Abstract
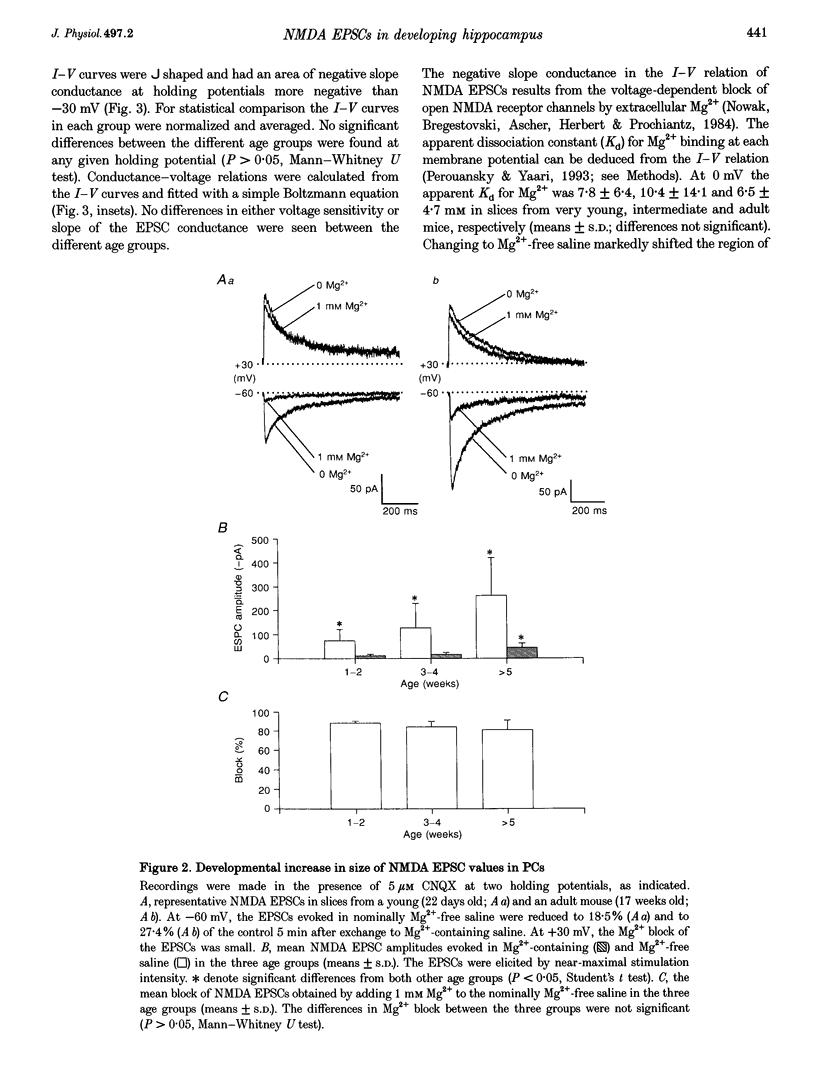
1. Whole-cell patch-clamp techniques were used to record pharmacologically isolated NMDA receptor-mediated EPSCs (NMDA EPSCs) from CA1 pyramidal cells (PCs) in hippocampal slices from 4-day-old to 36-week-old mice, in order to characterize developmental changes in functional properties and subunit composition of synaptic NMDA receptors. 2. During the first postnatal weeks the dendritic tree of CA1 PCs stained with biocytin increased both in size and in complexity. This was associated with an increase in amplitude of the focally evoked NMDA EPSCs recorded either in nominally Mg(2+)-free or Mg(2+)-containing saline. In adult PCs (> 5 weeks old) EPSC amplitude was 4-fold larger than in very young (up to 2 weeks old) neurones. 3. The sensitivity of NMDA EPSCs to blockade by Mg2+ did not change with age. In very young, intermediate and adult PCs the EPSC-voltage relation displayed an area of negative slope conductance at membrane potentials more negative than -30 mV. The apparent Kd values of the NMDA receptors for Mg2+ at 0 mV were 7.8 +/- 6.4, 10.4 +/- 14.1 and 6.5 +/- 4.7 mM in very young, intermediate and adult neurones, respectively. 4. The decay of the NMDA EPSC in both young and adult neurones could be described by the sum of a fast and a slow exponential function. Both EPSC rise time and fast and slow decay time constants measured at -60 mV, decreased with age. 5. The decay of NMDA EPSCs in young versus adult PCs was differentially modulated by membrane voltage. In young PCs depolarization slowed both the fast and the slow EPSC components. In adult PCs depolarization slightly accelerated the initial EPSC decay, though the overall duration of the EPSC did not change. The rise time of the EPSCs was not affected by voltage at any age. 6. The subunit-selective NMDA receptor antagonist ifenprodil similarly blocked iontophoretic NMDA-induced currents and NMDA EPSCs. In both young and adult PCs, the concentration-response curves for this effect disclosed distinct low and high affinity binding sites for ifenprodil. 7. In young PCs, low and high affinity binding sites for ifenprodil were about equally expressed (57 versus 43%, respectively), whereas in adult PCs, synaptic NMDA receptors expressed a majority (78%) of low affinity binding sites for ifenprodil. 8. The long duration of NMDA EPSCs (and by implication, of Ca2+ transfer through NMDA receptor channels) and its further prolongation by depolarization in young PCs are consistent with heightened NMDA-dependent neuronal plasticity early in development. The age-related changes in these properties may result from a developmental change in NMDA receptor subunit composition.
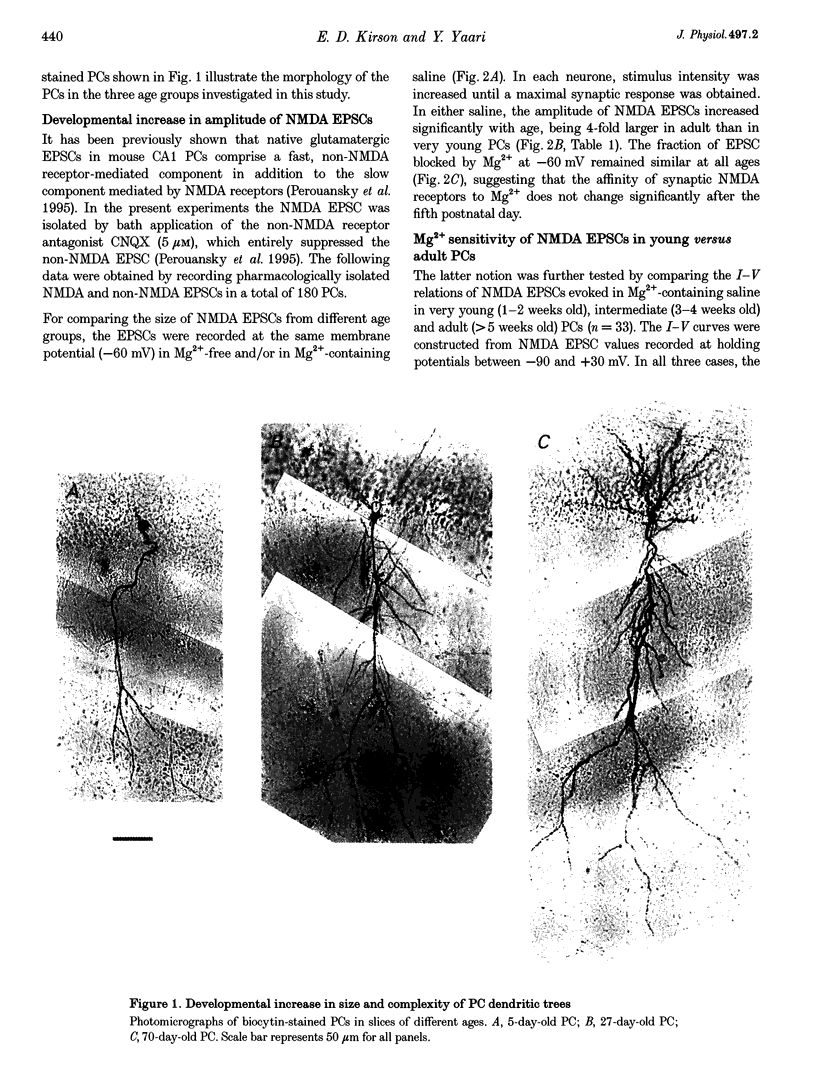
Full text
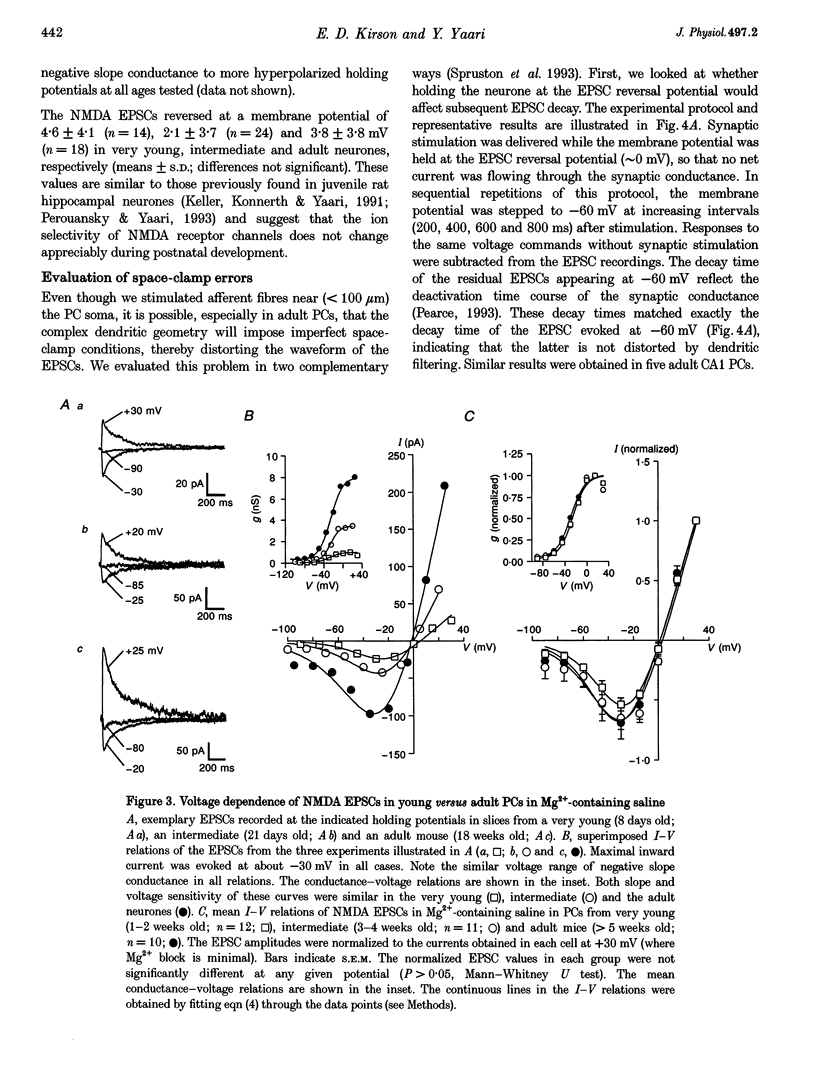
PDF





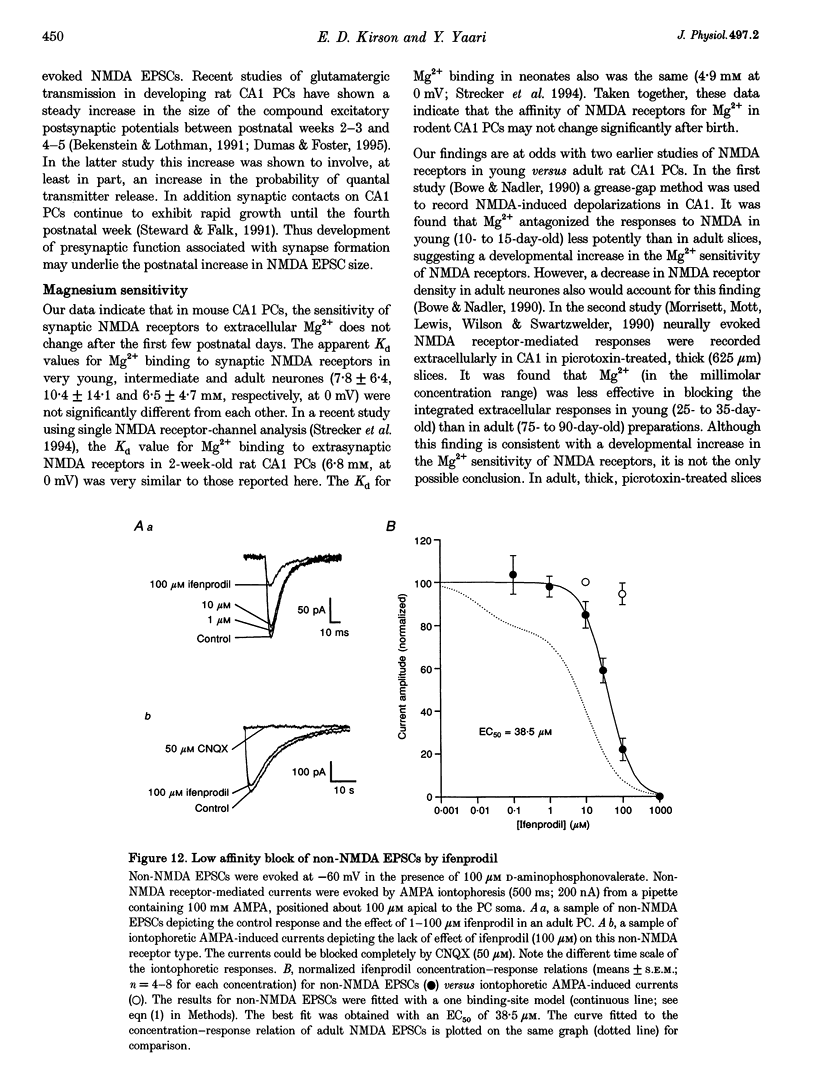
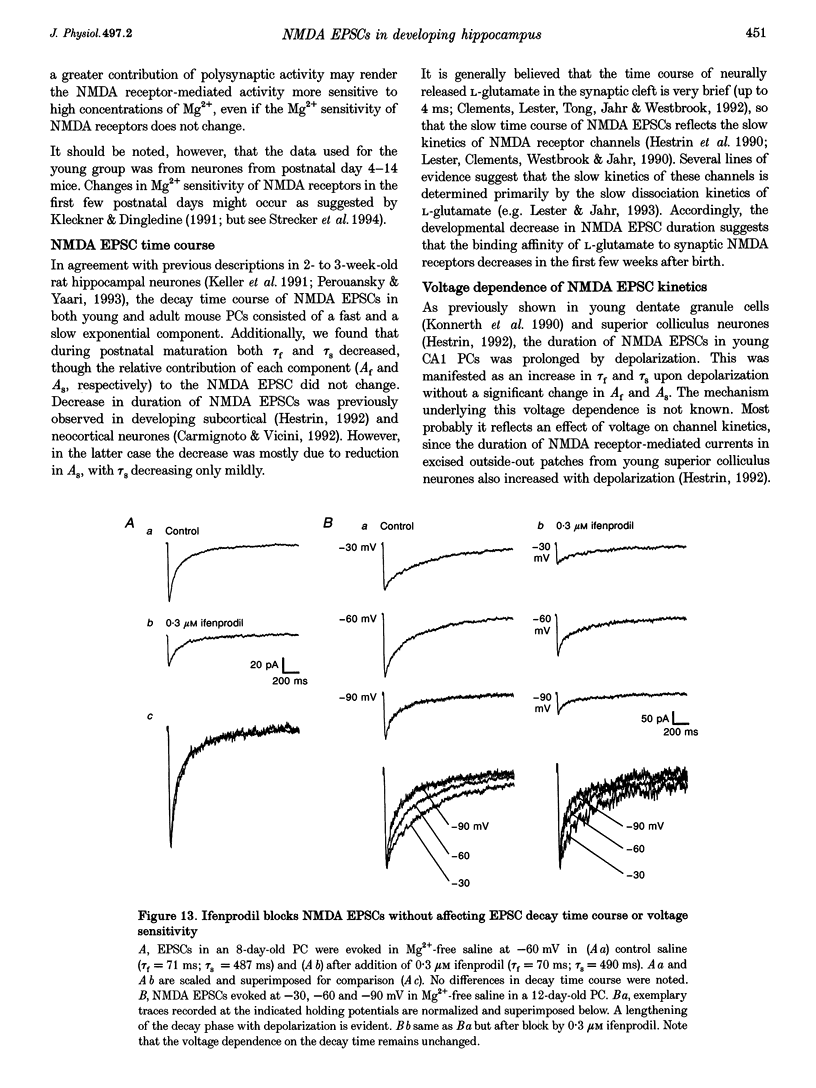

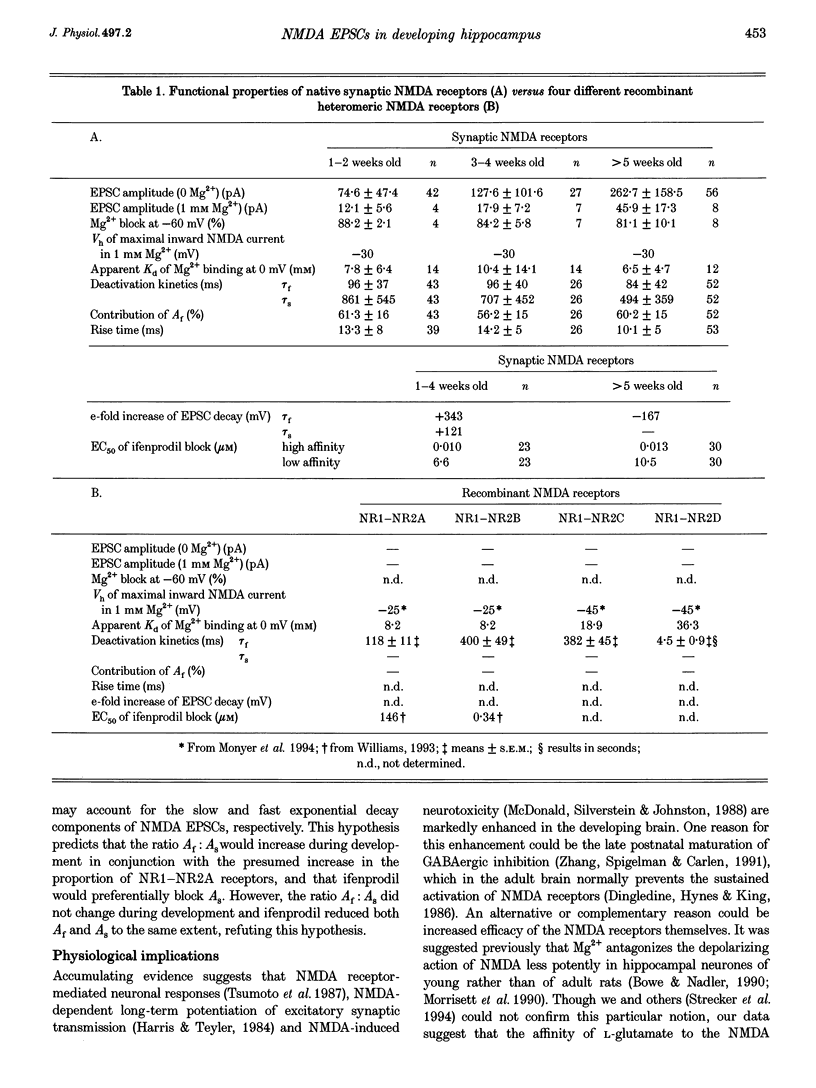


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong-James M., Welker E., Callahan C. A. The contribution of NMDA and non-NMDA receptors to fast and slow transmission of sensory information in the rat SI barrel cortex. J Neurosci. 1993 May;13(5):2149–2160. doi: 10.1523/JNEUROSCI.13-05-02149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekenstein J. W., Lothman E. W. A comparison of the ontogeny of excitatory and inhibitory neurotransmission in the CA1 region and dentate gyrus of the rat hippocampal formation. Brain Res Dev Brain Res. 1991 Nov 19;63(1-2):237–243. doi: 10.1016/0165-3806(91)90083-u. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bowe M. A., Nadler J. V. Developmental increase in the sensitivity to magnesium of NMDA receptors on CA1 hippocampal pyramidal cells. Brain Res Dev Brain Res. 1990 Oct 1;56(1):55–61. doi: 10.1016/0165-3806(90)90164-t. [DOI] [PubMed] [Google Scholar]
- Carmignoto G., Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992 Nov 6;258(5084):1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Church J., Fletcher E. J., Baxter K., MacDonald J. F. Blockade by ifenprodil of high voltage-activated Ca2+ channels in rat and mouse cultured hippocampal pyramidal neurones: comparison with N-methyl-D-aspartate receptor antagonist actions. Br J Pharmacol. 1994 Oct;113(2):499–507. doi: 10.1111/j.1476-5381.1994.tb17017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Lester R. A., Tong G., Jahr C. E., Westbrook G. L. The time course of glutamate in the synaptic cleft. Science. 1992 Nov 27;258(5087):1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Diemer N. H., Valente E., Bruhn T., Berg M., Jørgensen M. B., Johansen F. F. Glutamate receptor transmission and ischemic nerve cell damage: evidence for involvement of excitotoxic mechanisms. Prog Brain Res. 1993;96:105–123. doi: 10.1016/s0079-6123(08)63261-6. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Hynes M. A., King G. L. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol. 1986 Nov;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas T. C., Foster T. C. Developmental increase in CA3-CA1 presynaptic function in the hippocampal slice. J Neurophysiol. 1995 May;73(5):1821–1828. doi: 10.1152/jn.1995.73.5.1821. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Harris K. M., Teyler T. J. Developmental onset of long-term potentiation in area CA1 of the rat hippocampus. J Physiol. 1984 Jan;346:27–48. doi: 10.1113/jphysiol.1984.sp015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992 Jun 25;357(6380):686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Sah P., Nicoll R. A. Mechanisms generating the time course of dual component excitatory synaptic currents recorded in hippocampal slices. Neuron. 1990 Sep;5(3):247–253. doi: 10.1016/0896-6273(90)90162-9. [DOI] [PubMed] [Google Scholar]
- Keller B. U., Konnerth A., Yaari Y. Patch clamp analysis of excitatory synaptic currents in granule cells of rat hippocampus. J Physiol. 1991 Apr;435:275–293. doi: 10.1113/jphysiol.1991.sp018510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. W., Dingledine R. Regulation of hippocampal NMDA receptors by magnesium and glycine during development. Brain Res Mol Brain Res. 1991 Sep;11(2):151–159. [PubMed] [Google Scholar]
- Konnerth A., Keller B. U., Ballanyi K., Yaari Y. Voltage sensitivity of NMDA-receptor mediated postsynaptic currents. Exp Brain Res. 1990;81(1):209–212. doi: 10.1007/BF00230117. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T., Kashiwabuchi N., Mori H., Sakimura K., Kushiya E., Araki K., Meguro H., Masaki H., Kumanishi T., Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992 Jul 2;358(6381):36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Legendre P., Westbrook G. L. Ifenprodil blocks N-methyl-D-aspartate receptors by a two-component mechanism. Mol Pharmacol. 1991 Aug;40(2):289–298. [PubMed] [Google Scholar]
- Lester R. A., Clements J. D., Westbrook G. L., Jahr C. E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990 Aug 9;346(6284):565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Lester R. A., Jahr C. E. NMDA channel behavior depends on agonist affinity. J Neurosci. 1992 Feb;12(2):635–643. doi: 10.1523/JNEUROSCI.12-02-00635.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Silverstein F. S., Johnston M. V. Neurotoxicity of N-methyl-D-aspartate is markedly enhanced in developing rat central nervous system. Brain Res. 1988 Aug 30;459(1):200–203. doi: 10.1016/0006-8993(88)90306-x. [DOI] [PubMed] [Google Scholar]
- Monyer H., Burnashev N., Laurie D. J., Sakmann B., Seeburg P. H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994 Mar;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Mori H., Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995 Oct;34(10):1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Morrisett R. A., Mott D. D., Lewis D. V., Wilson W. A., Swartzwelder H. S. Reduced sensitivity of the N-methyl-D-aspartate component of synaptic transmission to magnesium in hippocampal slices from immature rats. Brain Res Dev Brain Res. 1990 Nov 1;56(2):257–262. doi: 10.1016/0165-3806(90)90090-l. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Pearce R. A. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993 Feb;10(2):189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- Perouansky M., Baranov D., Salman M., Yaari Y. Effects of halothane on glutamate receptor-mediated excitatory postsynaptic currents. A patch-clamp study in adult mouse hippocampal slices. Anesthesiology. 1995 Jul;83(1):109–119. doi: 10.1097/00000542-199507000-00014. [DOI] [PubMed] [Google Scholar]
- Perouansky M., Yaari Y. Kinetic properties of NMDA receptor-mediated synaptic currents in rat hippocampal pyramidal cells versus interneurones. J Physiol. 1993 Jun;465:223–244. doi: 10.1113/jphysiol.1993.sp019674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorný J., Yamamoto T. Postnatal ontogenesis of hippocampal CA1 area in rats. I. Development of dendritic arborisation in pyramidal neurons. Brain Res Bull. 1981 Aug;7(2):113–120. doi: 10.1016/0361-9230(81)90075-7. [DOI] [PubMed] [Google Scholar]
- Sah P., Hestrin S., Nicoll R. A. Properties of excitatory postsynaptic currents recorded in vitro from rat hippocampal interneurones. J Physiol. 1990 Nov;430:605–616. doi: 10.1113/jphysiol.1990.sp018310. [DOI] [PMC free article] [PubMed] [Google Scholar]
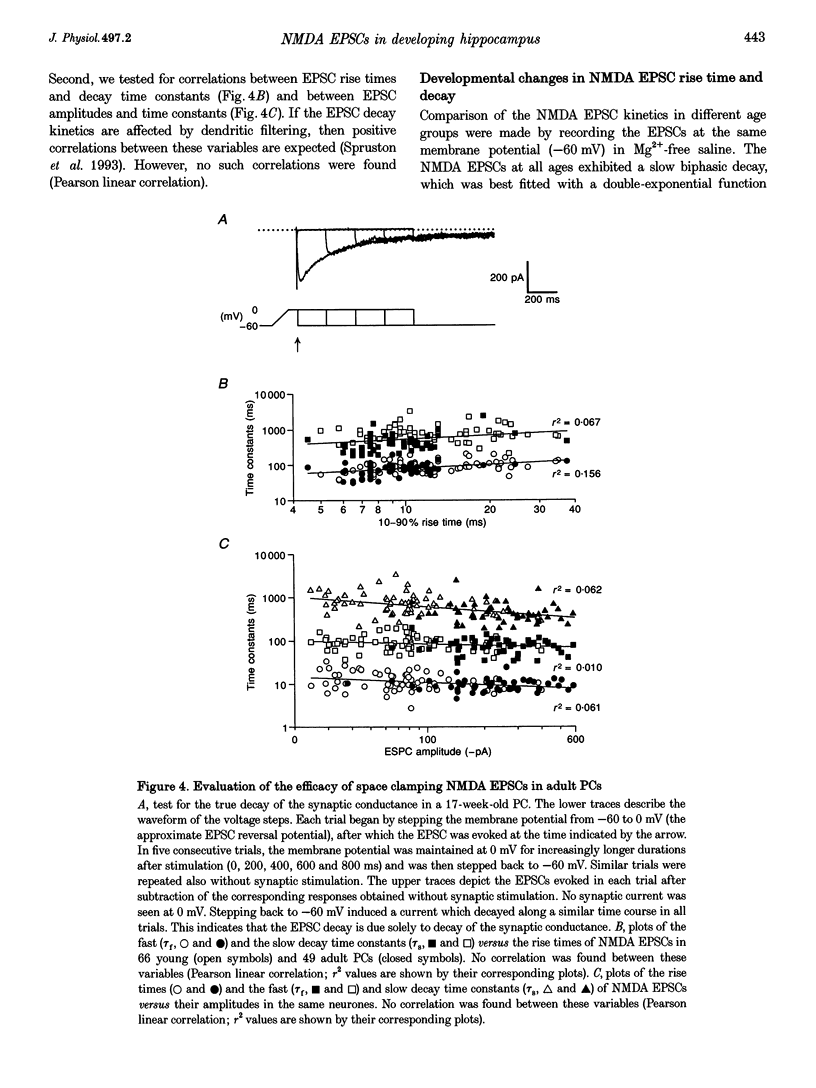
- Spruston N., Jaffe D. B., Williams S. H., Johnston D. Voltage- and space-clamp errors associated with the measurement of electrotonically remote synaptic events. J Neurophysiol. 1993 Aug;70(2):781–802. doi: 10.1152/jn.1993.70.2.781. [DOI] [PubMed] [Google Scholar]
- Steward O., Falk P. M. Selective localization of polyribosomes beneath developing synapses: a quantitative analysis of the relationships between polyribosomes and developing synapses in the hippocampus and dentate gyrus. J Comp Neurol. 1991 Dec 15;314(3):545–557. doi: 10.1002/cne.903140311. [DOI] [PubMed] [Google Scholar]
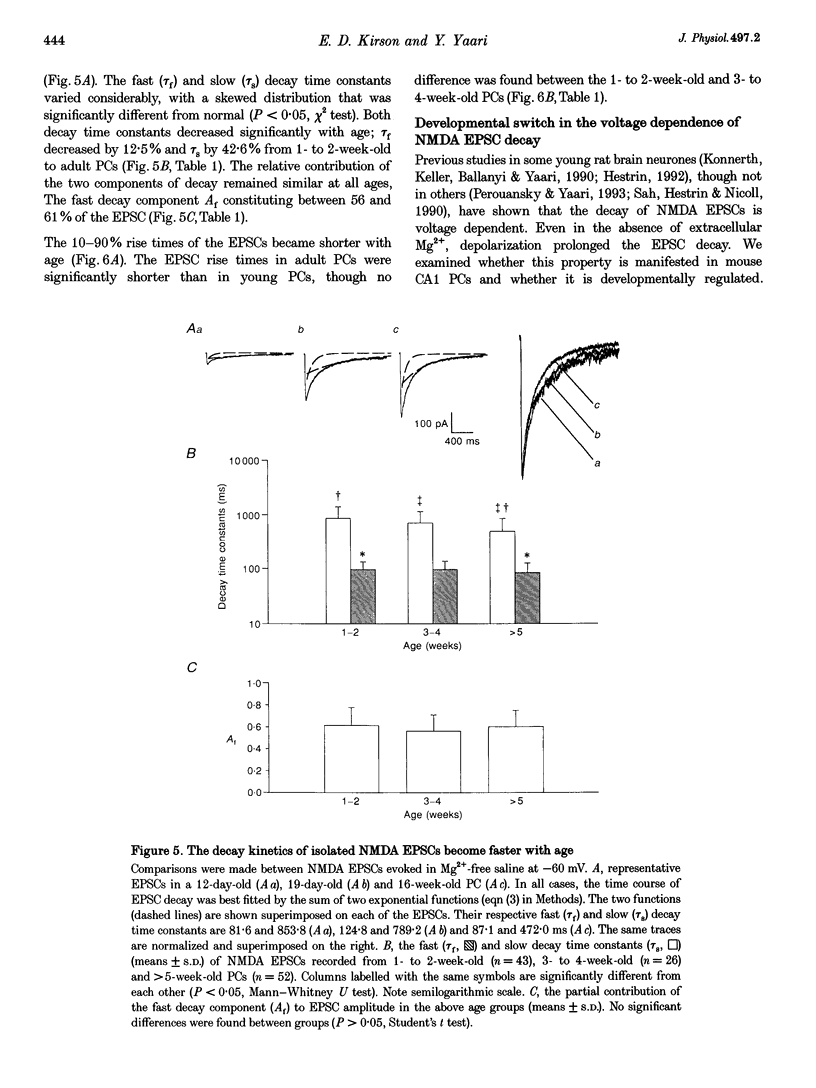
- Strecker G. J., Jackson M. B., Dudek F. E. Blockade of NMDA-activated channels by magnesium in the immature rat hippocampus. J Neurophysiol. 1994 Oct;72(4):1538–1548. doi: 10.1152/jn.1994.72.4.1538. [DOI] [PubMed] [Google Scholar]
- Tsumoto T., Hagihara K., Sato H., Hata Y. NMDA receptors in the visual cortex of young kittens are more effective than those of adult cats. Nature. 1987 Jun 11;327(6122):513–514. doi: 10.1038/327513a0. [DOI] [PubMed] [Google Scholar]
- Wafford K. A., Bain C. J., Le Bourdelles B., Whiting P. J., Kemp J. A. Preferential co-assembly of recombinant NMDA receptors composed of three different subunits. Neuroreport. 1993 Sep 30;4(12):1347–1349. doi: 10.1097/00001756-199309150-00015. [DOI] [PubMed] [Google Scholar]
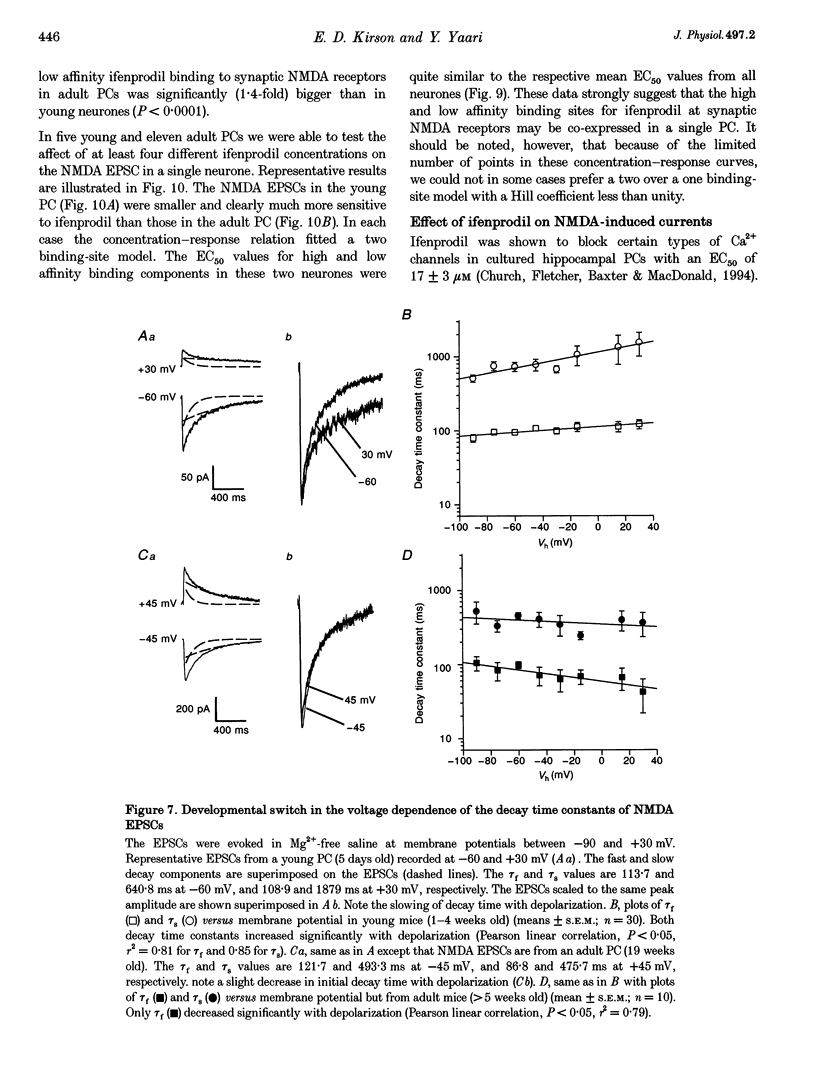
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993 Oct;44(4):851–859. [PubMed] [Google Scholar]
- Williams K. Pharmacological properties of recombinant N-methyl-D-aspartate (NMDA) receptors containing the epsilon 4 (NR2D) subunit. Neurosci Lett. 1995 Jan 30;184(3):181–184. doi: 10.1016/0304-3940(94)11201-s. [DOI] [PubMed] [Google Scholar]
- Williams K., Russell S. L., Shen Y. M., Molinoff P. B. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron. 1993 Feb;10(2):267–278. doi: 10.1016/0896-6273(93)90317-k. [DOI] [PubMed] [Google Scholar]
- Zhang L., Spigelman I., Carlen P. L. Development of GABA-mediated, chloride-dependent inhibition in CA1 pyramidal neurones of immature rat hippocampal slices. J Physiol. 1991 Dec;444:25–49. doi: 10.1113/jphysiol.1991.sp018864. [DOI] [PMC free article] [PubMed] [Google Scholar]