Abstract
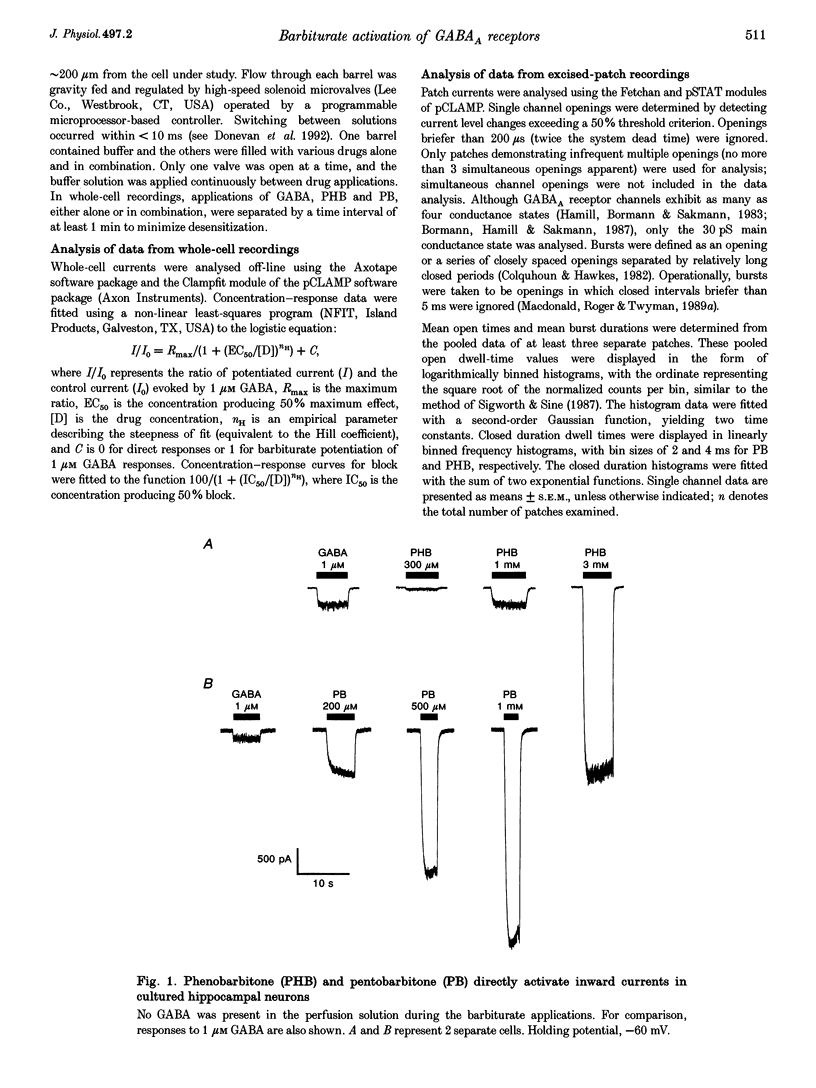
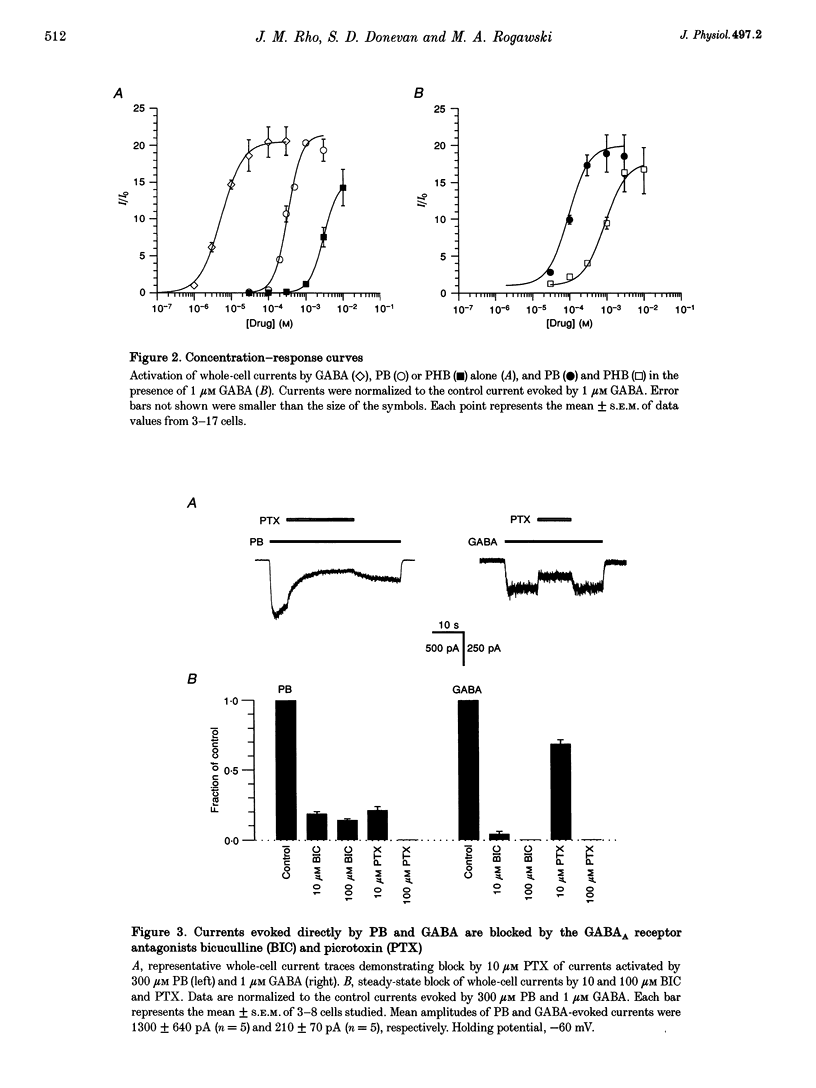
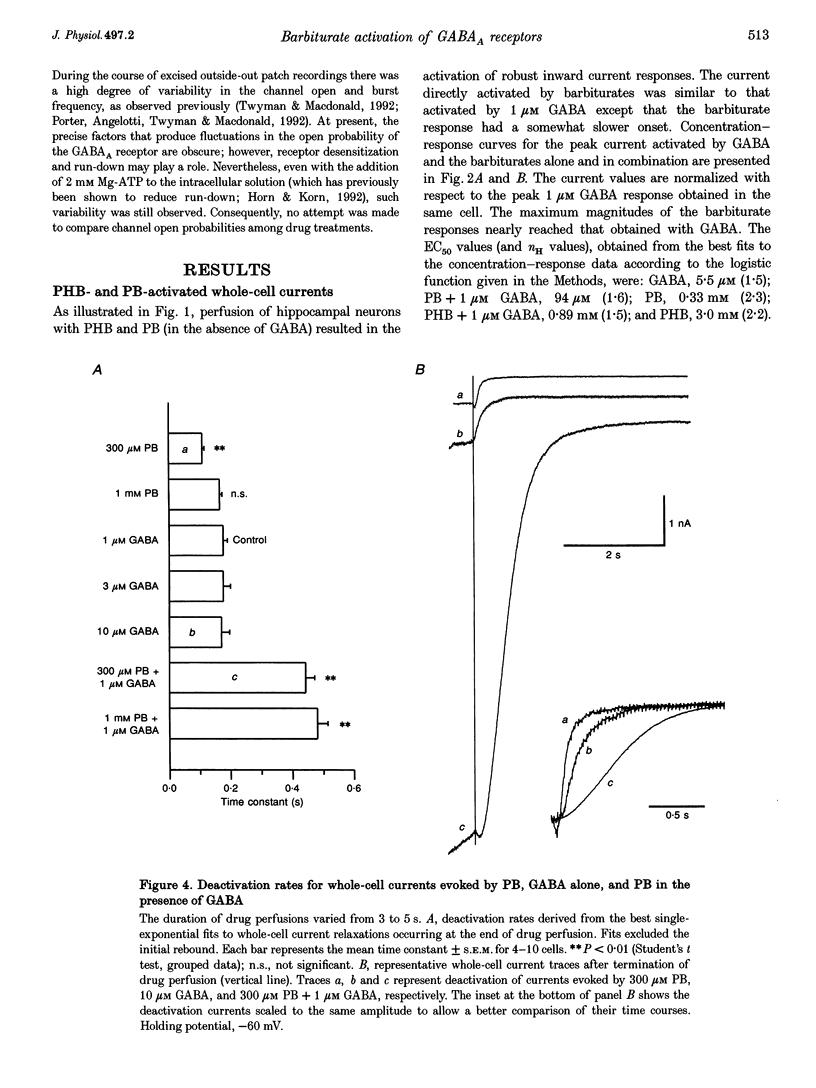
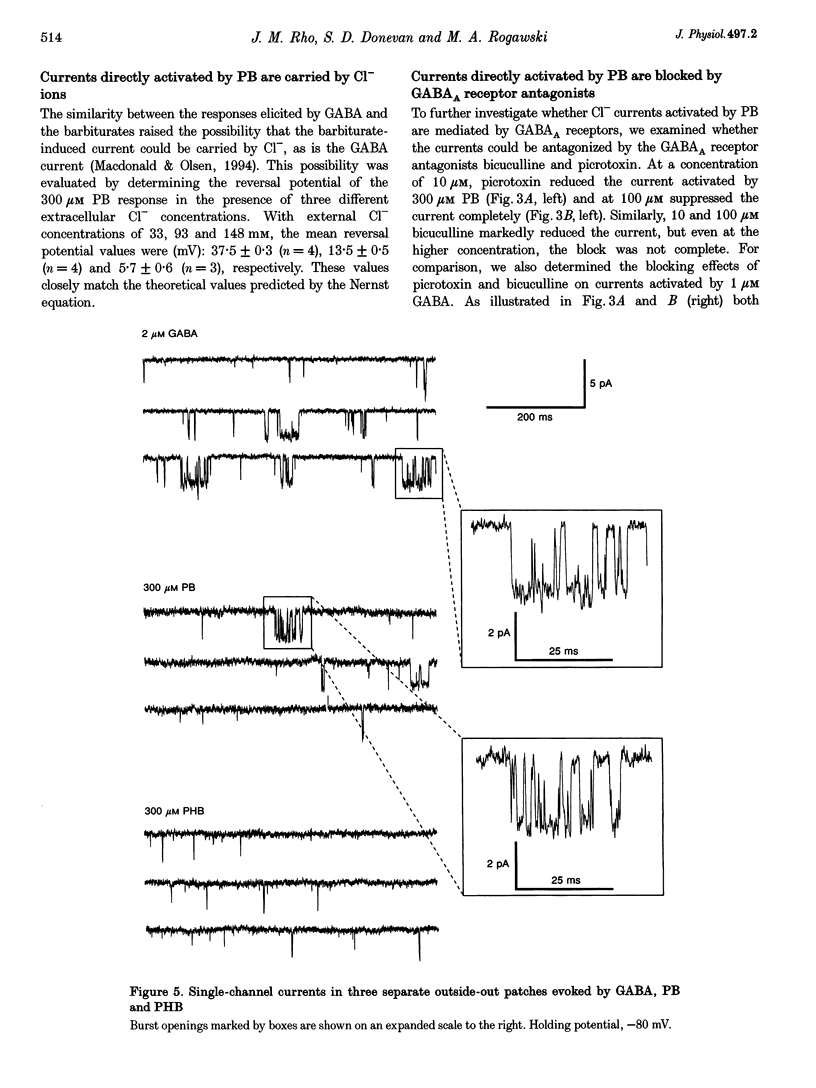
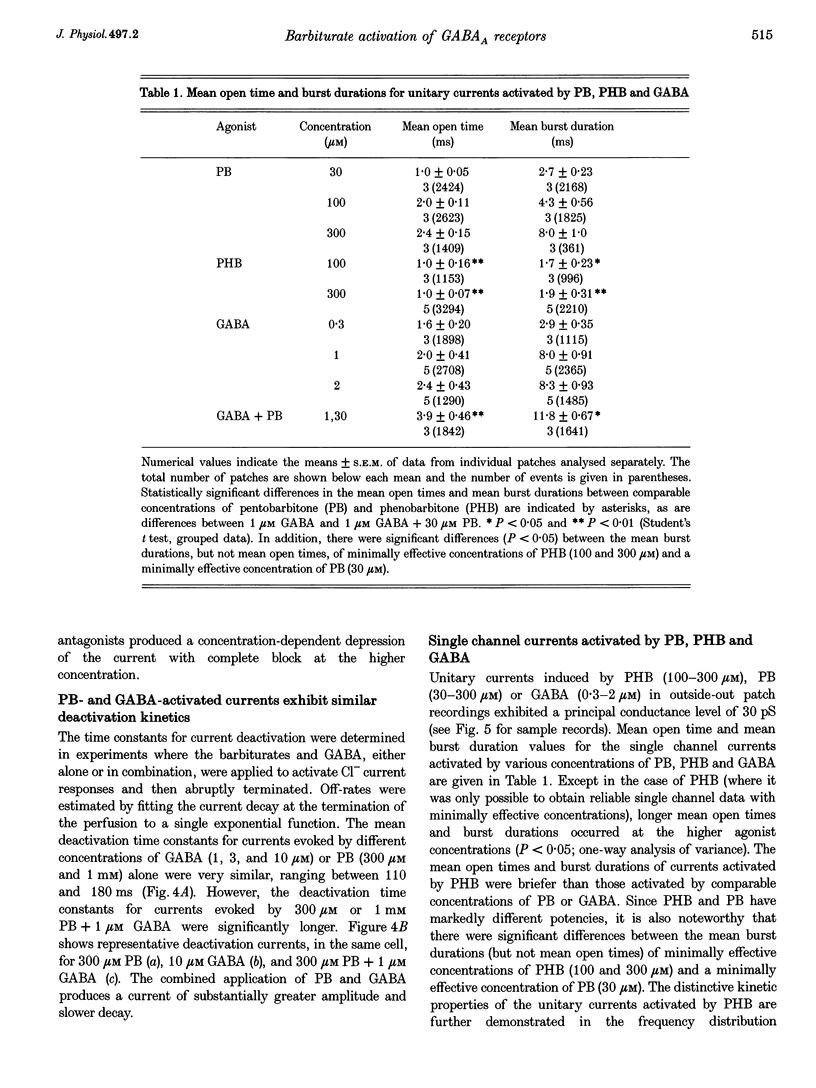
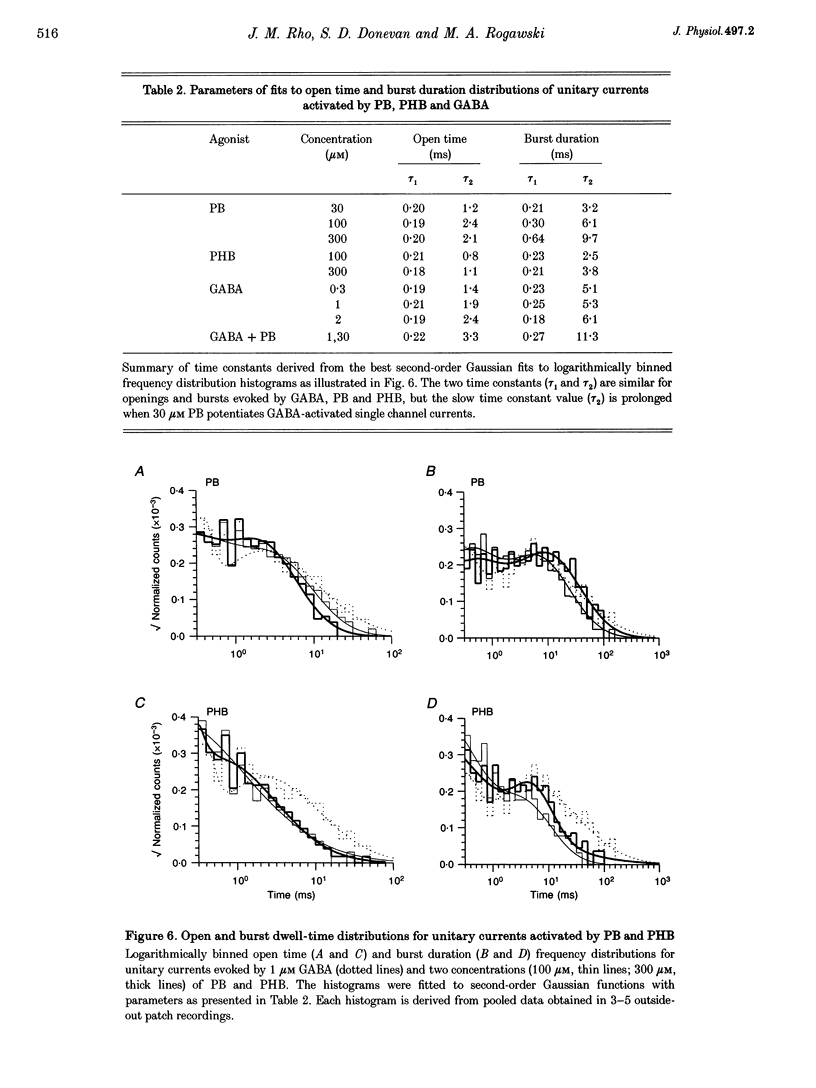
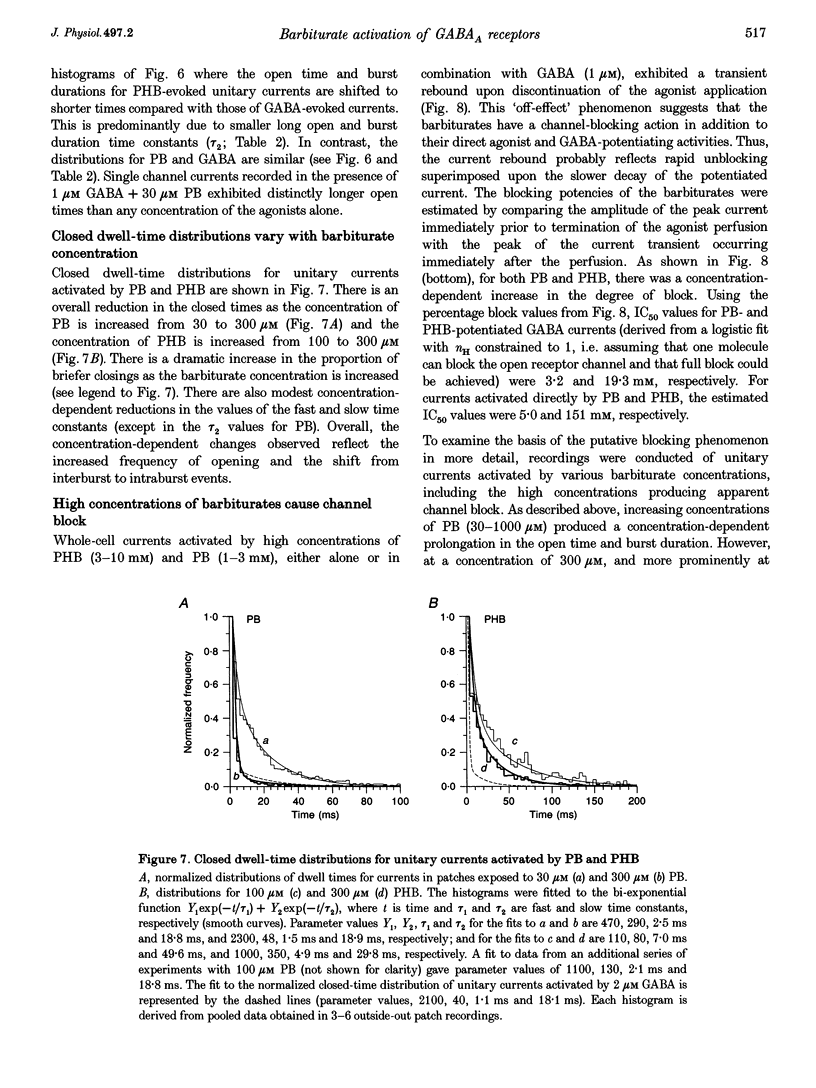
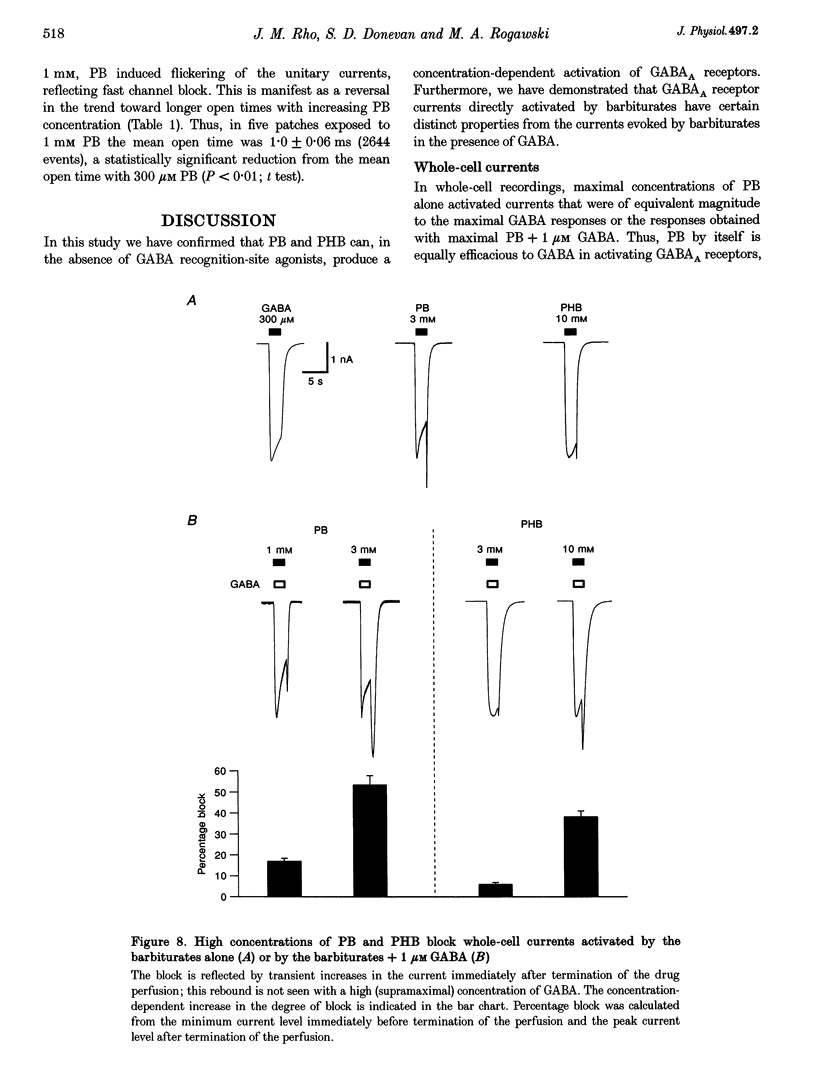
1. The direct activation of the GABAA receptor by pentobarbitone (PB) and phenobarbitone (PHB) was characterized in cultured rat hippocampal neurons using whole-cell voltage clamp and single channel recording techniques. 2. In whole-cell recordings, PB and PHB produced a concentration-dependent activation of Cl- current (EC50 values, 0.33 and 3.0 mM, respectively). The response to the barbiturates was similar to that produced by GABA, although GABA was more potent (EC50, 5.5 microM). PB and PHB were substantially more potent in enhancing the response to 1 microM GABA (EC50 values, 94 microM and 0.89 mM, respectively). The maximal magnitude of the responses to PB was similar to that of the maximal response to GABA or GABA + PB. PHB appeared to be modestly less efficacious. 3. The mean deactivation time constant for whole-cell Cl- currents evoked by 1 mM PB + 1 microM GABA was significantly longer (480 +/- 34 ms) than for 1 mM PB (170 +/- 9 ms) or 1 microM GABA (180 +/- 14 ms) alone. 4. Whole-cell currents directly activated by 300 microM PB and 1 microM GABA were blocked by the GABA receptor antagonists bicuculline and picrotoxin. 5. Unitary GABAA receptor channel currents evoked by 300 microM PB had similar main conductance, mean open time and mean burst duration as those activated by 2 microM GABA alone. Single channel openings and bursts were of shorter mean duration when 100 and 300 microM PHB were used. 6. High concentrations of PB (1-3 mM) and PHB (3-10 mM) produced a rapid block of currents activated by the barbiturate alone or by the barbiturate in the presence of 1 microM GABA. The estimated IC50 values for block of PB- and PHB-potentiated GABA currents were 2.8 and 12.9 mM, respectively. 7. Single channel currents activated by high concentrations of PB and PHB alone or in the presence of GABA demonstrated flickering, probably reflecting fast channel block. 8. We conclude that the gating of the GABAA receptor channel by PHB and PB is functionally similar to that produced by the natural agonist GABA alone, but distinct from that obtained when barbiturates modulate the response to GABA. At high concentrations, the barbiturates produce a channel blocking action that limits the maximum total current conducted by the channel.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Hattori K., Oomura Y., Carpenter D. O. Bicuculline and picrotoxin block gamma-aminobutyric acid-gated Cl- conductance by different mechanisms. Experientia. 1985 Jan 15;41(1):70–71. doi: 10.1007/BF02005880. [DOI] [PubMed] [Google Scholar]
- Akaike N., Shirasaki T., Yakushiji T. Quinolones and fenbufen interact with GABAA receptor in dissociated hippocampal cells of rat. J Neurophysiol. 1991 Aug;66(2):497–504. doi: 10.1152/jn.1991.66.2.497. [DOI] [PubMed] [Google Scholar]
- Amin J., Weiss D. S. GABAA receptor needs two homologous domains of the beta-subunit for activation by GABA but not by pentobarbital. Nature. 1993 Dec 9;366(6455):565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N., Mathers D. A. Convulsant-induced depression of amino acid responses in cultured mouse spinal neurones studied under voltage clamp. Br J Pharmacol. 1983 Dec;80(4):619–629. doi: 10.1111/j.1476-5381.1983.tb10051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N. Phenobarbitone modulation of postsynaptic GABA receptor function on cultured mammalian neurons. Proc R Soc Lond B Biol Sci. 1979 Dec 31;206(1164):319–327. doi: 10.1098/rspb.1979.0108. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Donevan S. D., Jones S. M., Rogawski M. A. Arcaine blocks N-methyl-D-aspartate receptor responses by an open channel mechanism: whole-cell and single-channel recording studies in cultured hippocampal neurons. Mol Pharmacol. 1992 Apr;41(4):727–735. [PubMed] [Google Scholar]
- Guthrie P. B., Brenneman D. E., Neale E. A. Morphological and biochemical differences expressed in separate dissociated cell cultures of dorsal and ventral halves of the mouse spinal cord. Brain Res. 1987 Sep 15;420(2):313–323. doi: 10.1016/0006-8993(87)91252-2. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Korn S. J. Prevention of rundown in electrophysiological recording. Methods Enzymol. 1992;207:149–155. doi: 10.1016/0076-6879(92)07010-l. [DOI] [PubMed] [Google Scholar]
- MacDonald R. L., Barker J. L. Enhancement of GABA-mediated postsynaptic inhibition in cultured mammalian spinal cord neurons: a common mode of anticonvulsant action. Brain Res. 1979 May 11;167(2):323–336. doi: 10.1016/0006-8993(79)90826-6. [DOI] [PubMed] [Google Scholar]
- MacDonald R. L., Rogers C. J., Twyman R. E. Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurones in culture. J Physiol. 1989 Oct;417:483–500. doi: 10.1113/jphysiol.1989.sp017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L., Olsen R. W. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Rogers C. J., Twyman R. E. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1989 Mar;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalec W., Narahashi T. Use-dependent pentobarbital block of kainate and quisqualate currents. Brain Res. 1993 Apr 9;608(1):7–15. doi: 10.1016/0006-8993(93)90766-g. [DOI] [PubMed] [Google Scholar]
- Mathers D. A., Barker J. L. (-)Pentobarbital opens ion channels of long duration in cultured mouse spinal neurons. Science. 1980 Jul 25;209(4455):507–509. doi: 10.1126/science.6248961. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Wojtowicz J. M. The effects of pentobarbital and related compounds on frog motoneurons. Brain Res. 1980 Jun 2;191(1):225–237. doi: 10.1016/0006-8993(80)90325-x. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. GABA-benzodiazepine-barbiturate receptor interactions. J Neurochem. 1981 Jul;37(1):1–13. doi: 10.1111/j.1471-4159.1981.tb05284.x. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Snowman A. M. Chloride-dependent enhancement by barbiturates of gamma-aminobutyric acid receptor binding. J Neurosci. 1982 Dec;2(12):1812–1823. doi: 10.1523/JNEUROSCI.02-12-01812.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. Actions of pentobarbital on rat brain receptors expressed in Xenopus oocytes. J Neurosci. 1986 Aug;6(8):2290–2297. doi: 10.1523/JNEUROSCI.06-08-02290.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter N. M., Angelotti T. P., Twyman R. E., MacDonald R. L. Kinetic properties of alpha 1 beta 1 gamma-aminobutyric acidA receptor channels expressed in Chinese hamster ovary cells: regulation by pentobarbital and picrotoxin. Mol Pharmacol. 1992 Nov;42(5):872–881. [PubMed] [Google Scholar]
- Ransom B. R., Barker J. L. Pentobarbital selectively enhances GABA-mediated post-synaptic inhibition in tissue cultured mouse spinal neurons. Brain Res. 1976 Sep 24;114(3):530–535. doi: 10.1016/0006-8993(76)90977-x. [DOI] [PubMed] [Google Scholar]
- Robertson B. Actions of anaesthetics and avermectin on GABAA chloride channels in mammalian dorsal root ganglion neurones. Br J Pharmacol. 1989 Sep;98(1):167–176. doi: 10.1111/j.1476-5381.1989.tb16878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Rat hippocampal neurons in culture: responses to electrical and chemical stimuli. J Neurophysiol. 1983 Dec;50(6):1249–1264. doi: 10.1152/jn.1983.50.6.1249. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzdak P. D., Schwartz R. D., Skolnick P., Paul S. M. Ethanol stimulates gamma-aminobutyric acid receptor-mediated chloride transport in rat brain synaptoneurosomes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4071–4075. doi: 10.1073/pnas.83.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman R. E., Macdonald R. L. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol. 1992 Oct;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman R. E., Rogers C. J., Macdonald R. L. Pentobarbital and picrotoxin have reciprocal actions on single GABAA receptor channels. Neurosci Lett. 1989 Jan 2;96(1):89–95. doi: 10.1016/0304-3940(89)90248-6. [DOI] [PubMed] [Google Scholar]
- Willow M., Johnston G. A. Enhancement of GABA binding by pentobarbitone. Neurosci Lett. 1980 Jul;18(3):323–327. doi: 10.1016/0304-3940(80)90305-5. [DOI] [PubMed] [Google Scholar]
- Yang J. S., Olsen R. W. gamma-Aminobutyric acid receptor-regulated 36Cl- flux in mouse cortical slices. J Pharmacol Exp Ther. 1987 May;241(2):677–685. [PubMed] [Google Scholar]
- Yoon K. W., Covey D. F., Rothman S. M. Multiple mechanisms of picrotoxin block of GABA-induced currents in rat hippocampal neurons. J Physiol. 1993 May;464:423–439. doi: 10.1113/jphysiol.1993.sp019643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Mullen J. M., Barker J. L., Rogawski M. A. Calcium current block by (-)-pentobarbital, phenobarbital, and CHEB but not (+)-pentobarbital in acutely isolated hippocampal CA1 neurons: comparison with effects on GABA-activated Cl- current. J Neurosci. 1993 Aug;13(8):3211–3221. doi: 10.1523/JNEUROSCI.13-08-03211.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


