Abstract
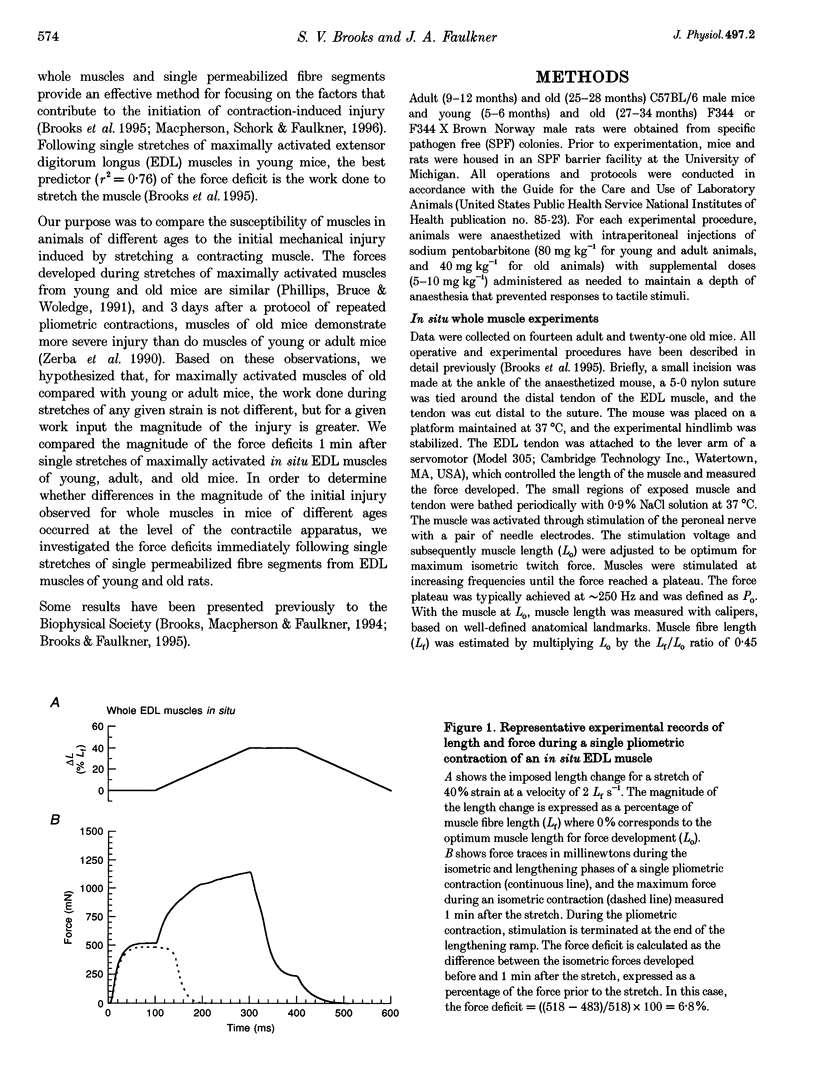
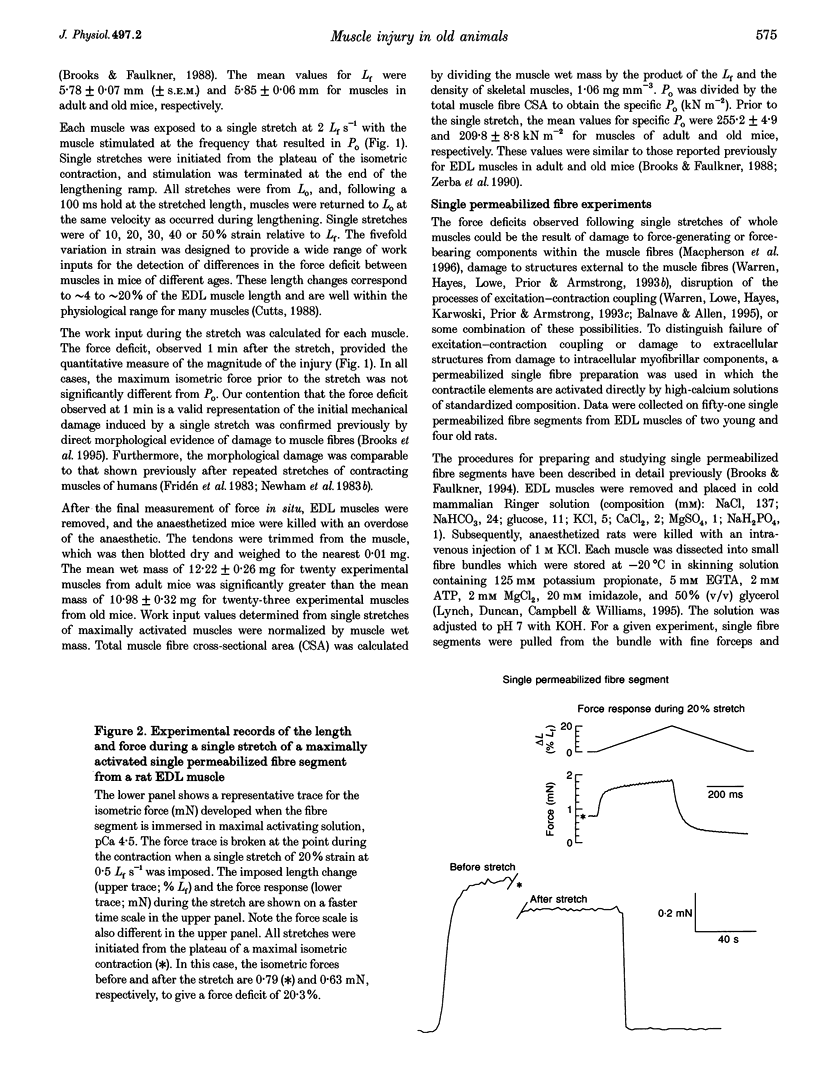
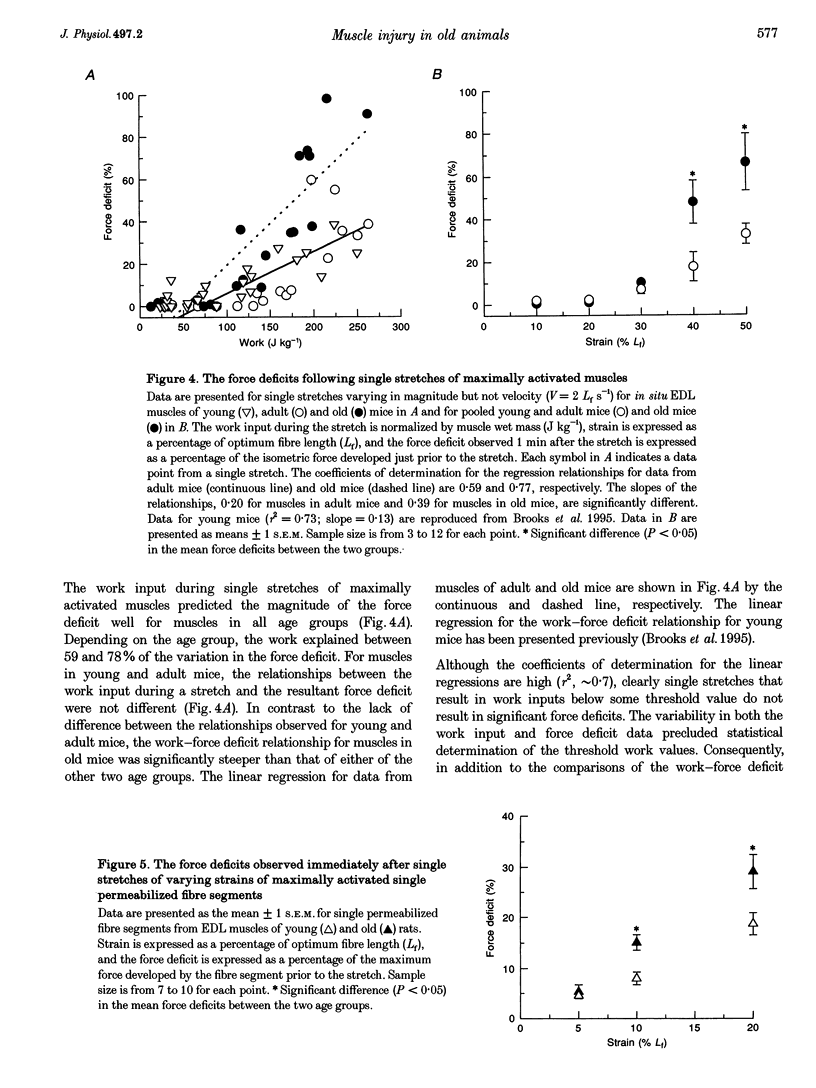
1. Our purpose was to compare the susceptibilities of muscles in animals of different ages to the injuries induced by stretching the contracting muscle. Single stretches provide an effective method for studying the factors that contribute to the initiation of contraction-induced injury. We hypothesized that, for maximally activated muscles in old compared with young or adult mice, the work input during a single stretch of any given strain is not different, but for a given work input the magnitude of the injury is greater. 2. The force deficit resulting from each single stretch was calculated as the decrease in the maximum isometric force expressed as a percentage of the maximum force prior to the stretch. Force deficits were compared 1 min after single stretches of in situ extensor digitorum longus (EDL) muscles of young, adult and old mice. In addition, measurements of force deficits immediately following single stretches of single permeabilized fibre segments from EDL muscles of young and old rats permitted investigation of the initial injury at the level of the contractile apparatus. 3. For maximally activated EDL muscles in young, adult and old mice, no differences were observed for the work input during stretches of any given strain. Furthermore, the relationships between the work and the resultant force deficit were not different for muscles in young and adult mice. In contrast, compared with the work-force deficit relationships for muscles in either young or adult mice, the relationship was significantly steeper for muscles in old mice. For single permeabilized fibres from muscles of old rats, the force deficits immediately after single stretches were greater than those observed for fibres from muscles of young rats. We conclude that the increased susceptibility of muscles in old animals to contraction-induced injury resides at least in part within the myofibrils.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnaqeeb M. A., Al Zaid N. S., Goldspink G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat. 1984 Dec;139(Pt 4):677–689. [PMC free article] [PubMed] [Google Scholar]
- Balnave C. D., Allen D. G. Intracellular calcium and force in single mouse muscle fibres following repeated contractions with stretch. J Physiol. 1995 Oct 1;488(Pt 1):25–36. doi: 10.1113/jphysiol.1995.sp020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. V., Faulkner J. A. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988 Oct;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. V., Faulkner J. A. Isometric, shortening, and lengthening contractions of muscle fiber segments from adult and old mice. Am J Physiol. 1994 Aug;267(2 Pt 1):C507–C513. doi: 10.1152/ajpcell.1994.267.2.C507. [DOI] [PubMed] [Google Scholar]
- Brooks S. V., Zerba E., Faulkner J. A. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J Physiol. 1995 Oct 15;488(Pt 2):459–469. doi: 10.1113/jphysiol.1995.sp020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K., Zagotta W. N., Baskin R. J. Sarcomere length behaviour along single frog muscle fibres at different lengths during isometric tetani. J Muscle Res Cell Motil. 1989 Feb;10(1):67–84. doi: 10.1007/BF01739857. [DOI] [PubMed] [Google Scholar]
- Cutts A. The range of sarcomere lengths in the muscles of the human lower limb. J Anat. 1988 Oct;160:79–88. [PMC free article] [PubMed] [Google Scholar]
- Fridén J., Sjöström M., Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983 Aug;4(3):170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- Higuchi H., Yoshioka T., Maruyama K. Positioning of actin filaments and tension generation in skinned muscle fibres released after stretch beyond overlap of the actin and myosin filaments. J Muscle Res Cell Motil. 1988 Dec;9(6):491–498. doi: 10.1007/BF01738754. [DOI] [PubMed] [Google Scholar]
- Jones D. A., Newham D. J., Round J. M., Tolfree S. E. Experimental human muscle damage: morphological changes in relation to other indices of damage. J Physiol. 1986 Jun;375:435–448. doi: 10.1113/jphysiol.1986.sp016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres at lengths above the optimum. J Physiol. 1980 Jul;304:529–539. doi: 10.1113/jphysiol.1980.sp013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L., Edström L. Effects of age on enzyme-histochemical fibre spectra and contractile properties of fast- and slow-twitch skeletal muscles in the rat. J Neurol Sci. 1986 Nov;76(1):69–89. doi: 10.1016/0022-510x(86)90143-7. [DOI] [PubMed] [Google Scholar]
- Lieber R. L., Fridén J. Muscle damage is not a function of muscle force but active muscle strain. J Appl Physiol (1985) 1993 Feb;74(2):520–526. doi: 10.1152/jappl.1993.74.2.520. [DOI] [PubMed] [Google Scholar]
- Lynch G. S., Duncan N. D., Campbell S. P., Williams D. A. Endurance training effects on the contractile activation characteristics of single muscle fibres from the rat diaphragm. Clin Exp Pharmacol Physiol. 1995 Jun-Jul;22(6-7):430–437. doi: 10.1111/j.1440-1681.1995.tb02035.x. [DOI] [PubMed] [Google Scholar]
- Mazzeo R. S., Brooks G. A., Horvath S. M. Effects of age on metabolic responses to endurance training in rats. J Appl Physiol Respir Environ Exerc Physiol. 1984 Nov;57(5):1369–1374. doi: 10.1152/jappl.1984.57.5.1369. [DOI] [PubMed] [Google Scholar]
- McCully K. K., Faulkner J. A. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J Appl Physiol (1985) 1986 Jul;61(1):293–299. doi: 10.1152/jappl.1986.61.1.293. [DOI] [PubMed] [Google Scholar]
- McCully K. K., Faulkner J. A. Injury to skeletal muscle fibers of mice following lengthening contractions. J Appl Physiol (1985) 1985 Jul;59(1):119–126. doi: 10.1152/jappl.1985.59.1.119. [DOI] [PubMed] [Google Scholar]
- Morgan D. L. New insights into the behavior of muscle during active lengthening. Biophys J. 1990 Feb;57(2):209–221. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newham D. J., Jones D. A., Edwards R. H. Large delayed plasma creatine kinase changes after stepping exercise. Muscle Nerve. 1983 Jun;6(5):380–385. doi: 10.1002/mus.880060507. [DOI] [PubMed] [Google Scholar]
- Newham D. J., McPhail G., Mills K. R., Edwards R. H. Ultrastructural changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983 Sep;61(1):109–122. doi: 10.1016/0022-510x(83)90058-8. [DOI] [PubMed] [Google Scholar]
- Palokangas H., Kovanen V., Duncan A., Robins S. P. Age-related changes in the concentration of hydroxypyridinium crosslinks in functionally different skeletal muscles. Matrix. 1992 Aug;12(4):291–296. doi: 10.1016/s0934-8832(11)80081-8. [DOI] [PubMed] [Google Scholar]
- Phillips S. K., Bruce S. A., Woledge R. C. In mice, the muscle weakness due to age is absent during stretching. J Physiol. 1991 Jun;437:63–70. doi: 10.1113/jphysiol.1991.sp018583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. K., Wiseman R. W., Woledge R. C., Kushmerick M. J. Neither changes in phosphorus metabolite levels nor myosin isoforms can explain the weakness in aged mouse muscle. J Physiol. 1993 Apr;463:157–167. doi: 10.1113/jphysiol.1993.sp019589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H. L., Corteselli S. A., Kushmerick M. J. Measurements on permeabilized skeletal muscle fibers during continuous activation. Am J Physiol. 1987 May;252(5 Pt 1):C575–C580. doi: 10.1152/ajpcell.1987.252.5.C575. [DOI] [PubMed] [Google Scholar]
- Warren G. L., Hayes D. A., Lowe D. A., Armstrong R. B. Mechanical factors in the initiation of eccentric contraction-induced injury in rat soleus muscle. J Physiol. 1993 May;464:457–475. doi: 10.1113/jphysiol.1993.sp019645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. L., Hayes D. A., Lowe D. A., Prior B. M., Armstrong R. B. Materials fatigue initiates eccentric contraction-induced injury in rat soleus muscle. J Physiol. 1993 May;464:477–489. doi: 10.1113/jphysiol.1993.sp019646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. L., Lowe D. A., Hayes D. A., Karwoski C. J., Prior B. M., Armstrong R. B. Excitation failure in eccentric contraction-induced injury of mouse soleus muscle. J Physiol. 1993 Aug;468:487–499. doi: 10.1113/jphysiol.1993.sp019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. A., Morgan D. L., Proske U. Effects of repeated eccentric contractions on structure and mechanical properties of toad sartorius muscle. Am J Physiol. 1993 Sep;265(3 Pt 1):C792–C800. doi: 10.1152/ajpcell.1993.265.3.C792. [DOI] [PubMed] [Google Scholar]
- Zerba E., Komorowski T. E., Faulkner J. A. Free radical injury to skeletal muscles of young, adult, and old mice. Am J Physiol. 1990 Mar;258(3 Pt 1):C429–C435. doi: 10.1152/ajpcell.1990.258.3.C429. [DOI] [PubMed] [Google Scholar]


