen M, Bengtson SH, Mohammadi M, Andreasen Struijk LNS, Moeslund TB, et al. A 4-DOF upper limb exoskeleton for physical assistance: Design, modeling, control and performance evaluation. Appl Sci. 2021;11(13):5865. DOI: 10.3390/app11135865 [DOI] [Google Scholar]
- 50.Martino Cinnera A, Pucello A, Lupo A, Gimigliano F, Mammucari E, Cicero DL, et al. Upper limb motor improvement in chronic stroke after combining botulinum toxin A injection and multi-joints robot-assisted therapy: A case report. Oxf Med Case Rep. 2019; 2019(10):omz097. DOI: 10.1093/omcr/omz097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li M, Liang ZT, He B, Zhao CG, Yao W, Xu GH, et al. Attention-controlled assistive wrist rehabilitation using a low-cost EEG sensor. IEEE Sens J. 2019;19(15):6497–6507. DOI: 10.1109/JSEN.2019.2910318 [DOI] [Google Scholar]
- 52.Kawase T, Sakurada T, Koike Y, Kansaku K.. A hybrid BMI-based exoskeleton for paresis: EMG control for assisting arm movements. J Neural Eng. 2017;14(1):016015. DOI: 10.1088/1741-2552/aa525f [DOI] [PubMed] [Google Scholar]
- 53.Dalla Gasperina S, Gandolla M, Manti A, Aquilante L, Longatelli V, D'Angelo MG, et al. Upper-limb actuated exoskeleton for muscular dystrophy patients: Preliminary results. Conf Proc IEEE Eng Med Biol Soc. 2019;4431–4435. DOI: 10.1109/EMBC.2019.8857725 [DOI] [PubMed]
- 54.Abramovich SG, Drobyshev VA, Pyatova AE, Yumashev AV, Koneva ES.. Comprehensive use of dynamic electrical neurostimulation and botulinum toxin therapy in patients with post-stroke spasticity. J Stroke Cerebrovasc Dis. 2020;29(11):105189. [DOI] [PubMed] [Google Scholar]
- 55.Wang HP, Guo AW, Bi ZY, Zhou YX, Wang ZG, Lu XY, et al. A novel distributed functional electrical stimulation and assessment system for hand movements using wearable technology. IEEE Biomed Circuits Syst Conf (BioCAS). 2016: 74–77. DOI: 10.1109/BioCAS.2016.7833728 [DOI]
- 56.Nakayama J, Horiki M, Denno K, Ogawa K, Oka H, Domen K.. Pneumatic-type dynamic traction and flexion splint for treating patients with extension contracture of the metacarpophalangeal joint. Prosthet Orthot Int. 2016;40(1):142–146. DOI: 10.1177/0309364615574165 [DOI] [PubMed] [Google Scholar]
- 57.Nakayama J, Sunagawa K, Ogawa K, Oka H.. Analysis of a new artificial muscle type dynamic orthosis for wrist joint disease using a three-dimensional motion analyzer. Prog Rehabil Med. 2021; 6:20210043. DOI: 10.2490/prm.20210043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bae JH, Kim YM, Moon I.. Wearable hand rehabilitation robot capable of hand function assistance in stroke survivors. 4th IEEE RAS & EMBS Int Conf Biomed Robot Biomechatronics (BioRob). 2012;1482–1487. DOI: 10.1109/BioRob.2012.6290736 [DOI]
- 59.Powell J, Pandyan AD, Granat M, Cameron M, Stott DJ.. Electrical stimulation of wrist extensors in poststroke hemiplegia. Stroke. 1999;30(7):1384–1389. DOI: 10.1161/01.STR.30.7.1384 [DOI] [PubMed] [Google Scholar]
- 60.De Araujo RC, Junior FL, Rocha DN, Sono TS, Pinotti M.. Effects of intensive arm training with an electromechanical orthosis in chronic stroke patients: A preliminary study. Arch Phys Med Rehabil. 2011;92(11):1746–1753. DOI: 10.1016/j.apmr.2011.05.021 [DOI] [PubMed] [Google Scholar]
- 61.Nam C, Rong W, Li W, Cheung C, Ngai W, Cheung T, et al. An Exoneuromusculoskeleton for Self-Help Upper Limb Rehabilitation after Stroke. Soft Robotics. 2022;9(1):14–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qian QY, Hu XL, Lai Q, Ng SC, Zheng YP, Poon WS.. Early stroke rehabilitation of the upper limb assisted with an electromyography-driven neuromuscular electrical stimulation-robotic arm. Front Neurol. 2017;8. DOI: 10.3389/fneur.2017.00447 [DOI] [PMC free article] [PubMed]
- 63.Nam C, Rong W, Li W, Xie Y, Hu X, Zheng Y.. The effects of upper-limb training assisted with an electromyography-driven neuromuscular electrical stimulation robotic hand on chronic stroke. Front Neurol. 2017;8:679. DOI: 10.3389/fneur.2017.00679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nam C, Zhang B, Chow T, Ye F, Huang Y, Guo Z, et al. Home-based self-help telerehabilitation of the upper limb assisted by an electromyography-driven wrist/hand exoneuromusculoskeleton after stroke. J Neuroeng Rehabil. 2021;18(1):137. DOI: 10.1186/s12984-021-00930-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zulauf-Czaja A, Al-Taleb MKH, Purcell M, Petric-Gray N, Cloughley J, Vuckovic A.. On the way home: a BCI-FES hand therapy self-managed by sub-acute SCI participants and their caregivers: a usability study. J Neuroeng Rehabil. 2021;18(1):44. DOI: 10.1186/s12984-021-00838-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chae J, Bethoux F, Bohinc T, Dobos L, Davis T, Friedl A.. Neuromuscular stimulation for upper extremity motor and functional recovery in acute hemiplegia. Stroke. 1998; 29(5):975–979. DOI: 10.1161/01.STR.29.5.975 [DOI] [PubMed] [Google Scholar]
- 67.Nobaveh AA, Caasenbrood B.. Design feasibility of an energy-efficient wrist flexion-extension exoskeleton using compliant beams and soft actuators. IEEE Int Conf Rehabil Rob. 2022. DOI: 10.1109/ICORR55369.2022.9896528 [DOI] [PubMed]
- 68.Rahman MH, Rahman MJ, Cristobal OL, Saad M, Kenné JP, Archambault PS.. Development of a whole arm wearable robotic exoskeleton for rehabilitation and to assist upper limb movements. Robotica. 2015;33(1):19–39. DOI: 10.1017/S0263574714000034 [DOI] [Google Scholar]
- 69.Lambelet C, Temiraliuly D, Siegenthaler M, Wirth M, Woolley DG, Lambercy O, et al. Characterization and wearability evaluation of a fully portable wrist exoskeleton for unsupervised training after stroke. J Neuroeng Rehabil. 2020;17(1). DOI: 10.1186/s12984-020-00749-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ceccarelli M, Morales-Cruz C.. A prototype characterization of ExoFinger, a finger exoskeleton. Int J Adv Robot. 2021;18(3). DOI: 10.1177/17298814211024880 [DOI] [Google Scholar]
- 71.Bengtson SH, Thøgersen MB, Mohammadi M, Kobbelgaard FV, Gull MA, Andreasen Struijk LNS, et al. Computer vision-based adaptive semi-autonomous control of an upper limb exoskeleton for individuals with tetraplegia. Appl Sci. 2022;12(9):4374. DOI: 10.3390/app12094374 [DOI] [Google Scholar]
- 72.Choi H, Kang BB, Jung BK, Cho KJ.. Exo-wrist: A soft tendon-driven wrist-wearable robot with active anchor for dart-throwing motion in hemiplegic patients. IEEE Robot Autom Lett. 2019;4(4):4499–4506. DOI: 10.1109/LRA.2019.2931607 [DOI] [Google Scholar]
- 73.Haarman CJW, Hekman EEG, Rietman JS, Van Der Kooij H.. Mechanical design and feasibility of a finger exoskeleton to support finger extension of severely affected stroke patients. IEEE Trans Neural Syst Rehabil Eng. 2023;31:1268–1276. DOI: 10.1109/TNSRE.2023.3243357 [DOI] [PubMed] [Google Scholar]
- 74.Martín-Odriozola A, Rodríguez-de-Pablo C, Caceres-Salegi A, García-Calleja A, Marín-Ojea JI, Hernández E, et al. Analysis of the movements generated by a multi-field functional electrical stimulation device for upper extremity rehabilitation. Artif Organs. 2022;46(10):2027–2033. DOI: 10.1111/aor.14346 [DOI] [PubMed] [Google Scholar]
- 75.Shimada Y, Chida S, Matsunaga T, Misawa A, Ito H, Sakuraba T, et al. Grasping power by means of functional electrical stimulation in a case of C6 complete tetraplegia. Tohoku J Exp Med. 2003; 201(2):91–96. DOI: 10.1620/tjem.201.91 [DOI] [PubMed] [Google Scholar]
- 76.Bustamante C, Brevis F, Canales S, Millón S, Pascual R.. Effect of functional electrical stimulation on the proprioception, motor function of the paretic upper limb, and patient quality of life: A case report. J Hand Ther. 2016;29(4):507–514. DOI: 10.1016/j.jht.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 77.Triolo ER, Stella MH, BuSha BF.. A force augmenting exoskeleton for the human hand designed for pinching and grasping. Annu Int Conf IEEE Eng Med Biol Soc. 2018;1875–1878. DOI: 10.1109/EMBC.2018.8512606 [DOI] [PubMed]
- 78.Hasegawa Y, Mikami Y, Watanabe K, Sankai Y, Ieee.. Five-fingered assistive hand with mechanical compliance of human finger. IEEE Int Conf Robot Autom. 2008;718–724. DOI: 10.1109/ROBOT.2008.4543290 [DOI]
- 79.Hasegawa Y, Watanabe K, Sankai Y, Ieee.. Performance evaluations of hand and forearm support system. IEEE/RSJ Int Conf Intell Robot Syst. 2010;2645–2650. DOI: 10.1109/IROS.2010.5650355 [DOI]
- 80.Ahmed T, Assad-Uz-Zaman M, Islam MR, Gottheardt D, McGonigle E, Brahmi B, et al. Flexohand: A hybrid exoskeleton-based novel hand rehabilitation device. Micromachines. 2021; 12(11). DOI: 10.3390/mi12111274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen WY, Li GY, Li N, Wang WX, Yu P, Wang RQ, et al. Restoring voluntary bimanual activities of patients with chronic hemiparesis through a foot-controlled hand/forearm exoskeleton. IEEE Trans Neural Syst Rehabil. 2023;31:769–778. DOI: 10.1109/TNSRE.2022.3233631 [DOI] [PubMed] [Google Scholar]
- 82.Duanmu D, Wang X, Li X, Wang Z, Hu Y.. Design of guided bending bellows actuators for soft hand function rehabilitation gloves. Actuators. 2022;11(12):346. DOI: 10.3390/act11120346 [DOI] [Google Scholar]
- 83.Bernocchi P, Mule C, Vanoglio F, Taveggia G, Luisa A, Scalvini S.. Home-based hand rehabilitation with a robotic glove in hemiplegic patients after stroke: A pilot feasibility study. Top Stroke Rehabil. 2018;25(2):114–119. DOI: 10.1080/10749357.2017.1389021 [DOI] [PubMed] [Google Scholar]
- 84.Das S, Lowell C, Kurita Y.. Force your hand—PAM enabled wrist support. In: Hasegawa S, Konyo M, Kyung KU, Nojima T, Kajimoto H, editors. Haptic interaction. Singapore: Springer; 2016;41–50. DOI: 10.1007/978-981-10-4157-0_41 [DOI] [Google Scholar]
- 85.Popov D, Gaponov I, Ryu JH.. Portable exoskeleton glove with soft structure for hand assistance in activities of daily living. IEEE-ASME Trans Mechatron. 2017;22(2):865–875. DOI: 10.1109/TMECH.2016.2641932 [DOI] [Google Scholar]
- 86.Goutam S, Aw KC.. Development of a compliant hand assistive device. MESA - IEEE/ASME Int Conf Mechatronic Embedded Syst Appl, Conf Proc. 2014. DOI: 10.1109/MESA.2014.6935521 [DOI]
- 87.Zhang F, Lin L, Yang L, Fu Y.. Design of an active and passive control system of hand exoskeleton for rehabilitation. Appl Sci. 2019;9(11):2291. DOI: 10.3390/app9112291 [DOI] [Google Scholar]
- 88.Conti R, Allotta B, Meli E, Ridolfi A.. Development, design and validation of an assistive device for hand disabilities based on an innovative mechanism. Robotica. 2017; 35(4):892–906. DOI: 10.1017/S0263574715000879 [DOI] [Google Scholar]
- 89.Guo K, Lu J, Liu C, Yang H.. Development, research, optimization and experiment of exoskeleton robot for hand rehabilitation training. Appl Sci. 2022;12(20):10580. DOI: 10.3390/app122010580 [DOI] [Google Scholar]
- 90.Xiao FY, Gu L, Ma WZ, Zhu YH, Zhang Z, Wang Y.. Real time motion intention recognition method with limited number of surface electromyography sensors for A 7-DOF hand/wrist rehabilitation exoskeleton. Mechatronics. 2021;79. DOI: 10.1016/j.mechatronics.2021.102642 [DOI]
- 91.Shindo K, Fujiwara T, Hara J, Oba H, Hotta F, Tsuji T, et al. Effectiveness of hybrid assistive neuromuscular dynamic stimulation therapy in patients with subacute stroke: A randomized controlled pilot trial. Neurorehabil Neural Repair. 2011; 25(9):830–837. DOI: 10.1177/1545968311408917 [DOI] [PubMed] [Google Scholar]
- 92.Oshima O, Kawakami M, Okuyama K, Suda M, Oka A, Liu MG.. Effects of hybrid assistive neuromuscular dynamic stimulation therapy for hemiparesis after pediatric stroke: A feasibility trial. Disabil Rehabil. 2021; 43(6):823–827. DOI: 10.1080/09638288.2019.1643415 [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Metcalfe B, Zhao Y, Zhang D.. An assistive system for upper limb motion combining functional electrical stimulation and robotic exoskeleton. IEEE Trans Med Robot Bionics. 2020; 2(2):260–268. DOI: 10.1109/TMRB.2020.2990318 [DOI] [Google Scholar]
- 94.Meng Q, Shen Z, Nie Z, Meng Q, Wu Z, Yu H.. Modeling and evaluation of a novel hybrid-driven compliant hand exoskeleton based on human-machine coupling model. Appl Sci. 2021;11(22):10825. DOI: 10.3390/app112210825 [DOI] [Google Scholar]
- 95.Burridge JH, Turk R, Merrill D, Dibb B, Hughes AM, Sparrow O, et al. A personalized sensor-controlled microstimulator system for arm rehabilitation poststroke. Part 2: Objective outcomes and patients' perspectives. Neuromodulation. 2011;14(1):80–88. DOI: 10.1111/j.1525-1403.2010.00310.x [DOI] [PubMed] [Google Scholar]
- 96.Malesevic NM, Popovic Maneski LZ, Ilic V, Jorgovanovic N, Bijelic G, Keller T, et al. A multi-pad electrode based functional electrical stimulation system for restoration of grasp. J Neuroeng Rehabil. 2012;9(1):66. DOI: 10.1186/1743-0003-9-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bockbrader M, Annetta N, Friedenberg D, Schwemmer M, Skomrock N, Colachis, et al. Clinically significant gains in skillful grasp coordination by an individual with tetraplegia using an implanted brain-computer interface with forearm transcutaneous muscle stimulation. Arch Phys Med Rehabil. 2019;100(7):1201–1217. DOI: 10.1016/j.apmr.2018.07.445 [DOI] [PubMed] [Google Scholar]
- 98.Aubin P, Petersen K, Sallum H, Walsh C, Correia A, Stirling L.. A pediatric robotic thumb exoskeleton for at-home rehabilitation: The isolated orthosis for thumb actuation (IOTA). Int J Intell Comput Cybern. 2014;7(3):233–252. DOI: 10.1108/IJICC-10-2013-0043 [DOI] [PubMed] [Google Scholar]
- 99.Lee K, Jung P-G, Lee Y, Cha Y.. Wearable multifunctional additive hand system for enhancing the workspace and grasping capability of the human hand. IEEE Access. 2022;10:28094–28108. DOI: 10.1109/ACCESS.2022.3157881 [DOI] [Google Scholar]
- 100.Frullo JM, Elinger J, Pehlivan AU, Fitle K, Nedley K, Francisco GE, et al. Effects of assist-as-needed upper extremity robotic therapy after incomplete spinal cord injury: A parallel-group controlled trial. Front Neurorobot. 2017;11. DOI: 10.3389/fnbot.2017.00026 [DOI] [PMC free article] [PubMed]
- 101.Pehlivan AU, Celik O, O'Malley MK.. Mechanical design of a distal arm exoskeleton for stroke and spinal cord injury rehabilitation. IEEE Int Conf Rehabil Robot. 2011;5975428. DOI: 10.1109/ICORR.2011.5975428 [DOI] [PubMed]
- 102.Thorsen R, Ferrarin M, Spadone R, Frigo C.. Functional control of the hand in tetraplegics based on residual synergistic EMG activity. Artif Organs. 1999;23(5):470–473. DOI: 10.1046/j.1525-1594.1999.06362.x [DOI] [PubMed] [Google Scholar]
- 103.Thorsen R, Spadone R, Ferrarin M.. A pilot study of myoelectrically controlled FES of upper extremity. IEEE Trans Neural Syst Rehabil Eng. 2001;9(2):161–168. DOI: 10.1109/7333.928576 [DOI] [PubMed] [Google Scholar]
- 104.Ma D, Li X, Xu Q, Yang F, Feng YT, Wang WX, et al. Robot-assisted bimanual training improves hand function in patients with subacute stroke: A randomized controlled pilot study. Front Neurol. 2022;13. DOI: 10.3389/fneur.2022.884261 [DOI] [PMC free article] [PubMed]
- 105.Kyeong S, Na Y, Kim J.. A mechatronic mirror-image motion device for symmetric upper-limb rehabilitation. Int J Precis Eng Manuf. 2020; 21(5):947–956. DOI: 10.1007/s12541-019-00310-x [DOI] [Google Scholar]
- 106.Toshihiro K, Hiroyuki K, Yasuharu K.. A power assist device based on joint equilibrium point estimation from electromyography. J Robot Mechatron. 2012;24(1):205–218. DOI: 10.20965/jrm.2012.p0205 [DOI] [Google Scholar]
- 107.McPherson AIW, Patel VV, Downey PR, Abbas Alvi A, Abbott ME, Stuart HS.. Motor-augmented wrist-driven orthosis: Flexible grasp assistance for people with spinal cord injury. 42nd Annu Int Conf IEEE Eng Med Biol Soc (EMBC). 2020;4936–4940. DOI: 10.1109/EMBC44109.2020.9176037 [DOI] [PubMed]
- 108.Nizamis K, Ayvaz A, Rijken NHM, Koopman BFJM and Sartori M (2023) Real-time myoelectric control of wrist/hand motion in Duchenne muscular dystrophy: A case study. Front Robot AI. DOI: 10.3389/frobt.2023.1100411 [DOI] [PMC free article] [PubMed]
- 109.Dunaway S, Brianna Dezsi D, Perkins J, Tran D, Naft J.. Case report on the use of a custom myoelectric elbow–wrist–hand orthosis for the remediation of upper extremity paresis and loss of function in chronic stroke. Mil Med. 2017;1963–1968. DOI: 10.7205/MILMED-D-16-00399 [DOI] [PubMed]
- 110.Serruya MD, Napoli A, Satterthwaite N, Kardine J, McCoy J, Grampurohit N, et al. Neuromotor prosthetic to treat stroke-related paresis: N-of-1 trial. Commun Med. 2022;2(1):37. DOI: 10.1038/s43856-022-00105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peters HT, Page SJ, Persch A.. Giving them a hand: Wearing a myoelectric elbow-wrist-hand orthosis reduces upper extremity impairment in chronic stroke. Arch Phys Med Rehabil. 2017; 98(9):1821–1827. DOI: 10.1016/j.apmr.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 112.Hoppe-Ludwig S, Armitage J, Turner KL, O'Brien MK, Mummidisetty CK, Koch LM, et al. Usability, functionality, and efficacy of a custom myoelectric elbow-wrist-hand orthosis to assist elbow function in individuals with stroke. J Rehabil Assist Technol Eng. 2021;8. DOI: 10.1177/20556683211035057 [DOI] [PMC free article] [PubMed]
- 113.Lauretti C, Cordella F, Ciancio AL, Trigili E, Catalan JM, Badesa FJ, et al. Learning by demonstration for motion planning of upper-limb exoskeletons. Front Neurorobot. 2018;12:5. DOI: 10.3389/fnbot.2018.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alon G, Dar A, Katz-Behiri D, Weingarden H, Nathan S.. Efficacy of a hybrid upper limb neuromuscular electrical stimulation system in lessening selected impairments and dysfunctions consequent to cerebral damage. J Neuroeng Rehabil. 1998; 12(2):73–79. DOI: 10.1177/154596839801200205 [DOI] [Google Scholar]
- 115.Berner YN, Kimchi OL, Spokoiny V, Finkeltov B.. The effect of electric stimulation treatment on the functional rehabilitation of acute geriatric patients with stroke - A preliminary study. Arch Gerontol Geriatr. 2004;39(2):125–132. DOI: 10.1016/j.archger.2004.02.003 [DOI] [PubMed] [Google Scholar]
- 116.Snoek GJ, MJ IJ, in't Groen FA, Stoffers TS, Zilvold G.. Use of the NESS handmaster to restore handfunction in tetraplegia: Clinical experiences in ten patients. Spinal Cord. 2000;38(4):244–249. DOI: 10.1038/sj.sc.3100980 [DOI] [PubMed] [Google Scholar]
- 117.Ugurlu U, Ozkan M, Ozdogan AH.. Development of a “Neuro-orthosis” for the control of wrist movements in patients with carpal tunnel syndrome: Preliminary results. Annu Int Conf IEEE Eng Med Biol Soc. 2007;4794–4797. DOI: 10.1109/IEMBS.2007.4353412 [DOI] [PubMed]
- 118.Ugurlu U, Ozkan M, Ozdogan H.. The development of a new orthosis (neuro-orthosis) for patients with carpal tunnel syndrome: Its effect on the function and strength of the hand. Prosthet Orthot Int. 2008;32(4):403–421. DOI: 10.1080/03093640802366166 [DOI] [PubMed] [Google Scholar]
- 119.Rong W, Li WM, Pang MK, Hu JY, Wei XJ, Yang BB, et al. A neuromuscular electrical stimulation (NMES) and robot hybrid system for multi-joint coordinated upper limb rehabilitation after stroke. J Neuroeng Rehabil. 2017;14. DOI: 10.1186/s12984-017-0245-y [DOI] [PMC free article] [PubMed]
- 120.Mann G, Taylor P, Lane R.. Accelerometer-triggered electrical stimulation for reach and grasp in chronic stroke patients: A pilot study. Neurorehabil Neural Repair. 2011;25(8):774–780. DOI: 10.1177/1545968310397200 [DOI] [PubMed] [Google Scholar]
- 121.Tan DWH, Ng PK, Noor EEM, Saptari A, Hue CC, Ng YJ.. Development and usability testing of a finger grip enhancer for the elderly. Robotics (Basel). 2022;11(1):5. DOI: 10.3390/robotics11010005 [DOI] [Google Scholar]
- 122.Ferreira F, Rubio GD, Dutra RMA, Van Petten A, Vimieiro CBS.. Development of portable robotic orthosis and biomechanical validation in people with limited upper limb function after stroke. Robotica. 2022;40(12):4238–4256. DOI: 10.1017/S0263574722000881 [DOI] [Google Scholar]
- 123.Connelly L, Jia YC, Toro ML, Stoykov ME, Kenyon RV, Kamper DG.. A pneumatic glove and immersive virtual reality environment for hand rehabilitative training after stroke. IEEE Trans Neural Syst Rehabil. 2010;18(5):551–559. DOI: 10.1109/TNSRE.2010.2047588 [DOI] [PubMed] [Google Scholar]
- 124.Al-Fahaam H, Davis S, Nefti-Meziani S, Theodoridis T.. Novel soft bending actuator-based power augmentation hand exoskeleton controlled by human intention. Intel Serv Robotics. 2018;11, 247–268. DOI: 10.1007/s11370-018-0250-4 [DOI] [Google Scholar]
- 125.Hara Y, Ogawa S, Tsujiuchi K, Muraoka Y.. A home-based rehabilitation program for the hemiplegic upper extremity by power-assisted functional electrical stimulation. Disabil Rehabil. 2008;30(4):296–304. DOI: 10.1080/09638280701265539 [DOI] [PubMed] [Google Scholar]
- 126.Mandeljc A, Rajhard A, Munih M, Kamnik R.. Robotic device for out-of-clinic post-stroke hand rehabilitation. Appl Sci. 2022;12(3): 1092. DOI: 10.3390/app12031092 [DOI] [Google Scholar]
- 127.Yao J, Sullivan JE, Dewald J.. A novel EMG-driven functional electrical stimulator for post-stroke individuals to practice activities of daily living. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS. 2018;1436–1439. DOI: 10.1109/EMBC.2018.8512543 [DOI] [PubMed]
- 128.Camona C, Wilkins KB, Drogos J, Sullivan JE, Dewald JPA, Yao J.. Improving hand function of severely impaired chronic hemiparetic stroke individuals using task-specific training with the Reln-Hand system: A case series. Front Neurol. 2018;9. DOI: 10.3389/fneur.2018.00923 [DOI] [PMC free article] [PubMed]
- 129.Butzer T, Lambercy O, Arata J, Gassert R.. Fully wearable actuated soft exoskeleton for grasping assistance in everyday activities. Soft Robot. 2021;8(2):128–143. DOI: 10.1089/soro.2019.0135 [DOI] [PubMed] [Google Scholar]
- 130.Meyer JT, Werner C, Hermann S, Demkó L, Lambercy O, Gassert R.. Understanding technology-induced compensation: Effects of a wrist-constrained robotic hand orthosis on grasping kinematics. In: Moreno JC, Masood J, Schneider U, Maufroy C, Pons JL, editors. Wearable robotics: Challenges and trends. Biosyst Biorob. Cham: Springer. 2020;69–79. DOI: 10.1007/978-3-030-69547-7_69 [DOI] [Google Scholar]
- 131.Guo K, Orban M, Yang HB, Li ZL.. Research on rope-driven flexible robot for hand rehabilitation and experimental study based on eeg signals. J Mech Med Biol. 2022;22(09). DOI: 10.1142/S0219519422400437 [DOI] [Google Scholar]
- 132.Balasubramanian S, Wei HR, Perez M, Shepard B, Koeneman E, Koeneman J, et al. Rupert: An exoskeleton robot for assisting rehabilitation of arm functions. Virtual Rehabil. 2008;163–167. DOI: 10.1109/ICVR.2008.4625154 [DOI]
- 133.He J, Koeneman EJ, Schultz R, Herring D, Wanberg J, Huang H, et al. RUPERT: A device for robotic upper extremity repetitive therapy. IEEE Eng Med Biol 27th Ann Conf. 2005;6844–6847. DOI: 10.1109/IEMBS.2005.1616077 [DOI] [PubMed]
- 134.Butler A, Blanton S, Rowe V, Wolf S.. Attempting to improve function and quality of life using the FTM Protocol: Case report. J Neurol Phys Ther. 2006;30(3):148–156. DOI: 10.1097/01.npt.0000281952.93934.6b [DOI] [PubMed] [Google Scholar]
- 135.Meadmore KL, Exell TA, Hallewell E, Hughes AM, Freeman CT, Kutlu M, et al. The application of precisely controlled functional electrical stimulation to the shoulder, elbow and wrist for upper limb stroke rehabilitation: A feasibility study. J Neuroeng Rehabil. 2014;11(1). DOI: 10.1186/1743-0003-11-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ates S, Mora-Moreno I, Wessels M, Stienen AHA.. Combined active wrist and hand orthosis for home use: Lessons learned. IEEE Int Conf Rehabil Robot (ICORR). 2015: 398–403. DOI: 10.1109/ICORR.2015.7281232 [DOI]
- 137.Amirabdollahian F, Ates S, Basteris A, Cesario A, Buurke J, Hermens H, et al. Design, development and deployment of a hand/wrist exoskeleton for home-based rehabilitation after stroke - SCRIPT project. Robotica. 2014;32(8):1331–1346. DOI: 10.1017/S0263574714002288 [DOI] [Google Scholar]
- 138.Palmcrantz S, Plantin J, Borg J.. Factors affecting the usability of an assistive soft robotic glove after stroke or multiple sclerosis. J Rehabil Med. 2020;52(3). DOI: 10.2340/16501977-2650 [DOI] [PubMed] [Google Scholar]
- 139.Kaneishi D, Matthew RP, Leu JE, O'Donnell J, Zhang B, Tomizuka M, et al. Hybrid control interface of a semi-soft assistive glove for people with spinal cord injuries. IEEE Int Conf Rehabil Robot. 2019;132–138. DOI: 10.1109/ICORR.2019.8779427 [DOI] [PubMed]
- 140.Jeong J, Hyeon K, Han J, Park CH, Ahn SY, Bok SK, et al. Wrist assisting soft wearable robot with stretchable coolant vessel integrated SMA muscle. IEEE/ASME Trans Mechatron. 2022; 27(2):1046–1058. DOI: 10.1109/TMECH.2021.3078472 [DOI] [Google Scholar]
- 141.Jeong U, In H-K, Cho K-J.. Implementation of various control algorithms for hand rehabilitation exercise using wearable robotic hand. Intell Serv Robot. 2013;6(4):181–189. DOI: 10.1007/s11370-013-0135-5 [DOI] [Google Scholar]
- 142.Wang JB, Liu ZY, Fei YQ.. Design and testing of a soft rehabilitation glove integrating finger and wrist function. J Mech Robot - Trans ASME. 2019;11(1). DOI: 10.1115/1.4041789 [DOI] [Google Scholar]
- 143.Liu Q, Liu Y, Li Y, Zhu C, Meng W, Ai Q, et al. Path planning and impedance control of a soft modular exoskeleton for coordinated upper limb rehabilitation. Front Neurorobot. 2021. DOI: 10.3389/fnbot.2021.745531 [DOI] [PMC free article] [PubMed]
- 144.Koizumi S, Chang TH, Nabae H, Endo G, Suzumori K, Mita M, et al. Soft robotic gloves with thin mckibben muscles for hand assist and rehabilitation. IEEE/SICE Int Symp Syst Integr (SII). 2020;93–98. DOI: 10.1109/SII46433.2020.9025832 [DOI]
- 145.Hussain I, Salvietti G, Spagnoletti G, Prattichizzo D.. The soft-sixthfinger: A wearable EMG controlled robotic extra-finger for grasp compensation in chronic stroke patients. IEEE Robot Autom Lett. 2016;1(2):1000–1006. DOI: 10.1109/LRA.2016.2530793 [DOI] [Google Scholar]
- 146.Hussain I, Spagnoletti G, Salvietti G, Prattichizzo D.. Toward wearable supernumerary robotic fingers to compensate missing grasping abilities in hemiparetic upper limb. Int J Robot Res. 2017; 36(13-14):1414–1436. DOI: 10.1177/0278364917712433 [DOI] [Google Scholar]
- 147.Rossero M, Ciullo AS, Grioli G, Catalano MG, Bicchi A.. Analysis of compensatory movements using a supernumerary robotic hand for upper limb assistance. Front Robot AI. 2020;7. DOI: 10.3389/frobt.2020.587759 [DOI] [PMC free article] [PubMed]
- 148.Wu FY, Asada HH.. Implicit and intuitive grasp posture control for wearable robotic fingers: A data-driven method using partial least squares. IEEE Trans Rob. 2016;32(1):176–186. DOI: 10.1109/TRO.2015.2506731 [DOI] [Google Scholar]
- 149.Guo N, Wang XJ, Duanmu DH, Huang X, Li XD, Fan YL, et al. SSVEP-based brain computer interface controlled soft robotic glove for post-stroke hand function rehabilitation. IEEE Trans Neural Syst Rehabil Eng. 2022;30:1737–1744. DOI: 10.1109/TNSRE.2022.3185262 [DOI] [PubMed] [Google Scholar]
- 150.Greco C, Weerakkody TH, Cichella V, Pagnotta L, Lamuta C.. Lightweight bioinspired exoskeleton for wrist rehabilitation powered by twisted and coiled artificial muscles. Robotics. 2023;12(1). DOI: 10.3390/robotics12010027 [DOI] [Google Scholar]
- 151.Zhao L, Liu ZB, Sun QF, Li HL.. Effect of transcranial direct current stimulation combined with a smart hand joint training device on hand dysfunction in patients with early stroke. Folia Neuropathologica. 2022;60(2):177–184. DOI: 10.5114/fn.2022.117534 [DOI] [PubMed] [Google Scholar]
- 152.Ostadabbas S, Housley SN, Sebkhi N, Richards K, Wu D, Zhang Z, et al. Tongue-controlled robotic rehabilitation: A feasibility study in people with stroke. J Rehabil Res Dev. 1006;53(6):989–1006. DOI: 10.1682/JRRD.2015.06.0122 [DOI] [PubMed] [Google Scholar]
- 153.Pieber K, Herceg M, Wick F, Grim-Stieger M, Bernert G, Paternostro-Sluga T.. Functional electrical stimulation combined with botulinum toxin type A to improve hand function in children with spastic hemiparesis - a pilot study. Wiener Klinische Wochenschrift. 2011;123(3-4):100–105. DOI: 10.1007/s00508-010-1518-7 [DOI] [PubMed] [Google Scholar]
- 154.Haarman CJW, Hekman EEG, Maas EM, Rietman JS, Van Der Kooij H.. Design and feasibility of the T-GRIP thumb exoskeleton to support the lateral pinch grasp of spinal cord injury patients. IEEE Int Conf Rehabil Robot. 2022;1–6. DOI: 10.1109/ICORR55369.2022.9896595 [DOI] [PubMed]
- 155.Prochazka A, Gauthier M, Wieler M, Kenwell Z.. The bionic glove: An electrical stimulator garment that provides controlled grasp and hand opening in quadriplegia. Arch Phys Med Rehabil. 1997;78(6):608–614. DOI: 10.1016/S0003-9993(97)90426-3 [DOI] [PubMed] [Google Scholar]
- 156.Rahman A, Al-Jumaily A.. Design and development of a bilateral therapeutic hand device for stroke rehabilitation. Int J Adv Robot Syst. 2013;10(12):405. DOI: 10.5772/56809 [DOI] [Google Scholar]
- 157.Hsu HY, Yang KC, Yeh CH, Lin YC, Lin KR, Su FC, et al. A tenodesis-induced-grip exoskeleton robot (TIGER) for assisting upper extremity functions in stroke patients: A randomized control study. Disabil Rehabil. 2022;44(23):7078–7086. DOI: 10.1080/09638288.2021.1980915 [DOI] [PubMed] [Google Scholar]
- 158.Kuo LC, Yang KC, Lin YC, Lin YC, Yeh CH, Su FC, et al. Internet of things (IoT) enables robot-assisted therapy as a home program for training upper limb functions in chronic stroke: A randomized control crossover study. Arch Phys Med Rehabil. 2023;104(3):363–371. DOI: 10.1016/j.apmr.2022.08.976 [DOI] [PubMed] [Google Scholar]
- 159.Meng QL, Xie QL, Shao HC, Cao WJ, Wang F, Wang LL, et al. Pilot study of a powered exoskeleton for upper limb rehabilitation based on the wheelchair. Biomed Res Int. 2019. DOI: 10.1155/2019/9627438 [DOI] [PMC free article] [PubMed]
- 160.Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM, et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;533:247–250. DOI: 10.1038/nature17435 [DOI] [PubMed] [Google Scholar]
- 161.Kang YS, Park YG, Lee BS, Park HS.. Biomechanical evaluation of wrist-driven flexor hinge orthosis in persons with spinal cord injury. J Rehabil Res Dev. 2013;50(8):1129–1137. DOI: 10.1682/JRRD.2012.10.0189 [DOI] [PubMed] [Google Scholar]
- 162.Borboni A, Faglia R, Mor M.. Compliant device for hand rehabilitation of stroke patient. ASME Bienn Conf Eng Syst Des Anal, ESDA. 2014;1. DOI: 10.1115/ESDA2014-20081 [DOI]
- 163.Zhou Y, Desplenter T, Chinchalkar S, Trejos AL.. A wearable mechatronic glove for resistive hand therapy exercises. IEEE Int Conf Rehabil Robot. 2019;1097–1102. DOI: 10.1109/ICORR.2019.8779502 [DOI] [PubMed]
- 164.Gopura R, Kiguchi K.. An exoskeleton robot for human forearm and wrist motion assist-hardware design and EMG-based controller. J Adv Mech Des Syst Manuf. 2008;2(6):1067–1083. DOI: 10.1299/jamdsm.2.1067 [DOI] [Google Scholar]
- 165.Gatto A, Carey S, Sundarrao S.. Design and development of a wrist-hand orthosis for individuals with a spinal cord injury [thesis]. Gerontech. 2018;17:104. DOI: 10.4017/gt.2018.17.s.101.00 [DOI] [Google Scholar]
- 166.Jovičić NS, Saranovac LV, Popović DB.. Wireless distributed functional electrical stimulation system. J Neuroeng Rehabil. 2012;9(1). DOI: 10.1186/1743-0003-9-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wheeler CA, Peckham PH.. Wireless wearable controller for upper-limb neuroprosthesis. J Rehabil Res Dev. 2009;46(2):243–256. DOI: 10.1682/JRRD.2008.03.0037 [DOI] [PubMed] [Google Scholar]
- 168.Higuma T, Kiguchi K, Arata J.. Low-profile two-degree-of-freedom wrist exoskeleton device using multiple spring blades. IEEE Robot Autom Lett. 2018;3(1):305–311. DOI: 10.1109/LRA.2017.2739802 [DOI] [Google Scholar]
- 169.Fulton PV, Lohlein S, Paredes-Acuna N, Berberich N, Cheng G.. Wrist exoskeleton design for pronation and supination using mirrored movement control. Int Conf Adv Robot. 2021: 575–580. DOI: 10.1109/ICAR53236.2021.9659397 [DOI]
- 170.Triandafilou KM, Ochoa J, Kang X, Fischer HC, Stoykov ME, Kamper DG.. Transient impact of prolonged versus repetitive stretch on hand motor control in chronic stroke. Top Stroke Rehabil. 2011;18(4):316–324. DOI: 10.1310/tsr1804-316 [DOI] [PubMed] [Google Scholar]
- 171.Popović DB. Advances in functional electrical stimulation (FES). J Electromyogr Kinesiol. 2014;24(6):795–802. DOI: 10.1016/j.jelekin.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 172.Lohr KN. Outcome measurement: Concepts and questions. Inquiry. 1988;25(1):37–50. https://www.jstor.org/stable/29771929 [PubMed] [Google Scholar]
- 173.Santisteban L, Térémetz M, Bleton JP, Baron JC, Maier MA, Lindberg PG.. Upper limb outcome measures used in stroke rehabilitation studies: A systematic literature review. PLoS One. 2016;11(5):e0154792. DOI: 10.1371/journal.pone.0154792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ashford S, Slade M, Malaprade F, Turner-Stokes L.. Evaluation of functional outcome measures for the hemiparetic upper limb: A systematic review. J Rehabil Med. 2008;40(10):787–795. DOI: 10.2340/16501977-0276 [DOI] [PubMed] [Google Scholar]
- 175.Lamers I, Kelchtermans S, Baert I, Feys P.. Upper limb assessment in multiple sclerosis: a systematic review of outcome measures and their psychometric properties. Arch Phys Med Rehabil. 2014;95(6):1184–1200. DOI: 10.1016/j.apmr.2014.02.023 [DOI] [PubMed] [Google Scholar]
- 176.Klingels K, Mayhew AG, Mazzone ES, Duong T, Decostre V, Werlauff U, et al. Development of a patient-reported outcome measure for upper limb function in Duchenne muscular dystrophy: DMD Upper Limb PROM. Dev Med Child Neurol. 2017; 59(2):224–231. DOI: 10.1111/dmcn.13277 [DOI] [PubMed] [Google Scholar]
- 177.Malkin RA. Barriers for medical devices for the developing world. Expert Rev Med Devices. 2007;4(6):759–763. DOI: 10.1586/17434440.4.6.759 [DOI] [PubMed] [Google Scholar]
- 178.ISO13485:2016-Medical devices — Quality management systems — Requirements for regulatory purposes [Internet]. Standardization. IOf. 2016. [cited 2024, August 12]. Available from: https://www.iso.org/standard/59752.html [Google Scholar]
- 179.Arnould A, Hendricusdottir R, Bergmann J.. The complexity of medical device regulations has increased, as assessed through data-driven techniques. Prosthes. 2021;3(4):314–330. DOI: 10.3390/prosthesis3040029 [DOI] [Google Scholar]
- 180.Martin JL, Norris BJ, Murphy E, Crowe JA.. Medical device development: The challenge for ergonomics. Appl Ergon. 2008; 39(3):271–283. DOI: 10.1016/j.apergo.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 181.Bergsland J, Elle OJ, Fosse E.. Barriers to medical device innovation. Med Devices (Auckl). 2014;7:205–209. DOI: 10.2147/MDER.S43369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sathian B, Asim M, Banerjee I, Pizarro AB, Roy B, Van Teijlingen ER, et al. Impact of COVID-19 on clinical trials and clinical research: A systematic review. Nepal J Epidemiol. 2020; 10(3):878–887. DOI: 10.3126/nje.v10i3.31622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Moynihan R, Sanders S, Michaleff ZA, Scott AM, Clark J, To EJ, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: A systematic review. BMJ Open. 2021;11(3):e045343. DOI: 10.1136/bmjopen-2020-045343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pokhrel S, Chhetri R.. A literature review on impact of COVID-19 pandemic on teaching and learning. High Educ Future. 2021; 8(1):133–141. DOI: 10.1177/234763112098348 [DOI] [Google Scholar]
- 185.Wormald JC, Geoghegan L, Sierakowski K, Price A, Peters M, Jain A, et al. Site-specific patient-reported outcome measures for hand conditions: Systematic review of development and psychometric properties. Plast Reconstr Surg Glob Op. 2019;7(5). DOI: 10.1097/GOX.0000000000002256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bengler K, Harbauer CM, Fleischer M.. Exoskeletons: A challenge for development. Wearable Technol. 2023;4:e1. DOI: 10.1017/wtc.2022.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Martinez-Hernandez U, Metcalfe B, Assaf T, Jabban L, Male J, Zhang D.. Wearable assistive robotics: A perspective on current challenges and future trends. Sensors. 2021;21(20). DOI: 10.3390/s21206751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Orekhov G, Fang Y, Cuddeback CF, Lerner ZF.. Usability and performance validation of an ultra-lightweight and versatile untethered robotic ankle exoskeleton. J Neuroeng Rehabil. 2021; 18(1):163. DOI: 10.1186/s12984-021-00954-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhu M, Biswas S, Dinulescu SI, Kastor N, Hawkes EW, Visell Y.. Soft, wearable robotics and haptics: Technologies, trends, and emerging applications. Proc IEEE. 2022;110(2):246–272. DOI: 10.1109/JPROC.2021.3140049 [DOI] [Google Scholar]
- 190.Artemiadis P, Thalman C.. A review of soft wearable robots that provide active assistance: Trends, common actuation methods, fabrication, and applications. Wearable Technol. 2020;1:e3. DOI: 10.1017/wtc.2020.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Prattichizzo D, Pozzi M, Baldi TL, Malvezzi M, Hussain I, Rossi S, et al. Human augmentation by wearable supernumerary robotic limbs: review and perspectives. Prog Biomed Eng. 2021;3(4):042005. DOI: 10.1088/2516-1091/ac2294 [DOI] [Google Scholar]
- 192.Liao Z, Chen B, Chang T, Zheng Q, Liu K, Lv J.. A human augmentation device design review: Supernumerary robotic limbs. Industrial Robot. 2023;50(2), 256–274. DOI: 10.1108/IR-03-2022-0079 [DOI] [Google Scholar]
- 193.Konrad P.. The ABC of EMG - A practical introduction to kinesiological electromyography [Internet]. NORAXON. 2005; [cited 2024, August 12]. Available from: https://hermanwallace.com/download/The_ABC_of_EMG_by_Peter_Konrad.pdf [Google Scholar]
- 194.Singh RM, Chatterji S, Kumar A.. Trends and challenges in EMG based control scheme of exoskeleton robots-a review. Int J Sci Eng Res. 2012;3(8):933–940. Available from: https://www.ijser.org/researchpaper/Trends-and-Challenges-in-EMG-Based-Control-Scheme-of-Exoskeleton-Robots-A-Review.pdf [Google Scholar]
- 195.Baud R, Manzoori AR, Ijspeert A, Bouri M.. Review of control strategies for lower-limb exoskeletons to assist gait. J Neuroeng Rehabil. 2021;18(1):119. DOI: 10.1186/s12984-021-00906-3 [DOI] [PMC free article] [PubMed] [Google Scholar]