Abstract
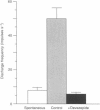
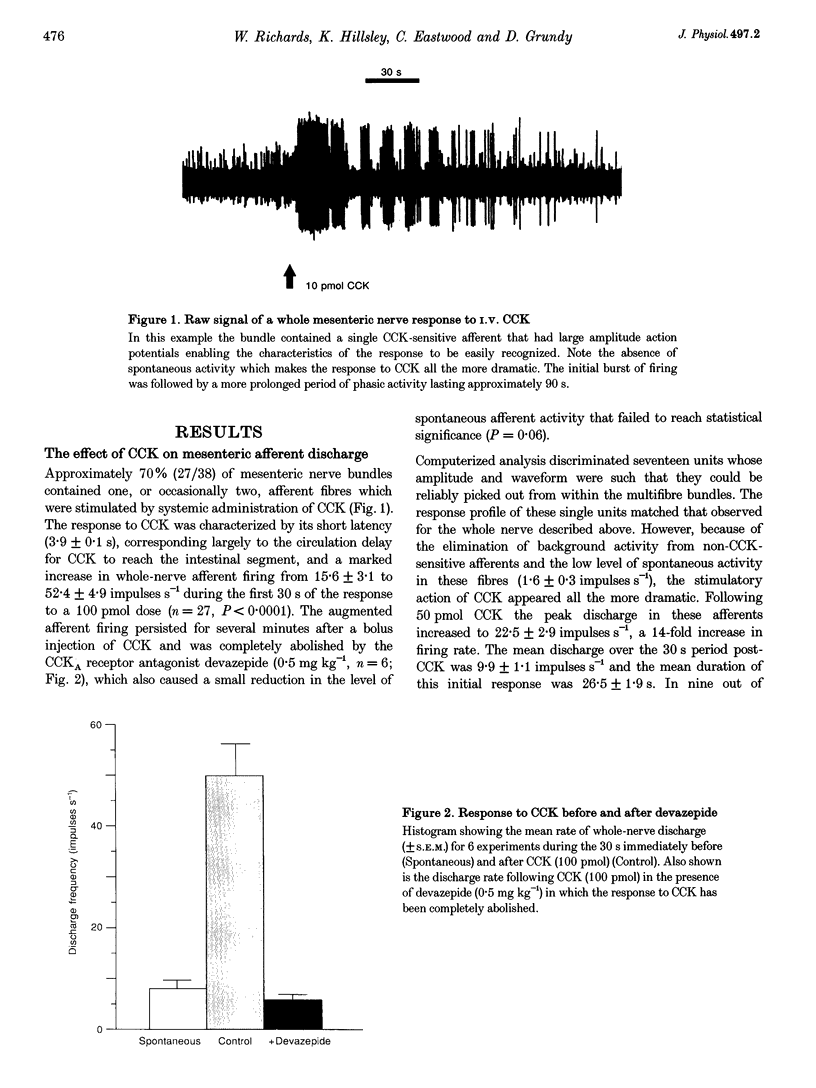
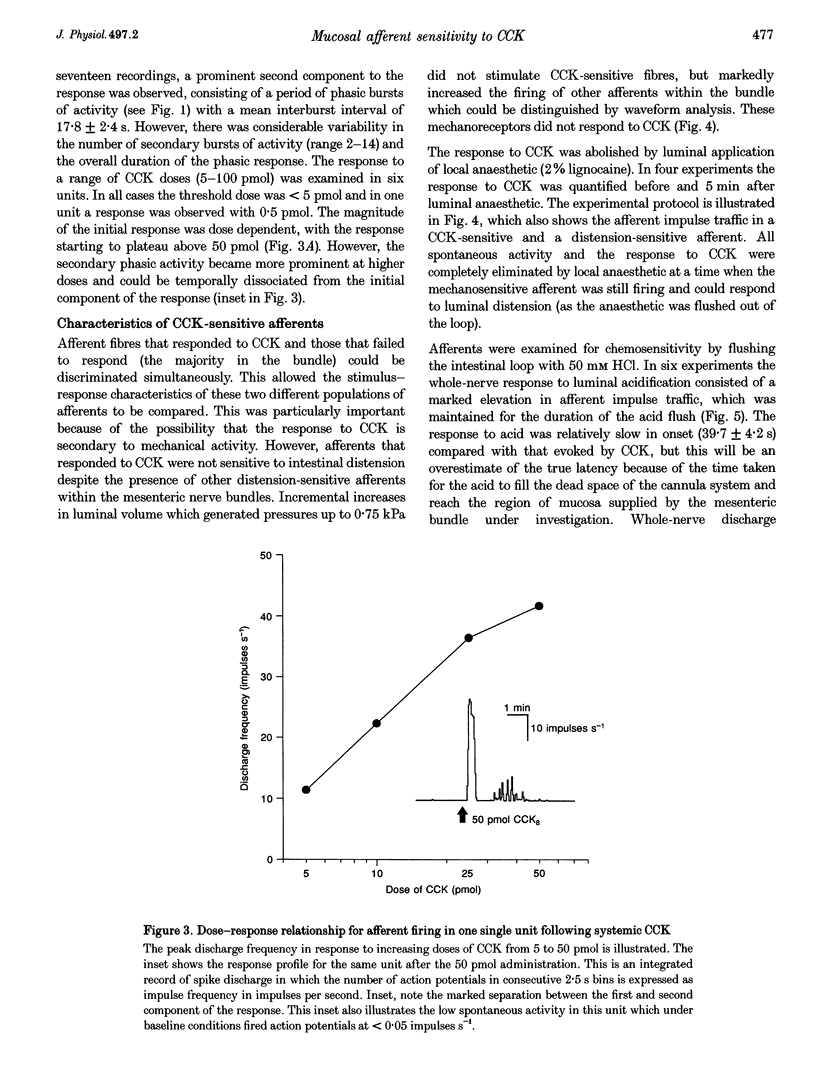
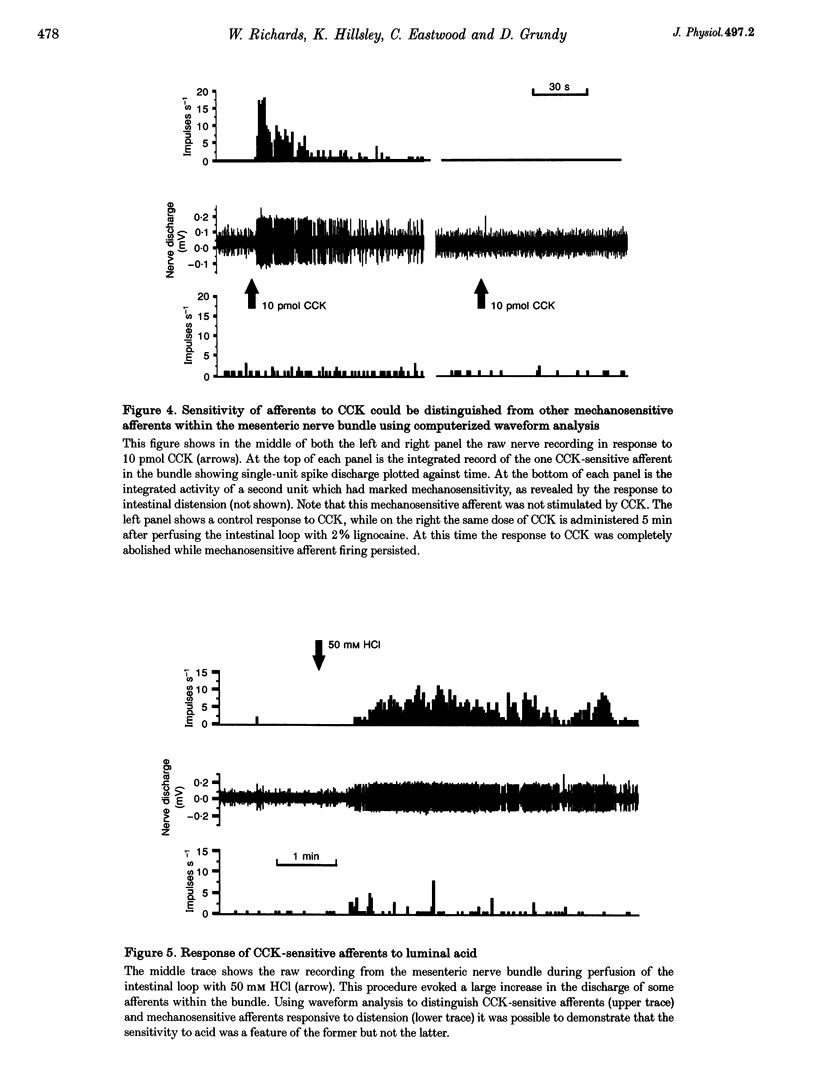
1. Extracellular recordings from rat mesenteric paravascular nerve bundles were made in order to characterize the responses of different populations of afferents supplying the small intestine to intravenous cholecystokinin (CCK; in the form of sulphated CCK8). 2. Approximately 70% of mesenteric nerve bundles contained CCK-sensitive afferent fibres. Responsive afferents had low spontaneous discharge (1.6 +/- 0.3 impulses s-1) and showed a 14-fold increase in firing at the peak of the response to 50 pmol CCK with the overall response lasting several minutes. The onset of the response occurred after a latency of (3.9 +/- 0.1 s) following i.v. administration of CCK, which corresponds largely to the circulation delay in these animals. The threshold dose of CCK was < 5 pmol. 3. The response to 100 pmol CCK was completely abolished by devazepide (0.5 mg kg-1) and by chronic subdiaphragmatic vagotomy performed 10-14 days prior to experimentation, indicating that CCK sensitivity was via CCKA receptors and exclusively mediated via vagal afferents rather than splanchnic or enteric afferents. 4. Evidence that CCK-sensitive afferents had mucosal receptive fields was indicated by the lack of any response to luminal distension and the sensitivity of the CCK response to luminal anaesthesia. Furthermore, CCK-sensitive afferents responded to luminal hydrochloric acid (50 mM) in a slowly adapting manner. The response to acid was significantly reduced (P < 0.005), but not abolished, by devazepide at a time when the response to exogenous CCK had been completely eliminated. 5. The exquisite sensitivity of some vagal mucosal afferents to CCK suggests that they may play a physiological role in the reflex and behavioural consequences of CCK release from the small intestine, possibly acting in a paracrine fashion. However, this sensitivity to CCK represents only one aspect of the broad chemosensitivity of these mucosal afferents and is not an obligatory component of the signal transduction pathway.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthoud H. R., Kressel M., Raybould H. E., Neuhuber W. L. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995 Mar;191(3):203–212. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- Blackshaw L. A., Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst. 1990 Dec;31(3):191–201. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Calingasan N., Ritter S., Ritter R., Brenner L. Low-dose near-celiac arterial cholecystokinin suppresses food intake in rats. Am J Physiol. 1992 Sep;263(3 Pt 2):R572–R577. doi: 10.1152/ajpregu.1992.263.3.R572. [DOI] [PubMed] [Google Scholar]
- Cervero F., Sharkey K. A. An electrophysiological and anatomical study of intestinal afferent fibres in the rat. J Physiol. 1988 Jul;401:381–397. doi: 10.1113/jphysiol.1988.sp017168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell D. F., Iggo A. Mucosal enteroceptors with vagal afferent fibres in the proximal duodenum of sheep. J Physiol. 1984 Sep;354:497–522. doi: 10.1113/jphysiol.1984.sp015390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster E. R., Green T., Elliot M., Bremner A., Dockray G. J. Gastric emptying in rats: role of afferent neurons and cholecystokinin. Am J Physiol. 1990 Apr;258(4 Pt 1):G552–G556. doi: 10.1152/ajpgi.1990.258.4.G552. [DOI] [PubMed] [Google Scholar]
- Gores G. J., LaRusso N. F., Miller L. J. Hepatic processing of cholecystokinin peptides. I. Structural specificity and mechanism of hepatic extraction. Am J Physiol. 1986 Mar;250(3 Pt 1):G344–G349. doi: 10.1152/ajpgi.1986.250.3.G344. [DOI] [PubMed] [Google Scholar]
- Grundy D., Bagaev V., Hillsley K. Inhibition of gastric mechanoreceptor discharge by cholecystokinin in the rat. Am J Physiol. 1995 Feb;268(2 Pt 1):G355–G360. doi: 10.1152/ajpgi.1995.268.2.G355. [DOI] [PubMed] [Google Scholar]
- Kuramoto H., Furness J. B. Distribution of enteric nerve cells that project from the small intestine to the coeliac ganglion in the guinea-pig. J Auton Nerv Syst. 1989 Aug;27(3):241–248. doi: 10.1016/0165-1838(89)90117-3. [DOI] [PubMed] [Google Scholar]
- Li Y., Owyang C. Vagal afferent pathway mediates physiological action of cholecystokinin on pancreatic enzyme secretion. J Clin Invest. 1993 Jul;92(1):418–424. doi: 10.1172/JCI116583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran T. H., Smith G. P., Hostetler A. M., McHugh P. R. Transport of cholecystokinin (CCK) binding sites in subdiaphragmatic vagal branches. Brain Res. 1987 Jul 7;415(1):149–152. doi: 10.1016/0006-8993(87)90278-2. [DOI] [PubMed] [Google Scholar]
- Raybould H. E., Taché Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am J Physiol. 1988 Aug;255(2 Pt 1):G242–G246. doi: 10.1152/ajpgi.1988.255.2.G242. [DOI] [PubMed] [Google Scholar]
- Reidelberger R. D. Abdominal vagal mediation of the satiety effects of exogenous and endogenous cholecystokinin in rats. Am J Physiol. 1992 Dec;263(6 Pt 2):R1354–R1358. doi: 10.1152/ajpregu.1992.263.6.R1354. [DOI] [PubMed] [Google Scholar]
- Reidelberger R. D., O'Rourke M. F. Potent cholecystokinin antagonist L 364718 stimulates food intake in rats. Am J Physiol. 1989 Dec;257(6 Pt 2):R1512–R1518. doi: 10.1152/ajpregu.1989.257.6.R1512. [DOI] [PubMed] [Google Scholar]
- Reidelberger R. D., Varga G., Liehr R. M., Castellanos D. A., Rosenquist G. L., Wong H. C., Walsh J. H. Cholecystokinin suppresses food intake by a nonendocrine mechanism in rats. Am J Physiol. 1994 Oct;267(4 Pt 2):R901–R908. doi: 10.1152/ajpregu.1994.267.4.R901. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., McHugh P. R., Moran T. H. Gastric loads and cholecystokinin synergistically stimulate rat gastric vagal afferents. Am J Physiol. 1993 Oct;265(4 Pt 2):R872–R876. doi: 10.1152/ajpregu.1993.265.4.R872. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Moran T. H. Sub-diaphragmatic vagal afferent integration of meal-related gastrointestinal signals. Neurosci Biobehav Rev. 1996;20(1):47–56. doi: 10.1016/0149-7634(95)00039-h. [DOI] [PubMed] [Google Scholar]
- Shillabeer G., Davison J. S. Proglumide, a cholecystokinin antagonist, increases gastric emptying in rats. Am J Physiol. 1987 Feb;252(2 Pt 2):R353–R360. doi: 10.1152/ajpregu.1987.252.2.R353. [DOI] [PubMed] [Google Scholar]
- Smith G. P., Jerome C., Cushin B. J., Eterno R., Simansky K. J. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981 Aug 28;213(4511):1036–1037. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- Weller A., Smith G. P., Gibbs J. Endogenous cholecystokinin reduces feeding in young rats. Science. 1990 Mar 30;247(4950):1589–1591. doi: 10.1126/science.2321020. [DOI] [PubMed] [Google Scholar]