Abstract
Introduction
Despite shared features with pulmonary arterial hypertension, acute vasoreactivity in pulmonary hypertension with interstitial lung disease (PH-ILD) is not well characterised, including its potential ability to predict therapeutic outcomes. We sought to determine whether acute vasoreactivity in PH-ILD to oxygen (O2) and inhaled nitric oxide (iNO) predicts inhaled treprostinil (iTre) outcomes.
Materials and methods
In this retrospective cohort analysis, we identified treatment-naive PH-ILD patients with vasoreactivity testing using O2 and O2+iNO. 6-month iTre outcome was assessed. “iTre improvement” required fulfilment of criteria on objective assessment without clinical worsening. “iTre failure” was defined by lack of objective improvement or a clinical worsening event.
Results
Among 75 PH-ILD patients, mean pulmonary arterial pressure (mPAP) decreased by −3 mmHg (−12.6%) and pulmonary vascular resistance (PVR) by −1.3 WU (−23.7%) with O2+iNO. With O2+iNO, mPAP decreased ≥10 mmHg to <40 mmHg in four patients (5.3%) and 23 (30.7%) had ≥20% reduction in mPAP and PVR. Among 33 iTre-treated patients, there were 13 improvements and 20 failures. The microvascular response, measured by distensibility, to O2 alone versus O2+iNO correlated with 6-month iTre outcome. Patients with 6-month iTre improvement had large relative distensibility increases with O2+iNO (versus failure, 76.0% versus 15.3%, p=0.004). Conversely, iTre failure was associated with increased distensibility with O2 alone (versus improvement, 26.8% versus −3.9%, p=0.045).
Conclusions
In PH-ILD, the microvascular response to O2 versus O2+iNO testing was associated with 6-month iTre outcome, likely reflecting the differential contributions of hypoxic vasoconstriction and remodelling. Acute vasoreactivity may inform therapeutic decision-making in PH-ILD.
Shareable abstract
In PH-ILD, the acute microvascular response to O2 versus O2+iNO testing predicted 6-month iTre outcome. Acute vasoreactivity exists in PH-ILD and may inform therapeutic decision-making. https://bit.ly/3NTPV6b
Introduction
Pulmonary hypertension (PH) is classified into five groups based on the presence of cardiopulmonary and other systemic comorbidities as well as the haemodynamic profiles observed during diagnostic right heart catheterisation (RHC). Acute vasoreactivity testing with oxygen (O2) and inhaled nitric oxide (iNO) may be performed during RHC in an effort to gain insight into a patient's relative burden of hypoxia-mediated disease, reversible vasoconstriction and fixed vasculopathy [1–4]. In clinical practice, however, this assessment is generally only used routinely in pulmonary arterial hypertension (PAH) to identify a subset of patients that would be candidates for calcium channel blocker therapy. The haemodynamic responses to O2 and iNO as well as their prognostic value for therapeutic decision-making are of increasing interest in other groups of patients with pre-capillary PH, but they have not been fully explored in PH associated with interstitial lung disease (ILD) [3].
PH-ILD shares features with PAH, including pre-capillary and microvascular remodelling and dysregulated nitric oxide signalling [5, 6]. Based on the common pulmonary vascular pathology with PAH as well as the potential additional contribution of hypoxic vasoconstriction to PH-ILD, we sought to determine whether O2 and iNO vasodilator testing could predict therapeutic response to inhaled treprostinil (iTre) in PH-ILD.
Materials and methods
Data source
Treatment-naive patients aged ≥18 years with World Symposium on Pulmonary Hypertension (WSPH) Group 3 PH-ILD who underwent RHC with iNO were retrospectively identified from the Brigham and Women's Hospital Pulmonary Vascular Disease Registry (supplementary figure 1) [7–9]. The Mass General Brigham (MGB) system was also queried to identify all additional treatment-naive PH-ILD patients who underwent RHC with iNO testing and who were subsequently initiated on iTre therapy. RHC data from May 2008 to October 2022 were utilised, with follow-up terminated on 31 May 2023. The study protocol was approved by the MGB Human Research Committee (2011P000272 and 2021P001494).
Inclusion and exclusion criteria
PH was defined as mean pulmonary arterial pressure (mPAP) ≥21 mmHg, pulmonary arterial wedge pressure (PAWP) ≤15 mmHg and PVR ≥3 Wood Units (WU) [6]. The PVR threshold of ≥3 WU was selected to be consistent with contemporary trial criteria, including the INCREASE study of iTre in PH-ILD [10].
Consistent with previous clinical trials of patients with PH-ILD, patients with fibrotic diffuse parenchymal lung disease (DPLD) or combined pulmonary fibrosis and emphysema (CPFE) were included [10–14]. Patients were diagnosed with ILD based on physician consensus prior to their enrolment RHC. The diagnosis of ILD required the presence of fibrotic DPLD on chest computed tomography (CT) scan prior to the time of RHC, adjudicated in accordance with the joint consensus definition from the American Thoracic Society (ATS) and European Respiratory Society (ERS) [10, 14]. Patients with ILD were additionally required to have a restrictive defect on pulmonary function tests (PFTs), evidenced by total lung volume (TLC) or forced vital capacity (FVC) <70% predicted [14, 15]. Based on the 2022 ATS/ERS statement, CPFE was defined by coexisting emphysema ≥5% of lung volume with pulmonary fibrosis [16, 17].
The full exclusion criteria for the study cohort are detailed in the supplementary material but included pulmonary capillary wedge pressure >15 mmHg; significant (≥2+) mitral or aortic valvular regurgitation; left ventricular ejection fraction <0.50; more than mild untreated obstructive sleep apnoea; unresolved chronic thromboemboli, and pulmonary veno-occlusive disease.
Vasoreactivity assessment
Within the MGB hospital system, acute vasoreactivity is assessed with iNO. Baseline measurements are first obtained, preferably breathing room air (RA). If possible, supplemental O2 therapy are stopped to obtain baseline measurements. The current protocol entails the serial addition of 1) O2 at ≥2 L·min−1 to resolve any hypoxaemia and 2) iNO at 40–80 ppm in combination with O2 (INOMAX, Mallinckrodt Pharmaceuticals, Hazelwood, MO, USA) [18]. Haemodynamics (mPAP, PAWP, cardiac output (CO) and PVR) are re-measured 5 min after the initiation of each intervention, when the patient has achieved a haemodynamic steady-state. CO was measured by thermodilution or the assumed Fick equation, with the same method utilised throughout the case to ensure that CO changes were not related to measurement technique. Stroke volume (SV) was computed as CO divided by heart rate.
Three states are generated to examine the distinct effects of the individual inhaled pulmonary vasodilators. “O2” describes the haemodynamic state following the initiation of supplemental O2, as the change referent to RA. It therefore identifies the impact of O2 alone. Patients who were on O2 for initial haemodynamics are therefore not reflected in this measure.
“O2+iNO” describes the state on the combination of O2 and iNO. It is measured as the haemodynamic difference with O2+iNO from O2 alone, such that it isolates the effects of iNO addition. Finally, the “baseline to iNO” state reflects the overall effect of iNO, and it is reported as the change from the baseline measurements (on RA or O2) to the addition of iNO. A small number of patients received iNO via the carrier of medical air instead of via supplemental O2; this subgroup is included only in the “baseline to iNO” category.
Indices of pulmonary vascular stiffness
Right ventricular (RV) afterload is the major determinant of outcomes in PH. It consists of both static and pulsatile elements that can be further classified by their location in the pulmonary circuit. Pulmonary arterial (PA) compliance measures the global ability of the entire PA circuit to respond to pulsatile flow and it is computed as SV divided by PA pulse pressure (systolic–diastolic PA pressure) [19, 20]. PVR is a static metric describing resistance in the small distal arteries [21]. Distensibility (α) is a measure of the microvasculature that quantifies its response to alterations in pulmonary blood flow, such as with physiological intervention, including exercise or passive leg lift [1, 22, 23]. It is calculated at a constant haematocrit as the change in pulmonary vessel diameter for every mmHg rise in transmural wall pressure [19, 23, 24]:
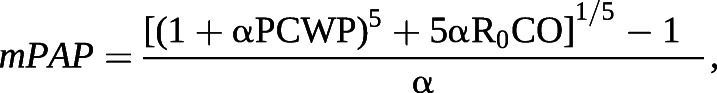
where R0 is the total pulmonary resistance; it can be calculated via solution for α or can be identified via a published online calculator [25]. Distensibility can also be pharmacologically modified with selective pulmonary vasodilator therapy and as we considered each inhaled vasodilator to be a medical intervention, we examined the related change between single-point values [26–28].
iTre regimen
Given the intrinsic ventilation–perfusion matching advantages of aerosolised therapies, we examined outcomes among patients who were treated with iTre. After RHC, candidacy for iTre treatment was determined at the discretion of the treating physician. iTre was started at 3 breaths (18 µg) four times daily and uptitrated as tolerated to ≥9 breaths per session (54 µg) [29].
iTre outcomes
iTre outcome was evaluated at 6 months with a composite end-point. Successful treatment with iTre (“iTre improvement”) required fulfilment of pre-specified criteria on objective assessment without a clinical worsening event [30]. Four objective tests were considered acceptable:
RHC: improvement in PVR by ≥30% [31].
Echocardiography: improvement in RV systolic pressure by ≥10 mmHg or ≥15% [32–34].
6-min walk test (6MWT): improvement in absolute distance by ≥33 m, as consistent with recently published work in PAH [35].
Submaximal exercise test: improvement by ≥10% in ≥2 of: VE/VCO2 slope, oxygen uptake efficiency slope, resting end-tidal partial pressure of carbon dioxide and peak predicted VO2 [36, 37].
If a patient underwent two or more forms of unique objective testing in follow-up, all were utilised for adjudication; iTre improvement required satisfaction of the above criteria on any available tests. A clinical worsening event was defined as one of 1) unsatisfactory response requiring vasodilator addition or iTre cessation; 2) cardiopulmonary hospitalisation; 3) lung transplantation ≥3 months; or 4) death [38, 39].
“iTre failure” was defined by lack of improvement on objective assessment or by a clinical worsening event. Patients with iTre nonadherence or who died, had a lung transplant or were lost to follow-up before optimal iTre uptitration were excluded.
Statistical analysis
Continuous variables are shown as mean±sd or median (interquartile range). Categorical variables are given as n (%). Differences in characteristics and haemodynamics were assessed via chi-squared, Wilcoxon rank-sum or t-tests depending on variable distribution and type. Receiver operating characteristic (ROC) curve analysis was used to assess the ability of distensibility to predict iTre response. Analyses were performed in SAS 9.4 (SAS Institute, Cary NC, USA), with graphs constructed in Prism 9.2 (GraphPad Software, La Jolla, CA, USA).
Results
Baseline characteristics
We first sought to determine the degree and frequency of response to vasodilator testing among a sample of PH-ILD patients evaluated at our institution. There were 75 PH-ILD patients who underwent acute vasodilator testing, of whom 22 (29.3%) had CPFE (supplementary figure 1). PH-ILD patients were predominantly male with moderate restriction and severely reduced diffusing capacity for carbon monoxide (DLCO) on PFTs (table 1). Baseline haemodynamics were consistent with moderate-to-severe PH (table 1). A total of 50 patients required intermittent or continuous home O2 therapy prior to RHC. Of note, continuous O2 supplementation was stopped to obtain baseline measurements on RA in 15 patients.
TABLE 1.
Baseline characteristics and haemodynamics in pulmonary hypertension with interstitial lung disease
| Variable | |
|---|---|
| Subjects, n | 75 |
| Age, years | 70.2 (60.1 to 75.2) |
| Male gender | 38 (50.7) |
| Body mass index, kg·m−2 | 27.3±5.0 |
| Home oxygen therapy, intermittent or continuous | 50 (66.7) |
| Interstitial lung disease | 53 (70.7) |
| Idiopathic pulmonary fibrosis | 24 (32.0) |
| Connective tissue disease-related | 19 (25.3) |
| Hypersensitivity pneumonitis | 3 (4.0) |
| Sarcoidosis | 2 (2.7) |
| Undifferentiated | 5 (6.7) |
| Combined pulmonary fibrosis and emphysema | 22 (29.3) |
| Comorbidities | |
| Obstructive sleep apnoea | 17 (22.7) |
| Current or former tobacco use | 50 (66.7) |
| Connective tissue disease | 23 (30.1) |
| Any home oxygen use | 50 (66.7) |
| Pulmonary function tests | |
| FEV1, % predicted | 60.6±18.4 |
| FVC, % predicted | 60.7±19.8 |
| FEV1/FVC | 81.5 (73.0 to 87.0) |
| TLC, % predicted | 61.0 (52.0 to 74.0) |
| DLCO, % predicted | 26.5 (20.0 to 34.5) |
| Baseline haemodynamics | |
| Mean RAP, mmHg | 6.0 (3.0 to 9.0) |
| RVEDP, mmHg | 9.0 (6.0 to 11.0) |
| Mean PA pressure, mmHg | 37.0 (32.0 to 45.0) |
| PAWP, mmHg | 9.0 (6.0 to 13.0) |
| CO, L·min−1 | 4.3 (3.8 to 5.0) |
| CI, L·min−1·m−2 | 2.3 (2.0 to 2.7) |
| PVR, Wood Units | 5.9 (4.9 to 8.3) |
| PA compliance, mL per mmHg# | 1.7 (1.2 to 2.2) |
| Pulmonary vascular distensibility, % per mmHg# | 0.31 (0.2 to 0.5) |
| Baseline SpO2, %¶ | 95.0 (92.0 to 98.0) |
Data are presented as mean±sd, median (interquartile range) or number (%), unless stated otherwise. Missing data (listed as variable, n) are FEV1, 11; FVC, 9; TLC, 29; DLCO, 29; SvO2, 1; and SpO2, 4. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; TLC: total lung capacity; DLCO: diffusing capacity for carbon monoxide; RAP: right atrial pressure; RVEDP: right ventricular end diastolic pressure; PA: pulmonary artery; PAWP: pulmonary arterial wedge pressure; CO: cardiac output; CI: cardiac index; PVR: pulmonary vascular resistance. #: reference equations are as follows:
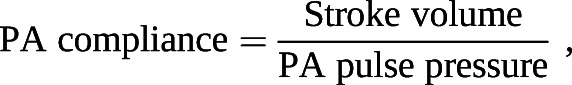
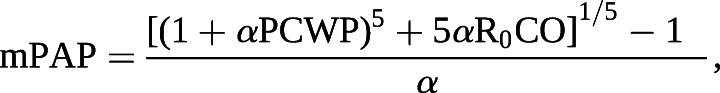 where α is the pulmonary vascular distensibility and R0 is the total pulmonary resistance. ¶: includes patients with baseline SpO2 measurement on home supplemental O2.
where α is the pulmonary vascular distensibility and R0 is the total pulmonary resistance. ¶: includes patients with baseline SpO2 measurement on home supplemental O2.
Acute vasoreactivity in PH-ILD
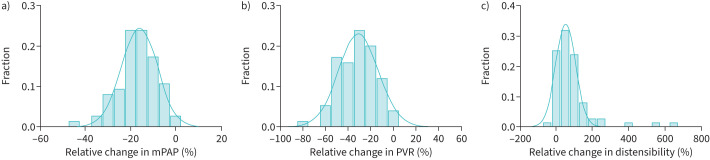
In PH-ILD, there were a wide range of responses to vasoreactivity testing. The overall change from baseline to iNO led to absolute median reductions in mPAP and PVR of 6.0 mmHg and 1.8 WU, respectively, which corresponded to relative values of 16.3% and 28.7% (figure 1). There was an absolute median increase of 0.15% per mmHg in distensibility (53.0%; figure 1c). With the initiation of O2 alone, there were small relative reductions in mPAP and PVR (supplementary table 1) but with O2+iNO, there were absolute median reductions in mPAP and PVR of 4.0 mmHg (−11.3%) and 1.4 WU (−23.4%).
FIGURE 1.
Distributions of vasoreactive response in pulmonary hypertension with interstitial lung disease, showing overall change from baseline with inhaled nitric oxide. a) Mean pulmonary arterial pressure (mPAP). b) Pulmonary vascular resistance (PVR). c) Distensibility.
We examined the frequency of PH-ILD patients meeting PAH-specific vasoreactivity definitions with the addition of O2+iNO. Four patients (5.3%) met the classic criteria for vasoreactivity of mPAP ≥10 mmHg reduction to <40 mmHg. There were 23 patients (30.7%) with a ≥20% reduction in both mPAP and PVR.
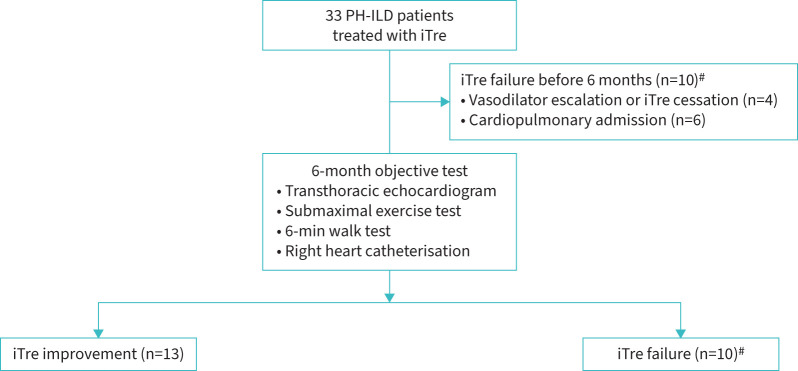
Acute vasoreactivity and outcomes in iTre-treated PH-ILD
Among the subset of PH-ILD patients who received iTre (n=33), we sought to determine whether the acute vasodilator response was associated with iTre outcomes. 33 PH-ILD patients were initiated on iTre following their diagnostic RHC with vasoreactivity assessment. The median time to iTre initiation after diagnostic RHC was 1.7 months (interquartile range, 0.5–3.3 months). Within this subset, there were 18 patients in whom oxygen was not stopped or was not used as an intermediary to iNO testing. As such, only 15 patients had haemodynamics measured from RA to O2.
Patients in this cohort had moderate-to-severe PH based on an mPAP of 41.8 mmHg (±12.0 mmHg) and PVR of 6.8 WU (4.8–8.6 WU) (supplementary table 2). Due to pulmonary vascular disease severity or coexisting Raynaud's phenomenon, six patients received concurrent PDE5i therapy.
13 patients met the criteria for iTre improvement, whereas there were 20 patients who failed iTre. Figure 2 displays a flow diagram of outcomes among iTre-treated patients over the 6-month treatment period. Objective testing in the iTre improvement cohort included two RHCs, seven transthoracic echocardiograms, two 6MWTs and four submaximal exercise tests. Changes between pre- and post-iTre objective metrics are shown in supplementary table 2. There were no apparent differences in clinical characteristics or haemodynamics, including initial PVR and distensibility between iTre improvement and failure cohorts (supplementary table 3).
FIGURE 2.
Flow diagram of inhaled treprostinil (iTre) outcomes in pulmonary hypertension with interstitial lung disease (PH-ILD). #: 20 PH-ILD patients met iTre failure criteria. 10 failed before the 6-month evaluation mark. 10 additional patients failed due to lack of objective improvement.
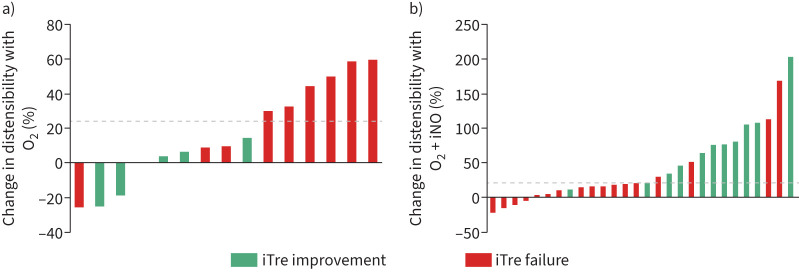
The acute vasodilator response at initial RHC was associated with the 6-month outcome in iTre-treated patients. Specifically, patterns of microvascular response to O2 only and O2+iNO varied between iTre improvement and failure cohorts, as shown in waterfall plots in figure 3. Patients with longitudinal improvement on iTre had large increases in distensibility with O2+iNO (versus iTre failure, relative change 76.0% versus 15.3%, p=0.004). These patients had no change in distensibility with O2 alone. Conversely, there were larger increases in distensibility with O2 only in patients who failed iTre (versus iTre improvement, 26.8% versus −3.9%, p=0.045). In the iTre failure cohort, the further addition of iNO caused minimal change in distensibility.
FIGURE 3.
Percentage change in distensibility in reference to 6-month inhaled treprostinil (iTre) outcome with a) O2 only and b) O2 + inhaled nitric oxide (iNO). Each bar represents an individual patient. Dotted line denotes the optimal cut-off determined by the Youden index.
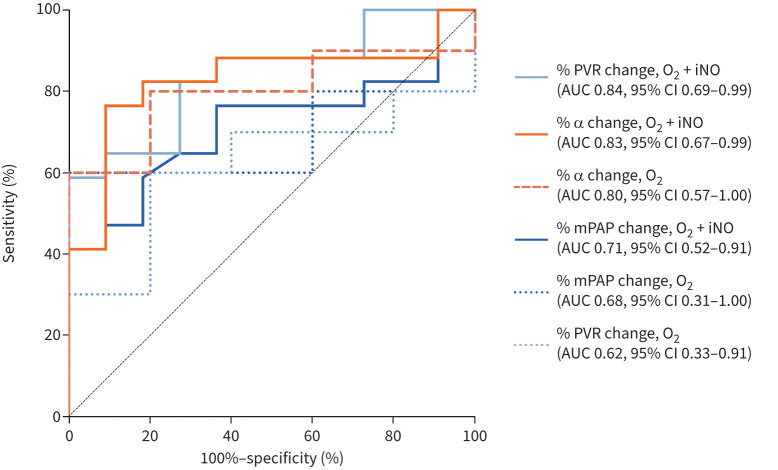
ROC analysis was used to assess the ability of acute vasoreactivity to predict the outcome of iTre-treated patients (figure 4). The performance of distensibility changes with O2+iNO was similar to the change in PVR with O2+iNO and superior to any mPAP measure. The area under the curve (AUC) of the distensibility change with O2 was 0.80, which exceeded the AUC of PVR change with O2 alone. The AUC of the distensibility change with O2+iNO was 0.83, with an optimal cut-off of ≥20.9% for predicting improvement with iTre.
FIGURE 4.
Receiver operating characteristic curve analysis for ability of acute vasoreactive measures to predict 6-month inhaled treprostinil outcome in pulmonary hypertension with interstitial lung disease, including distensibility (α) and traditional haemodynamics. PVR: pulmonary vascular resistance; iNO: inhaled nitric oxide; AUC: area under the curve; mPAP: mean pulmonary arterial pressure.
Finally, the relationship between changes in additional markers of RV afterload with 6-month outcome in iTre-treated patients was examined (tables 2 and 3). The change in compliance with acute exposure to inhaled vasodilators did not correlate with 6-month iTre outcome. Furthermore, the response of the small arteries to vasodilators, reflected by PVR, did not consistently correlate with iTre outcome.
TABLE 2.
Acute vasoreactive response (absolute change) stratified by 6-month inhaled treprostinil improvement versus failure
| Baseline to iNO | O2 | O2+iNO | ||||
|---|---|---|---|---|---|---|
| Improve (n=13) | Fail (n=20) | Improve (n=5) | Fail (n=10) | Improve (n=11) | Fail (n=17) | |
| mPAP, mmHg | −7.8±3.5 | −5.2±3.6* | −4.0±3.0 | −2.4±2.0 | −6.1±2.3 | −3.8±2.8* |
| PAWP, mmHg | 1.0±3.7 | 0.5±3.3 | −2.4±1.7 | 0.9±2.1* | 2.0 (−1.0 to 3.0) | −1.0 (−2.0 to 1.0) |
| CI, L·min−1·m−2 | 0.1 (0 to 0.2) | 0.2 (0 to 0.4) | 0.1±0.1 | 0.1±0.3 | 0.1±0.2 | 0.1±0.1 |
| PVR, Wood Units | −1.8 (−3.2 to −1.6) | −1.6 (−2.0 to −0.9) | −0.7 (−0.8 to −0.2) | −0.7 (−1.0 to 0.2) | −2.0 (−3.2 to −1.5) | −1.0 (−1.7 to −0.5)* |
| Compliance, mL per mmHg | 0.6±0.5 | 0.5±0.4 | 0.2 (0.1 to 1.1) | 0.5 (0.1 to 0.6) | 0.5±0.5 | 0.3±0.3 |
| Distensibility, % per mmHg | 0.14 (0.09 to 0.17) | 0.08 (0.04 to 0.17) | −0.03±0.07 | 0.07±0.08* | 0.15 (0.09 to 0.27) | 0.05 (0 to 0.11)* |
Data are presented as mean±sd or median (interquartile range). iNO: inhaled nitric oxide; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure; CI: cardiac index; PVR: pulmonary vascular resistance. *Significant at p<0.05.
TABLE 3.
Acute vasoreactive response (relative change) stratified by 6-month inhaled treprostinil improvement versus failure
| Baseline to iNO | O2 | O2+iNO | ||||
|---|---|---|---|---|---|---|
| Improve (n=13) | Fail (n=20) | Improve (n=5) | Fail (n=10) | Improve (n=11) | Fail (n=17) | |
| mPAP, % | −17.4±7.0 | −13.0±7.3 | −11.3 (−13.2 to −6.0) | −6.6 (−8.5 to 0) | −14.3±3.5 | −10.0±6.5 |
| PAWP, % | 3.3 (−9.1 to 11.9) | 4.5 (−0.2 to 16.6) | −22.0±13.6 | 10.3±21.9* | 25.0 (−7.7 to 44.4) | −10.0 (−25.0 to 7.7)* |
| PVR, % | −28.0±12.9 | −25.4±11.6 | −7.2±8.6 | −13.7±13.0 | −26.1 (−37.1 to −22.2) | −19.4 (−23.9 to −11.8)* |
| CI, % | 8.7±20.6 | 7.0±24.2 | 2.8±7.0 | 6.8±17.2 | 6.1 (0 to 12.3) | 7.2 (0 to 10.0) |
| Compliance, % | 42.0±28.9 | 33.8±22.1 | 21.8 (17.2 to 63.1) | 27.5 (7.4 to 36.3) | 31.9±25.8 | 18.4±15.4 |
| Distensibility, % | 45.4 (31.9 to 108.0) | 26.0 (16.5 to 81.7) | −3.9±17.1 | 26.8±28.1* | 76.0 (34.0 to 105.3) | 15.3 (3.0 to 20.0)* |
Data are presented as mean±sd or median (interquartile range). Missing data (listed as variable, n) are compliance, 2. iNO: inhaled nitric oxide; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure; PVR: pulmonary vascular resistance; CI: cardiac index.
*Significant at p<0.05.
Sensitivity analysis was performed to examine results after excluding the six patients who received concurrent PDE5i therapy. Results were similar (supplementary table 4).
Discussion
Within a treatment-naive PH-ILD cohort, there were a range of acute vasoreactive responses to O2 and iNO, and in a subset of patients treated with iTre, we observed that acute vasoreactivity during diagnostic RHC may represent a novel biomarker to predict longitudinal treatment outcome. Moreover, we observed that the response of the microvasculature to acute O2 and iNO testing was associated with distinct therapeutic outcomes [7, 24, 26]. Patients who had increased distensibility with the selective pulmonary vasodilator iNO—a dysregulated pathway in PH-ILD vasculopathy—tended to have improvement at 6 months with iTre. Conversely, patients who had distensibility increases with O2 alone did not improve with iTre.
Despite its potential utility across PH groups, acute vasoreactivity has been primarily studied in select PAH subsets. Our results suggest that it may have broader utility outside of PAH such that in patients with PH-ILD, initial vasoreactivity measures may be helpful to inform therapeutic decision-making. While the current definition of vasoreactivity relies on static global haemodynamics, we further observed that the differential microvascular response to acute O2 and iNO testing most accurately classified the 6-month iTre outcome into two groups. First, there was a cohort that demonstrated an increase in distensibility when challenged with iNO and who showed objective clinical improvement with iTre at 6 months. These patients had no significant change in distensibility when challenged with O2. Separately, there was another group with an increase in distensibility to O2—but minimal change with iNO—who failed iTre therapy.
As a low-resistance system, normal pulmonary circulation consists of serial vessels that branch into the microvasculature, an important reservoir that can accommodate increases in pulmonary blood flow to mitigate detrimental pressure and volume effects on the RV. In PH, the microvasculature is the primary site of remodelling as well as of the site where O2 and iNO would have the greatest effect [1, 21, 40]. Distensibility reflects the amount of microvascular reserve [1, 22, 41, 42]. It is reduced in PH and chronic hypoxia but can be improved with long-term vasodilator therapy [26, 43].
In PH-ILD, the contributions of hypoxic vasoconstriction and true vasculopathy to abnormal RV afterload can be difficult to separate; however, this delineation holds clear management implications. Our results suggest that O2 and iNO challenges during RHC can be used to separate these components of afterload and to inform therapeutic decision-making. iNO is a selective pulmonary vasodilator targeting abnormal vascular smooth muscle tone via the cyclic guanosine monophosphate pathway. We hypothesise that the cohort with iNO-driven increases in distensibility have significant vasculopathy and as such, they benefit most from longitudinal vasodilator therapy. While iTre acts via the prostacyclin pathway, it is also a pulmonary vasodilator that is directed towards smooth muscle tone [44]. Conversely, we suspect that hypoxic vasoconstriction is likely the prevailing pathology in the subset with larger O2 distensibility improvements. Importantly, no other characteristics correlated with therapeutic response. Despite previous work identifying elevated baseline PVR as a predictor of outcomes, we did not confirm this finding, which was potentially related to the already-high PVR in this cohort [2, 10].
Interestingly, the ability of the afterload metric to predict iTre treatment response appeared to be related to its location in the pulmonary circuit. Measures that were not specific for the microvasculature—such as the global measure of PA compliance—did not correlate well with iTre outcome, a finding that is not unexpected based on the shared location of both inhaled vasodilator effect and microvascular disease. Similarly, PVR—a measure of the small resistance arteries—was inconsistently associated with iTre outcome, with a relatively poor correlation when measured on O2 alone but a significant association with O2+iNO. Distensibility provided nuanced insight into the acute vasoreactivity response and ultimate iTre outcome, such that it represents an ideal and novel parameter to inform therapeutic selection in PH-ILD.
Our study has several limitations. From a procedural perspective, our vasoreactivity protocol entails haemodynamic measurements at the end of 5-min intervals, so distensibility was not derived from multi-point plots [23]. Supplemental O2 was not able to be stopped in a subgroup of patients during vasoreactivity testing—such that the haemodynamic impact of this therapy and therefore its implications for potential vasculopathy versus hypoxic vasoconstriction could not be understood—and this may have contributed to bias. Furthermore, our vasoreactivity protocol differs from that recommended in the most recent 2022 ESC/ERS guidelines for WSPH Group 1 PAH, in which iNO doses of 10–20 ppm for 5–10 min are utilised to assess the presence of vasoreactivity [2].
In terms of cohort selection, the sample size is small, and while we used strict selection criteria to define PH-ILD, we cannot exclude selection bias potentially related to haemodynamic severity in the iTre-treated subset. There may be further selection bias related to evolution in therapeutics over the period of our study and to the degree of haemodynamic abnormality. In particular, this includes the increased use of upfront combination therapy in the iTre improvement group (five patients, compared with one in the iTre failure arm), although distensibility changes between groups remained significant even when combination therapy patients were removed. While our cohort had significant PH, there may have been an additional reduction in pulmonary vascular distensibility from cigarette smoke exposure in select patients, potentially amplified in select subjects by concurrent hypoxia [45]. Furthermore, while this is the largest representative study of acute vasoreactivity in PH-ILD, our data are derived from a strictly defined population treated at a tertiary centre and our sample size was relatively small, which may limit generalizability.
From an outcome perspective, this is a retrospective study that is hypothesis-generating and is subject to associated limitations. Distensibility is not yet validated in predicting patient outcomes in PH, and we utilised an objective end-point composed of heterogenous metrics. While stringent criteria were required to define improvement on iTre therapy, certain assessments—most notably the 6MWT—can be influenced by both parenchymal and pulmonary vascular disease. Additionally, details on changes in other medications such as diuretics or steroids were not reliably available during the retrospective follow-up period. Statistically, the AUCs of distensibility and PVR with O2+iNO were similar, although the O2 distensibility AUC exceeds that for PVR. While specificity and sensitivity of distensibility are consistent with those of a high-quality, high-value test, vasoreactivity testing is not perfect and replication in larger sample sizes with varied ILD diagnoses and PH severities is necessary. Quantitative CT may also provide additional information in examining the regional distributions of both ILD and pulmonary vascular disease, as well as their impact on the vasoreactive distensible response [46].
Conclusions
Within a treatment-naive PH-ILD cohort, the response to acute vasoreactivity testing was associated with 6-month iTre outcome. Specifically, the vasodilator response of the microvasculature to O2 and iNO, as measured by distensibility, was able to accurately distinguish 6-month iTre treatment success or failure. Patients with increases in distensibility with the selective pulmonary vasodilator iNO clinically improved with 6 months of iTre therapy. Conversely, patients who had a vasodilator response with O2 only failed iTre therapy. This proof-of-concept analysis suggests that acute vasoreactivity testing may be useful in informing therapeutic selection in PH-ILD and that the microvasculature as reflected by distensibility may be important in quantifying this response. Additional investigations, however, are needed to further confirm the findings of this hypothesis-generating analysis and to examine the utility of acute vasoreactivity and distensibility in predicting treatment response in larger PH-ILD cohorts.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00201-2024.SUPPLEMENT (499.3KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Ethics statement: The registry protocol was approved by the MGB Human Research Committee (2011P000272, 2021P001494).
Author contributions: E.M. Harder, F.N. Rahaghi, J.A. Leopold, G.R. Washko and A.B. Waxman contributed to conception and design of the work, analysis, interpretation of the data and writing the manuscript. A.B. Waxman, J.A. Leopold and E.M. Harder contributed to collection of the data. All authors approved of the final version of the manuscript and agree to be accountable for all aspects of the work.
Conflict of interest: E.M. Harder reports support for the present work from the National Institutes of Health (NIH).
Conflict of interest: J.A. Leopold reports support for the present work from NIH/National Heart, Lung, and Blood Institute (NHLBI) U01 125215 and American Heart Association AIM 19AIML34980000; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from United Therapeutics, outside the submitted work; is a chair and member of NHLBI data safety monitoring boards outside the submitted work; and is Deputy Editor of the New England Journal of Medicine outside the submitted work.
Conflict of interest: G.R. Washko reports grants or contracts from the NIH, Department of Defense and Boehringer Ingelheim, outside the submitted work; consulting fees from Pulmonx, Vertex, Janssen Pharmaceuticals and Intellia Therapeutics, outside the submitted work; participation on a data safety monitoring or advisory board for Pulmonx outside the submitted work. G.R. Washko is a cofounder and equity share holder in Quantitative Imaging Solutions, a company that provides consulting services for image and data analytics. G.R. Washko's spouse works for Biogen.
Conflict of interest: A.B. Waxman reports being a board member for Insmed outside the submitted work; and grants from United Therapeutics, Acceleron/Merck, Janssen R&D and AI Therapeutics, outside the submitted work.
Conflict of interest: The remaining authors have nothing to disclose.
Support statement: E.M. Harder is supported by T32-HL0007633 (Brigham and Women's Hospital, Division of Pulmonary and Critical Care Medicine T32 grant). J.A. Leopold is supported by NIH/National Heart, Lung and Blood Institute U01 125215 and American Heart Association AIM 19AIML34980000. There were no other sources of funding for this manuscript. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Langleben D, Orfanos SE, Giovinazzo M, et al. Acute vasodilator responsiveness and microvascular recruitment in idiopathic pulmonary arterial hypertension. Ann Intern Med 2015; 162: 154–156. doi: 10.7326/M14-1402 [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 3.Frantz RP, Leopold JA, Hassoun PM, et al. Acute vasoreactivity testing during right heart catheterization in chronic thromboembolic pulmonary hypertension: Results from the pulmonary vascular disease phenomics study. Pulm Circ 2023; 13: e12181. doi: 10.1002/pul2.12181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tea I, Hussain I. Under pressure: right heart catheterization and provocative testing for diagnosing pulmonary hypertension. Methodist Debakey Cardiovasc J 2021; 17: 92–100. doi: 10.14797/AFUI4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 1995; 333: 214–221. doi: 10.1056/NEJM199507273330403 [DOI] [PubMed] [Google Scholar]
- 6.Dotan Y, Stewart J, Gangemi A, et al. Pulmonary vasculopathy in explanted lungs from patients with interstitial lung disease undergoing lung transplantation. BMJ Open Respir Res 2020; 7: e000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh I, Oliveira RKF, Naeije R, et al. Pulmonary vascular distensibility and early pulmonary vascular remodeling in pulmonary hypertension. Chest 2019; 156: 724–732. doi: 10.1016/j.chest.2019.04.111 [DOI] [PubMed] [Google Scholar]
- 8.Singh I, Rahaghi FN, Naeije R, et al. Dynamic right ventricular-pulmonary arterial uncoupling during maximum incremental exercise in exercise pulmonary hypertension and pulmonary arterial hypertension. Pulm Circ 2019; 9: 2045894019862435. doi: 10.1177/2045894019862435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolle JJ, Waxman AB, Van Horn TL, et al. Exercise-induced pulmonary arterial hypertension. Circulation 2008; 118: 2183–2189. doi: 10.1161/CIRCULATIONAHA.108.787101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med 2021; 384: 325–334. doi: 10.1056/NEJMoa2008470 [DOI] [PubMed] [Google Scholar]
- 11.Nathan SD, Cottin V, Behr J, et al. Impact of lung morphology on clinical outcomes with riociguat in patients with pulmonary hypertension and idiopathic interstitial pneumonia: a post hoc subgroup analysis of the RISE-IIP study. J Heart Lung Transplant 2021; 40: 494–503. doi: 10.1016/j.healun.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 12.Nathan SD, Behr J, Collard HR, et al. Riociguat for idiopathic interstitial pneumonia-associated pulmonary hypertension (RISE-IIP): a randomised, placebo-controlled phase 2b study. Lancet Respir Med 2019; 7: 780–790. doi: 10.1016/S2213-2600(19)30250-4 [DOI] [PubMed] [Google Scholar]
- 13.Hoeper MM, Halank M, Wilkens H, et al. Riociguat for interstitial lung disease and pulmonary hypertension: a pilot trial. Eur Respir J 2013; 41: 853–860. doi: 10.1183/09031936.00213911 [DOI] [PubMed] [Google Scholar]
- 14.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. doi: 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med 2002; 165: 277–304. doi: 10.1164/ajrccm.165.2.ats01 [DOI] [PubMed] [Google Scholar]
- 16.Cottin V, Nunes H, Brillet PY, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J 2005; 26: 586–593. doi: 10.1183/09031936.05.00021005 [DOI] [PubMed] [Google Scholar]
- 17.Cottin V, Selman M, Inoue Y, et al. Syndrome of combined pulmonary fibrosis and emphysema: an official ATS/ERS/JRS/ALAT research statement. Am J Respir Crit Care Med 2022; 206: e7–e41. doi: 10.1164/rccm.202206-1041ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang WHW, Wilcox JD, Jacob MS, et al. Comprehensive diagnostic evaluation of cardiovascular physiology in patients with pulmonary vascular disease: insights from the PVDOMICS program. Circ Heart Fail 2020; 13: e006363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naeije R, Vachiery J-L, Yerly P, et al. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 2013; 41: 217–223. doi: 10.1183/09031936.00074312 [DOI] [PubMed] [Google Scholar]
- 20.Thenappan T, Prins KW, Pritzker MR, et al. The critical role of pulmonary arterial compliance in pulmonary hypertension. Ann Am Thorac Soc 2016; 13: 276–284. doi: 10.1513/AnnalsATS.201509-599FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chemla D, Lau EMT, Papelier Y, et al. Pulmonary vascular resistance and compliance relationship in pulmonary hypertension. Eur Respir J 2015; 46: 1178–1189. doi: 10.1183/13993003.00741-2015 [DOI] [PubMed] [Google Scholar]
- 22.Lalande S, Yerly P, Faoro V, et al. Pulmonary vascular distensibility predicts aerobic capacity in healthy individuals. J Physiol 2012; 590: 4279–4288. doi: 10.1113/jphysiol.2012.234310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozitza CJ, Dharmavaram N, Tao R, et al. Pulmonary vascular distensibility with passive leg raise is comparable to exercise and predictive of clinical outcomes in pulmonary hypertension. Pulm Circ 2022; 12: e12029. doi: 10.1002/pul2.12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linehan JH, Haworth ST, Nelin LD, et al. A simple distensible vessel model for interpreting pulmonary vascular pressure-flow curves. J Appl Physiol (1985) 1992; 73: 987–994. doi: 10.1152/jappl.1992.73.3.987 [DOI] [PubMed] [Google Scholar]
- 25.Elliott J, Menakuru N, Martin KJ, et al. iCPET calculator: a web-based application to standardize the calculation of alpha distensibility in patients with pulmonary arterial hypertension. J Am Heart Assoc 2023; 12: e029667. doi: 10.1161/JAHA.123.029667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malhotra R, Dhakal BP, Eisman AS, et al. Pulmonary vascular distensibility predicts pulmonary hypertension severity, exercise capacity, and survival in heart failure. Circ Heart Fail 2016; 9: 10.1161/CIRCHEARTFAILURE.1115.003011 e003011. doi: 10.1161/CIRCHEARTFAILURE.115.003011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace WD, Nouraie M, Chan SY, et al. Treatment of exercise pulmonary hypertension improves pulmonary vascular distensibility. Pulm Circ 2018; 8: 2045894018787381. doi: 10.1177/2045894018787381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blyth KG, Syyed R, Chalmers J, et al. Pulmonary arterial pulse pressure and mortality in pulmonary arterial hypertension. Respir Med 2007; 101: 2495–2501. doi: 10.1016/j.rmed.2007.07.004 [DOI] [PubMed] [Google Scholar]
- 29.Faria-Urbina M, Oliveira RKF, Agarwal M, et al. Inhaled treprostinil in pulmonary hypertension associated with lung disease. Lung 2018; 196: 139–146. doi: 10.1007/s00408-017-0081-7 [DOI] [PubMed] [Google Scholar]
- 30.Sitbon O, Nikkho S, Benza R, et al. Novel composite clinical endpoints and risk scores used in clinical trials in pulmonary arterial hypertension. Pulm Circ 2020; 10: 2045894020962960. doi: 10.1177/2045894020962960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ventetuolo CE, Gabler NB, Fritz JS, et al. Are hemodynamics surrogate end points in pulmonary arterial hypertension? Circulation 2014; 130: 768–775. doi: 10.1161/CIRCULATIONAHA.114.009690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seko Y, Kato T, Morimoto T, et al. A decrease in tricuspid regurgitation pressure gradient associates with favorable outcome in patients with heart failure. ESC Heart Fail 2021; 8: 2826–2836. doi: 10.1002/ehf2.13355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee M, Mercurio V, Balasubramanian A, et al. Defining minimal detectable difference in echocardiographic measures of right ventricular function in systemic sclerosis. Arthritis Res Ther 2022; 24: 146. doi: 10.1186/s13075-022-02835-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mora B, Roth D, Bernardi MH, et al. Estimation of pulmonary artery pressure with transesophageal echocardiography: an observer-blinded test accuracy study. Medicine (Baltimore) 2021; 100: e26988. doi: 10.1097/MD.0000000000026988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moutchia J, McClelland RL, Al-Naamani N, et al. Minimal clinically important difference in the 6-minute-walk distance for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2023; 207: 1070–1079. doi: 10.1164/rccm.202208-1547OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oudiz RJ, Roveran G, Hansen JE, et al. Effect of sildenafil on ventilatory efficiency and exercise tolerance in pulmonary hypertension. Eur J Heart Fail 2007; 9: 917–921. doi: 10.1016/j.ejheart.2007.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groepenhoff H, Vonk-Noordegraaf A, van de Veerdonk MC, et al. Prognostic relevance of changes in exercise test variables in pulmonary arterial hypertension. PLOS ONE 2013; 8: e72013. doi: 10.1371/journal.pone.0072013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preston IR, Badesch D, Ghofrani HA, et al. Abstract 10188: sotatercept phase 3 program design and rationale: a novel treatment for pulmonary arterial hypertension. Circulation. 2021; 144: Suppl. 1, A10188. doi: 10.1161/circ.144.suppl_1.10188 [DOI] [Google Scholar]
- 39.Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. doi: 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- 40.Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal 2008; 10: 1185–1198. doi: 10.1089/ars.2007.1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunham-Snary KJ, Wu D, Sykes EA, et al. Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest 2017; 151: 181–192. doi: 10.1016/j.chest.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roos CM, Rich GF, Uncles DR, et al. Sites of vasodilation by inhaled nitric oxide vs. sodium nitroprusside in endothelin-constricted isolated rat lungs. J Appl Physiol (1985) 1994; 77: 51–57. doi: 10.1152/jappl.1994.77.1.51 [DOI] [PubMed] [Google Scholar]
- 43.Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol 2005; 288: L419–L425. doi: 10.1152/ajplung.00162.2004 [DOI] [PubMed] [Google Scholar]
- 44.Corboz MR, Plaunt AJ, Malinin V, et al. Treprostinil palmitil inhibits the hemodynamic and histopathological changes in the pulmonary vasculature and heart in an animal model of pulmonary arterial hypertension. Eur J Pharmacol 2022; 916: 174484. doi: 10.1016/j.ejphar.2021.174484 [DOI] [PubMed] [Google Scholar]
- 45.Ferrer E, Peinado VI, Castañeda J, et al. Effects of cigarette smoke and hypoxia on pulmonary circulation in the guinea pig. Eur Respir J 2011; 38: 617–627. doi: 10.1183/09031936.00105110 [DOI] [PubMed] [Google Scholar]
- 46.Harder E, Abtin F, Nardelli P, et al. Pulmonary hypertension in idiopathic interstitial pneumonia is associated with small vessel pruning. Am J Respir Crit Care Med 2024; 209: 1170–1173. doi: 10.1164/rccm.202312-2343LE [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00201-2024.SUPPLEMENT (499.3KB, pdf)