Abstract
Background
Autoimmune pulmonary alveolar proteinosis (aPAP) is a rare disease that may progress towards pulmonary fibrosis. Data about fibrosis prevalence and risk factors are lacking.
Methods
In this retrospective multicentre nationwide cohort, we included patients newly diagnosed with aPAP between 2008 and 2018 in France and Belgium. Data were collected from medical records using a standardised questionnaire.
Results
61 patients were included in the final analysis. We identified 5 patients (8%) with fibrosis on initial computed tomography (CT) and 16 patients (26%) with fibrosis on final CT after a median time of 3.6 years. Dust exposure was associated with pulmonary fibrosis occurrence (OR 4.3; p=0.038). aPAP patients treated with whole-lung lavage, rituximab or granulocyte–monocyte colony-stimulating factor therapy did not have more fibrotic evolution than patients who did not receive these treatments (n=25 out of 45, 57% versus n=10 out of 16, 62%; p=0.69). All-cause mortality was significantly higher in fibrotic than in nonfibrotic cases (n=4 out of 16, 25% versus n=2 out of 45, 4.4%; p=0.036, respectively).
Conclusion
In our population, a quarter of aPAP patients progressed towards pulmonary fibrosis. Dust exposure seems to be an important factor associated with this complication. More studies are needed to analyse precisely the impact of dust exposure impact, especially silica, in patients with aPAP.
Shareable abstract
Pulmonary fibrosis occurrence in aPAP patients is not rare, and seems to be associated with dust exposure and with higher mortality rate https://bit.ly/3xZZo7s
Introduction
Pulmonary alveolar proteinosis (PAP) is an extremely rare respiratory disease described in 1958 by S.H. Rosen and co-workers with a prevalence around seven cases per million inhabitants [1]. It is caused by accumulation of surfactant components filling alveoli with periodic acid-Schiff (PAS)-positive material, which can lead to hypoxaemia [2, 3].
PAP can be classified into three groups: primary PAP, with disruption of granulocyte–monocyte colony-stimulating factor (GM-CSF) signalling (divided into autoimmune PAP (aPAP) and hereditary PAP); secondary PAP; and genetic PAP [4]. The vast majority of PAP occurs as aPAP (90% of all PAP) [3, 5, 6], with serum anti-GM-CSF antibodies blocking activation of alveolar macrophages. Autoantibodies in PAP neutralise GM-CSF by blocking the initial binding to the low-affinity receptor and cause a functional GM-CSF deficiency [7, 8]. This reduces GM-CSF bioactivity, especially surfactant catabolism, by affecting alveolar macrophage differentiation [3].
Some studies suggest that a small subset of patients with aPAP may experience progression towards pulmonary fibrosis [9, 10] but available data are scarce. Akira et al. [10] showed that among 44 patients, 6 patients developed traction bronchiectasis, and 2 patients developed honeycombing. As stated in a recent review from Trapnell et al. [1], pulmonary fibrosis can occur in PAP but remains poorly understood in terms of frequency of occurrence, aetiology and pathogenesis. Fibrosis evolution may be multifactorial [11].
In this multicentre, retrospective, nationwide cohort study of aPAP patients, we aimed to assess the prevalence of fibrotic evolution and to identify associated risk factors.
Methods
Ethics
The study was approved by the Ethics Committee of the University Hospital of Rennes (approval number 19.104).
Study population and data collection
We screened the medical files of all patients who had detectable serum anti-GM-CSF antibodies between 2008 and 2018 in 25 centres in France and Belgium (listed in the supplementary material). We included only patients with at least one chest computed tomography (CT) scan at diagnosis and one chest CT scan 1 year later or more.
Anti-GM-CSF antibody assay was performed at the Immunology Department of Rennes University Hospital, the immunology referral centre for this dosage for participating countries. We included only adults (age >18 years) who fulfilled the diagnostic criteria for aPAP: typical or compatible chest CT scan, presence of PAS-positive acellular eosinophilic lipoprotein in bronchoalveolar lavage (BAL), which may be associated with foamy alveolar macrophages with PAS-positive intracellular inclusions, or surgical lung biopsy. Then, we used a standardised questionnaire to collect data, including demographics, comorbidities, imaging features, outcomes and microbiological data.
Dust exposure was defined as an inhalation of dust particles during occupational activity. In particular, we considered that all patients who had worked in the building sector were exposed to dust particles. This exposure was retained when it was reported in medical files.
Infection was defined by the association of compatible symptoms, imaging findings and/or microbiological data.
Anti-GM-CSF antibody assessment
We determined serum anti-GM-CSF antibody titres with a functional assay. We measured the ability of antibodies to neutralise the effect of recombinant cytokine GM-CSF on the growth of a GM-CSF-dependent erythroblastic cell line TF-1 [3, 12]. Briefly, serial serum dilutions were incubated with 1 ng·mL−1 GM-CSF (Cellgenix, Freiburg, Germany) and TF-1 cells. The proliferation of TF-1 cells was measured by incorporation of tritiated thymidine for each dilution. The resulting count-per-minute curve followed a sigmoidal curve, which allowed for the calculation of a standardised antibody titre inhibiting 50% of TF-1 cell proliferation (IC50) [3, 12]. Patients were considered positive for anti-GM-CSF antibody when the IC50 was >1.
All sera with detectable anti-GM-CSF antibodies using the functional method were also positive with the ELISA technique.
CT protocol
Chest CT was performed in a routine care setting, by using 64 or more detector row CT scans. All CT scans were acquired in volumetric mode with millimetre or submillimetre slice thickness. Of the 122 CT scans in the study, 21 were performed with contrast medium injection.
CT scan analysis
Visual quantification of pulmonary lesions was performed in consensus by three readers (with 17, 6 and 5 years of experience in chest imaging) blinded to other data, using a previously published method [13]. The extent of ground glass, reticular opacities, crazy paving, consolidation, honeycombing and the overall extent of lung disease was quantified in this way, with values rounded to the nearest 5%. In addition, the severity of fibrosis was scored using a categorical three-point scale as described by Hino et al. [14], where 0 indicates absence of fibrosis; 1 indicates traction bronchiectasis; 2 indicates parenchymal distortion; and 3 indicates honeycombing. Each item was defined according to the Fleischner Society [14–16]. Data were censored on 31 October 2021 for survival analyses. All CT analyses met standard quality criteria.
Statistical analysis
Results were expressed as median and interquartile range (IQR) or mean and sd for quantitative variables, and percentages for qualitative variables. Missing data for each variable were excluded from the denominator. Fisher's exact test was used for categorical variables. A Mann–Whitney U-test or t-test was used for continuous variable, as appropriate. Univariate logistic regression was used to determine potential risk factors for fibrotic evolution. No multivariate analysis was performed due to an insufficient total number of events (n=16). Results of logistic regression were expressed as an odds ratio (OR) and 95% confidence interval (CI). A p-value <0.05 was considered significant. Statistical analyses were conducted using R-Studio (R-Studio Team 2020: Integrated Development for R. R-Studio, PBC, Boston, MA, USA).
Results
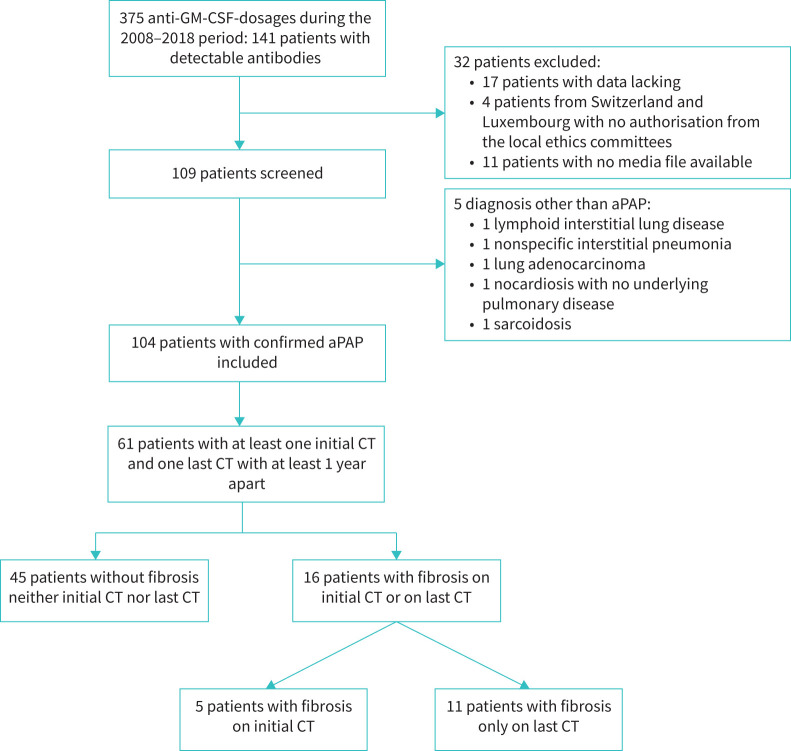
Between 2008 and 2018, anti-GM-CSF antibody analysis was performed in 375 patients, including 141 patients with detectable antibodies. Of these positive patients, 61 met the criteria for the present radiological analysis (figure 1). The diagnosis was obtained by BAL in 75% (46 out of 61) or by biopsy in 25% (15 out of 61) of cases. The mean follow-up was 4.6 years (±3.22 years).
FIGURE 1.
Flowchart of the study. GM-CSF: granulocyte–monocyte colony-stimulating factor; aPAP: autoimmune pulmonary alveolar proteinosis; CT: computed tomography.
Clinical characteristics of patients
The characteristics of patients are described in table 1. Compared with aPAP patients without pulmonary fibrosis, patients with fibrosis were significantly older (OR 47.6, 95% CI 40.5–55.0 versus 41.0, 33.0–49.0 years; p=0.046), were more frequently exposed to dust (62% versus 28%; p=0.015), and complained more frequently of dyspnoea (94% versus 65%; p=0.045).
TABLE 1.
Comparison of baseline characteristics between patients without fibrosis and patients with fibrosis on initial and on follow-up computed tomography (CT)
| Patients without fibrosis (n=45) | Patients with fibrosis on initial CT (n=5) | Patients with fibrosis on final CT (n=11) | Patients with fibrosis on initial or on final CT (n=16) | |
|---|---|---|---|---|
| Baseline characteristics at aPAP diagnosis | ||||
| Age, years | 41.0 (33.0–49.0) | 48.0 (47.0–56.0) | 41.0 (39.5–54.0)* | 47.6 (40.5–55.0)# |
| Male gender | 26 (58%) | 4 (80%) | 7 (64%) | 11 (69%) |
| GM-CSF titre | 600 (245–1155) | 300 (115–850) | 300 (100–750) | 300 (115–850) |
| Comorbidities | ||||
| Active smokers | 20 (47%) | 3 (60%) | 6 (55%) | 9 (56%) |
| COPD | 2 (4.5%) | 0 (0%) | 1 (9.1%) | 1 (6.2%) |
| Asthma | 4 (9.5%) | 0 (0%) | 1 (9.1%) | 1 (6.2%) |
| Dust exposure¶ | 12 (28%) | 3 (60%) | 7 (64%)* | 10 (62%)# |
| Diabetes mellitus | 3 (6.8%) | 0 (0%) | 2 (18%) | 2 (12%) |
| High blood pressure | 10 (23%) | 0 (0%) | 4 (36%) | 4 (25%) |
| Dyslipidaemia | 4 (9.1%) | 0 (0%) | 1 (9.1%) | 1 (6.2%) |
| Symptoms at diagnosis | ||||
| No symptoms | 5 (13%) | 0 (0%) | 1 (9.1%) | 1 (6.2%) |
| Cough | 20 (51%) | 3 (60%) | 7 (64%) | 10 (62%) |
| Dyspnoea | 28 (65%) | 5 (100%) | 10 (91%)* | 15 (94%)# |
| Sputum | 6 (15%) | 3 (60%) | 2 (18%) | 5 (31%) |
| Digital clubbing | 1 (2.6%) | 1 (20%) | 1 (9.1%) | 2 (12%) |
| Fever | 4 (10%) | 0 (0%) | 1 (9.1%) | 1 (6.2%) |
| Weight loss | 12 (32%) | 0 (0%) | 2 (18%) | 2 (13%) |
| Oxygen need | 8 (18%) | 2 (40%) | 3 (27%) | 5 (31%) |
| Functional data at aPAP diagnosis | ||||
| FVC (% predicted) | 82.5 (78.0–91.8) | 88.4 (80.0–90.0) | 86.5 (71.2–101) | 87.9 (73.2–103) |
| FEV1 (% predicted) | 83.2 (76.2–91.5) | 90.0 (87.0–90.8) | 85.0 (75.0–95.0) | 88.8 (80.0–97.0) |
| DLCO (% predicted) | 53.0 (36.8–70.0) | 60.0 (47.5–65.0) | 58.4 (44.0–63.5) | 59.0 (42.0–65.0) |
| 6MWT, m | 510 (472–521) | 490 (468–499) | 498 (470–509) | −2.00 (−4.00–9.00) |
| Desaturation during 6MWT | −0.500 (−6.50–5.75) | −8.00 (2.00–8.00) | −2.00 (−3.00–14.0) | 7.00 (2.00–9.00) |
| PaO2, mmHg | 72.0 (64.0–88.0) | 59.0 (47.0–61.0) | 68.0 (57.0–83.0) | 67.0 (56.0–82.0) |
| PCO2, mmHg | 34.7 (32.0–39.0) | 35.0 (34.2–35.8) | 36.0 (32.5–37.5) | 35.0 (32.8–37.2) |
| Treatments | ||||
| Whole-lung lavage | 25 (57%) | 3 (60%) | 7 (64%) | 10 (62%) |
| Inhaled GM-CSF therapy | 8 (21%) | 0 (0%) | 4 (36%) | 4 (25%) |
| Subcutaneous injection of GM-CSF | 15 (36%) | 1 (20%) | 4 (36%) | 5 (31%) |
| Rituximab | 12 (29%) | 2 (40%) | 1 (9.1%) | 3 (19%) |
| Other immunosuppressive treatment | 1 (2.4%) | 0 (0%) | 1 (10%) | 1 (7.1%) |
| Plasmapheresis | 0 (0%) | 0 (0%) | 1 (10%) | 1 (6.7%) |
| Infections | ||||
| All infections | 19 (42%) | 4 (80%) | 7 (64%) | 11 (69%) |
| Non-opportunistic infections | 16 (36%) | 2 (40%) | 6 (55%) | 8 (50%) |
| Opportunistic infections | 9 (20%) | 3 (60%) | 4 (36%) | 7 (44%) |
| Nocardia spp. | 4 (8.9%) | 0 (0%) | 1 (9.1%) | 1 (6.2%) |
| Clinical outcomes | ||||
| Complete resolution | 13 (32%) | 3 (60%) | 3 (30%) | 6 (40%) |
| All-cause mortality | 2 (4.4%) | 1 (20%) | 3 (27%)* | 4 (25%)# |
Data are expressed as median (interquartile range) or number (%). aPAP: autoimmune pulmonary alveolar proteinosis; GM-CSF: granulocyte–monocyte colony-stimulating factor; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; DLCO: diffusing capacity of the lung for carbon monoxide; 6MWT: 6-min walking test; PaO2: arterial oxygen tension; PCO2: carbon dioxide tension. ¶: Dust exposure includes patients who had worked in the building sector. *: p<0.05 compared with the column of 45 patients without fibrosis. #: p<0.05 compared with the column of 45 patients without fibrosis. Bold indicates significant values.
There was no significant difference regarding tobacco smoke exposure (47% versus 56%; p=0.51).
During follow-up, the infection rate was numerically higher in patients with fibrosis than in patients without fibrosis (62% versus 49%; p=0.068) and mortality was significantly higher (25% versus 4.4%; p=0.036).
Fibrotic evolution in aPAP patients treated with whole-lung lavage (WLL), rituximab or GM-CSF therapy was not more common than in patients who did not receive these treatments.
Radiological characteristics
Initial and follow-up CT findings are summarised in table 2. The median interval between initial and follow-up CT was 3.96 (2.00–6.88) years in patients without fibrosis and 3.60 (2.83–6.86) years in the fibrosis group (p=0.54). A fibrotic pattern was observed in 16 out of 61 (26.2%) aPAP patients in this cohort, including 5 at diagnosis and an 11 additional patients during follow-up.
TABLE 2.
Comparison of initial computed tomography (CT) and follow-up CT between patients with and without pulmonary fibrosis
| Patients without fibrosis (n=45) | Patients with fibrosis on initial CT (n=5) | Patients with fibrosis on final CT (n=11) | Patients with fibrosis on initial or on follow-up CT (n=16) | |
|---|---|---|---|---|
| Initial CT findings | ||||
| Total extent (%) | 45.0 (23.0–73.0) | 65.0 (50.0–70.0) | 40.0 (14.0–59.0) | 47.5 (17.3–68.5) |
| Crazy paving (%) | 40.0 (23.0–63.0) | 27.5 (13.5–55.2) | 40.0 (12.7–50.8) | 37.2 (12.6–56.7) |
| Reticular opacities (%) | 0 (0–0) | 0 (0–0) | 0 (0–0.9)* | 0 (0–0.49)* |
| Ground-glass opacification (%) | 2.25 (0–4.65) | 9.30 (3.50–9.75) | 0.90 (0–4.75) | 2.28 (0–6.9) |
| Condensations (%) | 0 (0–3.75) | 0 (0–1.50) | 0 (0–0) | 0 (0–0) |
| Honeycomb (%) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Emphysema | 13 (29%) | 3 (60%) | 2 (18%) | 5 (31%) |
| Adenopathy | 7 (16%) | 0 (0%) | 1 (9.1%) | 1 (6.2%) |
| Final CT findings | ||||
| Total extent (%) | 10.0 (5.00–28.0) | 10.0 (5.00–30.0) | 50.0 (34.0–79.0)* | 39.0 (19.5–78.5)* |
| Crazy paving (%) | 5.00 (0–24.7) | 0 (0–1.50) | 22.5 (3.90–64.5) | 7.5 (0–41.4) |
| Reticular opacities (%) | 0 (0–0) | 0 (0–9.00) | 0 (0–15.8)* | 0 (0–13.9)* |
| Ground-glass opacification (%) | 2.00 (0–4.65) | 2.50 (1.00–4.75) | 6.00 (1.74–10.6)* | 4.6 (1.1–8.3) |
| Condensations (%) | 0 (0–0) | 2.50 (0–4.75) | 0 (0–0) | 0 (0–2.6) |
| Honeycomb (%) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Emphysema | 13 (29%) | 2 (40%) | 3 (27%) | 5 (31%) |
| Adenopathy | 2 (4.4%) | 0 (0%) | 1 (9.1%) | 1 (6.2%) |
| Follow-up duration, years | 3.96 (2.00–6.88) | 3.60 (2.83–6.86) | 3.55 (2.88–6.65) | 3.60 (2.83–6.86) |
Data are expressed as median (interquartile range) or n (%). *: p<0.05 compared with the column of 45 patients without fibrosis. Bold indicates significant values.
5 out of 61 (8.2%) patients already had grade 1 fibrosis (traction bronchiectasis) at diagnosis. These five patients still had grade 1 fibrosis on the follow-up CT. 10 patients did not have fibrosis on the initial CT and developed grade 1 fibrosis on the follow-up CT, and 1 patient did not have fibrosis on the initial CT and developed grade 3 fibrosis (honeycombing) on the follow-up CT (figure 2). Among patients without fibrosis, the median overall extent of parenchymal abnormalities on the initial CT was 45% and decreased to 10% on the follow-up CT. In those patients, the median extent of the different patterns on initial and follow-up CT scans were crazy paving from 40.0% to 5.0% and ground-glass opacities from 2.2% to 2.0%. Patients with fibrosis on initial or follow-up CT had a median overall extent of parenchymal abnormalities on initial CT of 47.5% (17.3–68.5%), which decreased to 39% (19.5–78.5%) on follow-up CT. In those patients, the median extent of the patterns on initial and follow-up CT scans were crazy paving from 37.2% (12.6–56.7) to 7.5% (0.0–41.4) and ground-glass opacities from 2.3% (0.0–6.9) to 4.6% (1.1–8.3). Compared with aPAP patients without pulmonary fibrosis, there was no significant difference regarding the prevalence of the different patterns.
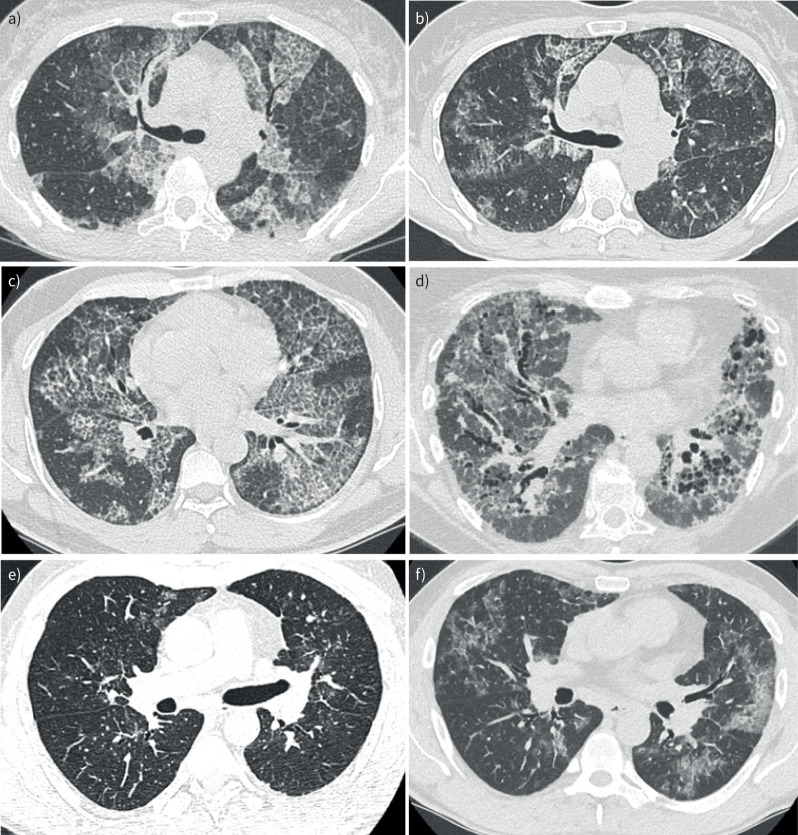
FIGURE 2.
Chest computed tomography (CT) scan images of three groups. a, b) Patient without fibrosis. Diagnosis, no fibrosis (a) and 26-month follow-up, no fibrosis (b). c, d) Patient without fibrosis on initial CT and with fibrosis on final CT. Diagnosis, no fibrosis (c) and 124-month follow-up, grade 3 fibrosis (d). e, f) Patient with fibrosis on initial CT and on final CT. Diagnosis, grade 1 fibrosis (e) and 37-month follow-up, grade 1 fibrosis (f).
Risk factors associated with occurrence of pulmonary fibrosis in aPAP patients
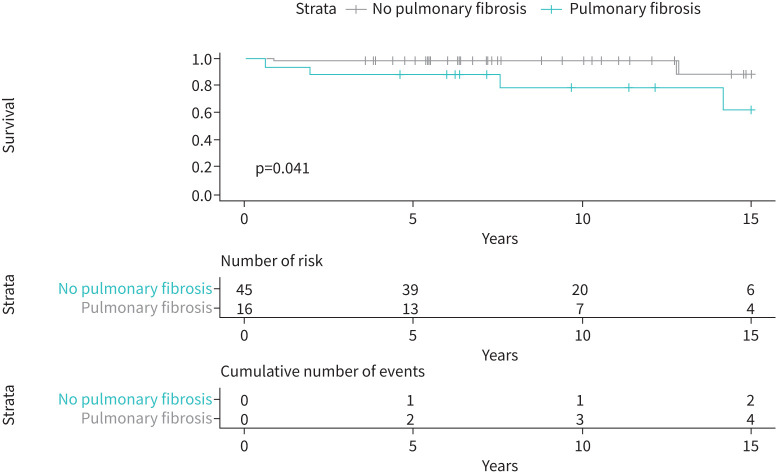
Among 56 patients without fibrosis at diagnosis (5 patients were excluded from this analysis due to fibrosis on the initial CT), 11 (19.6%) patients developed fibrosis between the initial CT and the follow-up CT (table 1). In univariate analysis, an older age (p=0.046; OR 1.06, 95% CI 1.00–1.12) and dust exposure (p=0.015; OR 4.3, 95% CI 1.32–15.3) were associated with the development of pulmonary fibrosis. No association was found for other covariates, including infections, WLL, tobacco smoke exposure and GM-CSF serum titre. The occurrence of fibrosis was associated with all-cause mortality (p=0.036; OR 7.17, 95% CI 1.25–56.44) (figure 3).
FIGURE 3.
Kaplan–Meier curve of survival.
Discussion
In this multicentre cohort of patients diagnosed with aPAP, we showed fibrotic changes on high-resolution CT, suggesting that fibrosis was frequent (26%) and associated with occupational dust exposure. We also demonstrated that patients with fibrosis had higher mortality rate compared with patients without fibrosis.
To the best of our knowledge, this cohort study of exclusively aPAP patients focused on CT analysis is the largest described in a non-Asian population so far. We found an occurrence of pulmonary fibrosis during follow-up in 19.6% of patients without any sign of parenchymal distortion at diagnosis. Akira et al. [10] found the presence of traction bronchiectasis on the initial CT scan in 4 out of 33 (12%) aPAP patients, which is approximately similar to our 5 out of 61 (8.2%). Furthermore, on their follow-up CT, Akira et al. [10] found 9 out of 33 (27%) traction bronchiectasis and 1 out of 33 (3%) honeycombing, which is also comparable with our population with 15 out of 61 (26%) of pulmonary fibrosis on follow-up CT, including 1 out of 61 patients with honeycombing (1.6%).
The relationship between dust exposure and pulmonary fibrosis is described in many studies outside the scope of PAP [17–20], especially the role of silica in systemic sclerosis-related interstitial lung disease [21–23]. Macrophages are the first line of defence against invading foreign bodies, which, in response to dust particles, produce transforming growth factor (TGF)-β. When TGF-β1 is activated, it promotes the transformation of fibroblasts to myofibroblasts, which is essential in the fibrotic processes. In fact, myofibroblasts can secrete type I and III collagen and are generally regarded as the main effector cells of fibrosis [24, 25].
These results may be extrapolated to our study. Dust exposure in the present aPAP cohort was significantly associated with pulmonary fibrosis occurrence. We can hypothesise that dust exposure in aPAP causes repeated damages to alveolar epithelial cells by activating inflammatory macrophages [26]. As previously mentioned, silica exposure activates the stimulator of interferon genes [27], therefore, this pathway may explain the fibrosis evolution in patients with aPAP exposed to dust. The link between silica exposure and PAP has already been reported, but mainly in acute presentations known as silicoproteinosis [11]. These patients might also have anti-GM-CSF antibodies. By showing a link between silica exposure, proteinosis and chronic fibrosis, our study adds to our understanding of the pathophysiology of this rare disease.
Pulmonary fibrosis has been suggested to be a treatment-related adverse effect of WLL, GM-CSF augmentation or oxygen administration [1]. However, in our cohort study, we did not find any association between these treatments of aPAP and pulmonary fibrosis occurrence. Our hypothesis is that, as suggested by Seymour and Presneill [28], pulmonary fibrosis may be a late complication of aPAP, especially in patients with active and persistent disease who consequently need more WLL and/or more lines of treatments and so, leading towards associations between treatments and pulmonary fibrosis evolution. A further argument in support of this hypothesis is that patients with fibrosis were significantly older. We demonstrated a higher mortality rate among patients with fibrosis on initial or on follow-up CT. This is explained by the poor outcome of pulmonary fibrosis. Only one death was related to complications independent of fibrosis and was caused by Pseudomonas aeruginosa pneumonia. All other deaths were secondary to chronic respiratory failure. In the literature, the 5-year survival is currently around 95%, which is consistent with our results [4, 28, 29].
Our study has limitations. First, this is a retrospective study with biases inherent to this type of research. Second, we only included 61 patients, but given the rarity of the disease, this is one of the largest studies on aPAP in a non-Asian population. Third, CT analysis was conducted visually through a semi-quantitative method. Visual analysis is known to lack reproducibility. However, current automatic tools are not yet sufficiently standardised to reliably quantify pulmonary lesions at CT [30]. In particular, the quantification of each CT pattern remains a highly complex task for artificial intelligence algorithms. By carrying out a consensus analysis by three experienced readers, we achieved pattern-based quantification, while limiting reproducibility issues. In the future, new deep-learning algorithms are likely to enable a fully automatic and comprehensive analysis of CT patterns [31].
To conclude, we showed that in aPAP patients, pulmonary fibrosis occurrence was not exceptional, was associated with dust exposure and with a higher mortality rate. Further research is needed to clarify what type of dust is involved and to better understand the pathophysiology of pulmonary fibrosis in aPAP patients.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00314-2024.SUPPLEMENT (565.8KB, pdf)
Acknowledgements
The authors thank Sylvain Marchand-Adam (Service de Pneumologie, CHRU de Tours), Emmanuel Bergot (Service de Pneumologie, CHU de Caen), Charles-Hugo Marquette (Service de Pneumologie, CHU de Nice), Philippe Bonniaud (Service de Pneumologie, CHU Dijon Bourgogne), Luc Thiberville (Service de Pneumologie, oncologie thoracique et soins intensifs, CHU de Rouen), Pierre-Régis Burgel (Service de pneumologie - CRCM - AP-HP - Université de Paris - Hôpital Cochin), Sébastien Quétant (Service de Pneumologie, CHU Grenoble Alpes), Elodie Blanchard (Service de Pneumologie, CHU de Bordeaux), Sandrine Hirschi (Centre de compétence des maladies pulmonaires rares de l'adulte, CHU de Strasbourg), David Bonnet (Centre Hospitalier de la Côte Basque), Loïc Grandon (Polyclinique de Poitiers), Marie Jouvenot (Centre hospitalier du Mans), Stephanie Dirou (Service de Pneumologie, CHU de Nantes) and Jean-Pierre Mallet (Service de Pneumologie, CHU de Montpellier).
Provenance: Submitted article, peer reviewed.
Ethics statement: The study was approved by the Ethics Committee of the University Hospital of Rennes (approval number 19.104).
Conflict of interest: C. Chenivesse declares having received grants from AstraZeneca, GlaxoSmithKline, Novartis and Santelys, personal fees from ALK-Abello, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline and Sanofi, and congress support from AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis and Sanofi. M. Lederlin has received fees, funding or reimbursement for national and international conferences, boards, expert or opinion groups and research projects over the past 5 years from AstraZeneca, Boehringer, Fresenius-Kabi and Siemens Healthcare. S. Jouneau has received fees, funding or reimbursement for national and international conferences, boards, expert or opinion groups, and research projects over the past 5 years from Actelion, AIRB, AstraZeneca, Bellorophon Therapeutics, Biogen, BMS, Boehringer, Chiesi, Fibrogen, Gilead, GSK, LVL, Mundipharma, Novartis, Olam Pharm, Pfizer, Pliant Therapeutics, Roche and Savara-Serendex. The other authors have nothing to disclose.
References
- 1.Trapnell BC, Nakata K, Bonella F, et al. Pulmonary alveolar proteinosis. Nat Rev Dis Primer 2019; 5: 16. doi: 10.1038/s41572-019-0066-3 [DOI] [PubMed] [Google Scholar]
- 2.Mabo A, Borie R, Wemeau-Stervinou L, et al. Infections in autoimmune pulmonary alveolar proteinosis: a large retrospective cohort. Thorax 2023; 79: 68–74. doi: 10.1136/thorax-2023-220040 [DOI] [PubMed] [Google Scholar]
- 3.Uchida K, Nakata K, Trapnell BC, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood 2004; 103: 1089–1098. doi: 10.1182/blood-2003-05-1565 [DOI] [PubMed] [Google Scholar]
- 4.Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med 2008; 177: 752–762. doi: 10.1164/rccm.200708-1271OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jouneau S, Kerjouan M, Briens E, et al. La protéinose alvéolaire pulmonaire [Pulmonary alveolar proteinosis]. Rev Mal Respir 2014; 31: 975–991. doi: 10.1016/j.rmr.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 6.Jouneau S, Ménard C, Lederlin M. Pulmonary alveolar proteinosis. Respirology 2020; 25: 816–826. doi: 10.1111/resp.13831 [DOI] [PubMed] [Google Scholar]
- 7.Kitamura T, Tanaka N, Watanabe J, et al. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med 1999; 190: 875–880. doi: 10.1084/jem.190.6.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey B, Trapnell BC. The molecular basis of pulmonary alveolar proteinosis. Clin Immunol Orlando Fla 2010; 135: 223–235. doi: 10.1016/j.clim.2010.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Da Nam B, Kim TJ, Chung MP, et al. CT findings in pulmonary alveolar proteinosis: serial changes and prognostic implications. J Thorac Dis 2018; 10: 5774–5783. doi: 10.21037/jtd.2018.09.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akira M, Inoue Y, Arai T, et al. Pulmonary fibrosis on high-resolution CT of patients with pulmonary alveolar proteinosis. Am J Roentgenol 2016; 207: 544–551. doi: 10.2214/AJR.15.14982 [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Abdelmalak B, Inoue Y, et al. Pulmonary alveolar proteinosis in adults: pathophysiology and clinical approach. Lancet Respir Med 2018; 6: 554–565. doi: 10.1016/S2213-2600(18)30043-2 [DOI] [PubMed] [Google Scholar]
- 12.Uchida K, Beck DC, Yamamoto T, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med 2007; 356: 567–579. doi: 10.1056/NEJMoa062505 [DOI] [PubMed] [Google Scholar]
- 13.Ley B, Elicker BM, Hartman TE, et al. Idiopathic pulmonary fibrosis: CT and risk of death. Radiology 2014; 273: 570–579. doi: 10.1148/radiol.14130216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hino T, Hida T, Nishino M, et al. Progression of traction bronchiectasis/bronchiolectasis in interstitial lung abnormalities is associated with increased all-cause mortality: age gene/environment susceptibility-Reykjavik study. Eur J Radiol Open 2021; 8: 100334. doi: 10.1016/j.ejro.2021.100334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner society: glossary of terms for thoracic imaging. Radiology 2008; 246: 697–722. doi: 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 16.Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society white paper. Lancet Respir Med 2018; 6: 138–153. doi: 10.1016/S2213-2600(17)30433-2 [DOI] [PubMed] [Google Scholar]
- 17.Zhang N, Liu K, Wang K, et al. Dust induces lung fibrosis through dysregulated DNA methylation. Environ Toxicol 2019; 34: 728–741. doi: 10.1002/tox.22739 [DOI] [PubMed] [Google Scholar]
- 18.Li N, Shi F, Wang X, et al. Silica dust exposure induces pulmonary fibrosis through autophagy signaling. Environ Toxicol 2021; 36: 1269–1277. doi: 10.1002/tox.23124 [DOI] [PubMed] [Google Scholar]
- 19.Cottin V, Crestani B, Cadranel J, et al. French practical guidelines for the diagnosis and management of idiopathic pulmonary fibrosis – 2017 update. Full-length version. Environ Toxicol 2017; 34: 900–968. doi: 10.1016/j.rmr.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 20.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. doi: 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballerie A, Cavalin C, Lederlin M, et al. Association of silica exposure with chest HRCT and clinical characteristics in systemic sclerosis. Semin Arthritis Rheum 2020; 50: 949–956. doi: 10.1016/j.semarthrit.2020.08.014 [DOI] [PubMed] [Google Scholar]
- 22.Lescoat A, Ballerie A, Lecureur V, et al. The neglected association of crystalline silica exposure and systemic sclerosis. Rheumatology (Oxford) 2020; 59: 3587–3588. doi: 10.1093/rheumatology/keaa638 [DOI] [PubMed] [Google Scholar]
- 23.Lescoat A, Ballerie A, Lelong M, et al. Crystalline silica impairs efferocytosis abilities of human and mouse macrophages: implication for silica-associated systemic sclerosis. Front Immunol 2020; 11: 219. doi: 10.3389/fimmu.2020.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Tian H, Liu H, et al. The role of macrophage-derived TGF-β1 on SiO2-induced pulmonary fibrosis: a review. Toxicol Ind Health 2021; 37: 240–250. doi: 10.1177/0748233721989896 [DOI] [PubMed] [Google Scholar]
- 25.Li J, Yao W, Hou JY, et al. The role of fibrocyte in the pathogenesis of silicosis. Biomed Environ Sci 2018; 31: 311–316. doi: 10.3967/bes2018.040 [DOI] [PubMed] [Google Scholar]
- 26.Laskin DL, Malaviya R, Laskin JD. Role of macrophages in acute lung injury and chronic fibrosis induced by pulmonary toxicants. Toxicol Sci 2019; 168: 287–301. doi: 10.1093/toxsci/kfy309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benmerzoug S, Rose S, Bounab B, et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat Commun 2018; 9: 5226. doi: 10.1038/s41467-018-07425-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med 2002; 166: 215–235. doi: 10.1164/rccm.2109105 [DOI] [PubMed] [Google Scholar]
- 29.Borie R, Danel C, Debray M-P, et al. Pulmonary alveolar proteinosis. Eur Respir Rev 2011; 20: 98–107. doi: 10.1183/09059180.00001311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansell DM, Goldin JG, King TE, et al. CT staging and monitoring of fibrotic interstitial lung diseases in clinical practice and treatment trials: a position paper from the Fleischner Society. Lancet Respir Med 2015; 3: 483–496. doi: 10.1016/S2213-2600(15)00096-X [DOI] [PubMed] [Google Scholar]
- 31.Romei C, Tavanti LM, Taliani A, et al. Automated computed tomography analysis in the assessment of idiopathic pulmonary fibrosis severity and progression. Eur J Radiol 2020; 124: 108852. doi: 10.1016/j.ejrad.2020.108852 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00314-2024.SUPPLEMENT (565.8KB, pdf)