Abstract
Introduction
Chronic cough is considered a disorder of neuronal hypersensitivity in which patients frequently report abnormal laryngeal and chest sensations, and excessive triggers. To facilitate clinical assessment, we developed the Cough Hypersensitivity Questionnaire (CHQ).
Methods
Candidate questionnaire items were developed following interviews with patients with refractory chronic cough (n=10, United Kingdom), and review by a multidisciplinary team. The CHQ was evaluated in individuals with chronic cough (n=535, UK/South Korea), for unidimensionality and differential item functioning (with Rasch analysis), internal consistency, concurrent validity (against cough severity visual analogue scale (VAS) and Leicester Cough Questionnaire (LCQ) scores), and content validity (cognitive debriefing interviews, n=13).
Results
Concept elicitation created a pool of 34 items. Eleven items were removed following multidisciplinary team review of patient interviews. Rasch analysis confirmed the CHQ total score to be a unidimensional scale; one item was removed due to differential item functioning. The final 22 binary-item CHQ comprises 6 sensation-related and 16 trigger-related items. Median (interquartile range) total CHQ scores were 9 (6–12); sensations 4 (2–5) and triggers 5 (3–8). Internal consistency was good (person separation index 0.74). The CHQ total score was moderately associated with cough severity VAS (0.42, p=0.005) and LCQ total score (ρ=−0.52, p<0.001). In cognitive debriefing, patients found that the CHQ was relevant to their condition and simple to complete.
Conclusion
The CHQ is simple to use and has validity for assessing cough triggers and sensations in patients with chronic cough. Further studies are needed to assess its repeatability, responsiveness and clinical utility.
Shareable abstract
The Cough Hypersensitivity Questionnaire assesses 6 cough sensations and 16 triggers. It was developed with patient interviews and validated in patients with chronic cough (n=535). Patients found it easy, quick and relevant to all chronic coughs. https://bit.ly/3WaQjC0
Introduction
Chronic cough is a common condition that affects around 10% of the global population [1] and is associated with a substantial impairment of quality of life [1–4]. Chronic cough that persists despite optimal treatment of underlying conditions is deemed refractory, whereas cough with no clear precipitant is unexplained [3]. Chronic cough is now considered a disorder of sensory neuronal dysfunction [5–7]. The condition is associated with cough reflex hypersensitivity to tussive agents such as capsaicin, citric acid and ATP [8]. The symptom profile of chronic cough is consistent with hypersensitivity, with features of laryngeal paraesthesia (e.g. throat tickle), allotussia (e.g. cough triggered by stimuli that are not considered irritants, such as talking) and hypertussia (e.g. excessive cough to smoke and other inhaled irritants) [9]. These are akin to the core features of neuropathic pain; paraesthesia, allodynia and hyperalgesia, respectively [10]. Potential triggers of cough are numerous, vary significantly between patients, and may indicate underlying pathology [9, 11]. However, the extent of specific cough triggers and sensations are not frequently captured in clinical consultations despite advocation in recent guidelines and consensus statements [3, 12]. Patients infrequently report cough sensations or triggers spontaneously and, when prompted during consultation, have often not considered them. The trigger profile is important as it alerts the clinician to the possibility of cough reflex hypersensitivity as the basis of chronic cough [9, 13], thus having the potential to guide diagnosis and personalised management [3, 14–16]. Currently, there are few dedicated tools that assess the range of cough triggers and sensations associated with chronic cough. The aim of this study was to develop the Cough Hypersensitivity Questionnaire (CHQ), a user-friendly tool that assesses and quantifies triggers and sensations associated with chronic cough.
Methods
Participants
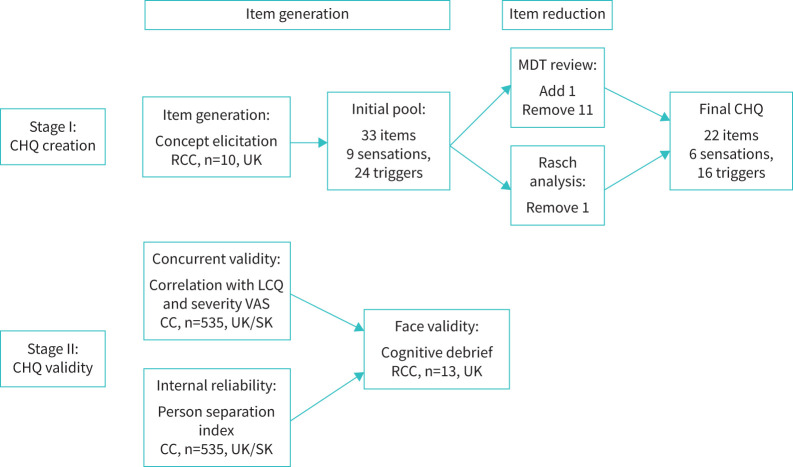
Patients with chronic cough were recruited from specialist cough clinics. Chronic cough was defined as cough lasting more than 8 weeks and all patients were managed as per European guidelines [3]. Concept elicitation interviews to generate the CHQ items were conducted in patients with refractory chronic cough (RCC), recruited from the United Kingdom and described in a previous study (Cohort 1) [17]. RCC is defined as a cough persisting despite guideline-driven treatment of treatable traits and includes patients with unexplained chronic cough, defined as a cough with no explanation despite extensive investigation [3, 16]. To evaluate the CHQ fit to a unidimensional model (Rasch analysis), a separate cohort of patients under investigation for chronic cough were recruited from specialist clinics in the United Kingdom and South Korea (Cohort 2). The Korean translation was developed by curation of an item concept framework, forward and backward translations by two experienced individuals, followed by reconciliation for differences. Concurrent validity was investigated in a subgroup of consecutive patients who completed cough severity and quality of life questionnaires (Cohort 2). Face validity of the final CHQ version was assessed in cognitive debriefing interviews in Cohort 3, all patients had RCC and were from the United Kingdom (figure 1). All participants gave informed consent, and the study was approved by East London and the City Research Ethics Committee (REC reference 10/H0703/6) and the participating institutions in South Korea (H-1602-024-739).
FIGURE 1.
Consort flow diagram of Stage I (CHQ creation) and Stage II (CHQ validity). CHQ: Cough Hypersensitivity Questionnaire; RCC: refractory or unexplained chronic cough; CC: chronic cough; SK: South Korea; MDT: multidisciplinary team; LCQ: Leicester Cough Questionnaire; VAS: visual analogue scale.
Item generation (Cohort 1)
Concept elicitation was performed with semi-structured interviews and qualitative methodology as previously described [17]. Open-ended discussions in consecutive patients with RCC (n=10) assessed a wide range of cough sensations and triggers [17]. Interviews were conducted by a single researcher trained in qualitative patient interviews, who was not directly involved in the patients’ clinical care. Items elicited by more than one participant were prompted in subsequent interviews. Concept saturation was predefined as no new concepts elicited in two consecutive interviews and was achieved after the eighth interview. Furthermore, multidisciplinary team (MDT) discussion considered additional items, and proposed a user-friendly scoring system. The MDT consensus was to use a broad recall period referred to as “recently” rather than specified, such as “past day”. This was to ensure that important triggers avoided by patients or encountered occasionally were not excluded.
Item reduction
A multidisciplinary team comprising respiratory physicians and physiotherapists reviewed items generated from concept elicitation. The review aimed to retain items elicited by >2 participants (Cohort 1) and considered removal of items that were potentially a consequence rather than a feature preceding coughing, overlapping concepts and those with confusing terminology. A floor effect was considered if ≥15% participants answered “no” in Cohort 2 [18]. Furthermore, items with high inter-item correlations (coefficients >0.80) were considered for elimination [19]. Items were also removed if they did not fit the Rasch analysis model of unidimensional scale.
Rasch analysis: testing fit to a unidimensional model (Cohort 2)
Rasch analysis, a type of psychometric analysis [20], was conducted using the data from Cohort 2. This analysis is conducted iteratively, inspecting a variety of psychometric criteria. If the overall model provides an adequate fit, item–trait interaction is expected to be nonsignificant. Rasch analysis allows items to be placed within a continuum of item difficulty, and a strong psychometric scale is expected to have a range of so-called easy as well as difficult items. Another important criterion is that item difficulty is equivalent across subgroups of interest (e.g. sex). Differential item functioning (DIF) was assessed for variables: sex, country and diagnosis.
Concurrent validity and internal reliability (Cohort 2)
Concurrent validity, a measure of the association between CHQ and other cough end-points, was assessed in Cohort 2 with cough severity visual analogue scale (VAS) (range 0–100 mm) and Leicester Cough Questionnaire (LCQ) (range 3–21, where lower scores indicate a worse cough-specific health status). Internal reliability, a measure of the interrelatedness of items, was assessed in Cohort 2 by the person separation index (PSI), which applies to categorical scales such as the CHQ, and is interpreted similarly to Cronbach's alpha, where values >0.70 indicate adequate reliability [20].
Cognitive debriefing (Cohort 3)
Patients with RCC from the United Kingdom underwent semi-structured interviews to assess the face validity of the final version of the CHQ (full methodology in supplementary material). Each CHQ item was assessed for simplicity and relevance in chronic cough, and illustrative quotes were documented. Overall opinions on the CHQ were sought, and participants were asked about questionnaire simplicity, ease of understanding, the binary scoring system and the total score concept.
Analysis
A D'Agostino–Pearson test was employed to evaluate normality. Parametric data were expressed as mean±sd and nonparametric data were presented as median (interquartile range (IQR)). Unpaired parametric data were subject to comparison using a t-test, and nonparametric data were compared using Mann–Whitney U-tests. Correlations between variables were assessed using the Spearman correlation coefficient (ρ) for nonparametric data. Comparison of categorical data between groups was performed using chi-squared tests. Rasch was conducted using the software package RUMM2030 [21]. All remaining analyses were performed on Prism® version 10.0.1 (GraphPad Software, San Diego, CA, USA) or IBM SPSS Statistics version 28.0.1 (IBM Corporation, Armonk, NY, USA).
Results
Participant demographics
Participant demographics are presented in table 1. Participants were predominantly middle-aged and female. There were no significant differences in age or sex between the participants recruited for CHQ development (Cohort 1, United Kingdom), validation (Cohort 2, United Kingdom n=57 and South Korea n=478) and cognitive debrief (Cohort 3, United Kingdom) or between UK versus South Korea patients (table 1). The participants in all cohorts had a moderate-to-severe cough severity (VAS) and impairment in quality of life (LCQ) (table 1).
TABLE 1.
Participant demographics
| Cohort 1 CHQ development (UK) |
Cohort 2 Validation (UK/South Korea) |
Cohort 3 Cognitive debrief (UK) |
|
|---|---|---|---|
| Number | 10 | 535 | 13 |
| Age, years | 63 (55–67) | 58 (45–67) | 56 (46–70) |
| Female sex, n (%) | 7 (70) | 371 (69) | 9 (69) |
| Smoking status, n (%) | |||
| Never | 7 (70) | 383 (73) | 9 (69) |
| Ex-smoker | 2 (20) | 102 (20) | 4 (31) |
| Current | 1 (10) | 37 (7) | 0 (0) |
| Duration of cough, months, n (%) | |||
| 0 to ≤6 | 0 | 214 (41) | 0 |
| 6 to ≤12 | 0 | 69 (13) | 0 |
| 12 to ≤60 | 3 (30) | 146 (28) | 6 (46) |
| >60 | 7 (70) | 98 (18) | 7 (54) |
| VAS cough severity | 74±12 | 61±20 | 64±21 |
| LCQ | |||
| Physical | 3.5 (2.7–3.9) | 4.0 (4.0–5.0) | 3.8 (2.7–5.9) |
| Psychological | 2.1 (1.7–2.6) | 4.0 (2.0–4.0) | 2.7 (1.9–4.9) |
| Social | 2.0 (1.8–3.1) | 3.0 (2.0–4.3) | 2.9 (1.8–4.7) |
| Total score | 7.5 (6.8–9.0) | 11.0 (9.0–13.0) | 9.7 (7.1–14.9) |
| CHQ | |||
| Sensations | 4.5 (2.8–5.0) | 4 (2–5) | 4 (3–5) |
| Triggers | 9.5 (7.5–12.3) | 5 (3–8) | 9 (8–12) |
| Total score | 15 (11–18) | 9 (6–12) | 14 (12–16) |
Data are displayed as mean±sd or median (interquartile range) unless otherwise stated. CHQ: Cough Hypersensitivity Questionnaire (total score range 0–22); VAS: visual analogue scale (range 0–100 mm); LCQ: Leicester Cough Questionnaire (total score range 3–21).
CHQ item generation and scale
33 distinct items were identified in a previous concept elicitation study in patients from the United Kingdom with RCC. MDT and patient consensus categorised these items into two broad domains: cough sensations and cough triggers, with concept elicitation identifying 9 sensations and 24 triggers [17]. A further sensation, “throat clearing”, was added following MDT discussion as this was considered a common symptom, increasing the pool to 34 items (supplementary table E5). A multidisciplinary team proposed a yes/no binary scoring system, for simplicity and ease of completing the questionnaire given the large number of triggers and sensations. A total score was calculated as the sum of all triggers and sensations answered “Yes” after a test of unidimensionality.
Item reduction
Multidisciplinary team review
11 items were eliminated after concept elicitation in UK patients with RCC, by MDT review (supplementary table E7). The reasons for item elimination were low-frequency items (≤2 participants, n=11), item content overlap (n=6), symptoms considered a consequence of cough rather than trigger or sensation (n=2) and relevance to a limited patient population (n=3).
Floor effects/inter-item correlations
There were no significant floor effects (<15% participants reporting “yes”). Ceiling effects were not assessed as it was considered important to identify triggers that occur commonly and could be useful diagnostically. Inter-item correlation coefficients ranged from −0.06 to 0.67 and were all <0.80, the threshold for eliminating high correlation items.
Rasch analysis (unidimensional model testing)
The amount of missing data was minimal from Cohort 2 (United Kingdom/South Korea), accounting for less than 1%. The baseline model contained all 23 items. Item–trait interaction was nonsignificant (χ2=(115)=130.80; p>0.05), indicating that the overall fit to the Rasch model was acceptable. No items exhibited elevated fit residuals. Given the binary nature of the response scale, there were no disordered thresholds. Significant DIF by sex was noted for the item “throat clearing”, whereby the item was easier to endorse for males than for females. As a result, this item was removed. Male participants also reported significantly higher throat clearing than females; 82% versus 68%, p=0.039. There was no DIF for sex or participant country of origin. The overall fit for the remaining 22-item CHQ to the model was adequate (nonsignificant), and PSI was 0.74. An equating Smith's test confirmed that the solution was unidimensional [22]. Item difficulty values are presented in supplementary table E6; negative values indicating easier items, and positive values more difficult to score. The easiest item was “tickle in throat” (item difficulty −1.53) and the most difficult item was “indigestion” (+1.38). The final version of CHQ had 22 items (table 2). Information about item difficulty is shown in supplementary table E6.
TABLE 2.
Cough Hypersensitivity Questionnaire final version
| This questionnaire assesses the sensations and triggers associated with your cough recently Please complete ALL questions, by SELECTING the responses that best apply to you |
|---|
| Have you experienced any of the following sensations in relation to your cough? |
| Noticeable urge to cough before coughing starts |
| Tickle in throat |
| Itchy throat |
| Dry throat |
| Irritation in throat |
| Cough originating from a sensation in the chest |
| Do any of the following trigger your cough? |
|---|
| Cold air |
| Hot air |
| Dry air |
| Damp |
| Perfumes and scents |
| Smoke or smoky atmosphere |
| Talking |
| Laughing |
| Eating or drinking |
| Heartburn |
| Indigestion |
| Change in body position (e.g. lying down) |
| Exercise |
| Brushing teeth |
| Sputum (phlegm) |
| Post-nasal drip (dripping sensation in the back of the throat) |
© Professor Surinder Birring, 2024.
Concurrent validity and internal reliability
There was a moderate correlation between the CHQ total score and cough severity VAS; ρ= 0.42, p=0.005 (figure 2; subgroup who underwent VAS assessment). There was also a moderate correlation between CHQ total score and LCQ total score; ρ=−0.52, p<0.001 (figure 3). The PSI for the CHQ total score was 0.74, indicative of good internal consistency reliability.
FIGURE 2.
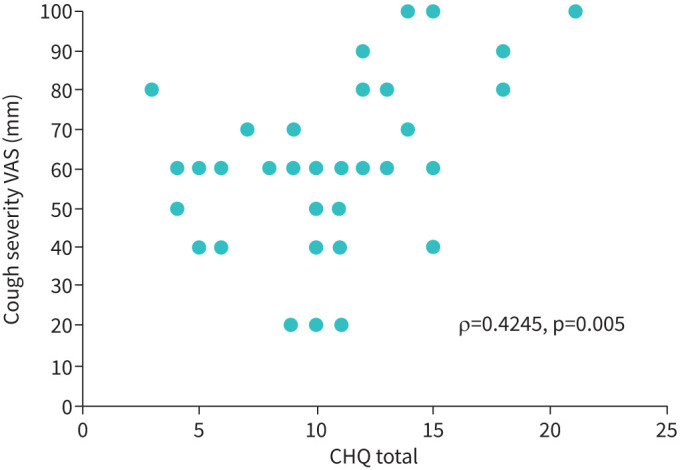
The relationship between the Cough Hypersensitivity Questionnaire (CHQ) and cough severity visual analogue scale (VAS) (Cohort 2).
FIGURE 3.
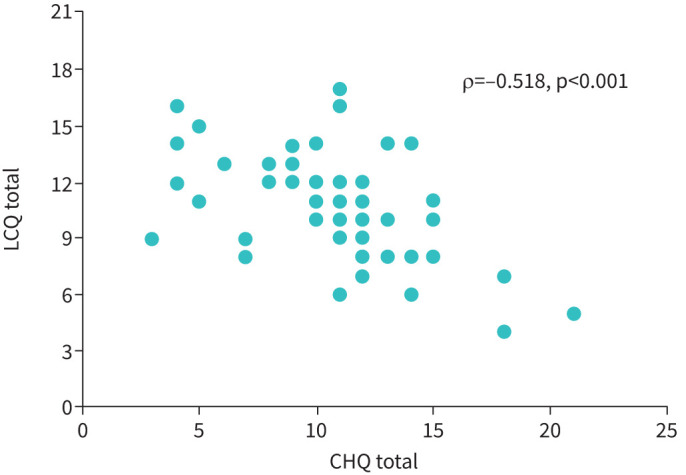
The relationship between the Cough Hypersensitivity Questionnaire (CHQ) and Leicester Cough Questionnaire (LCQ) (Cohort 2).
CHQ characteristics (Cohort 2)
The median (IQR) CHQ total and domain scores are presented in table 1. There was a weak correlation between age and CHQ total score (ρ=−0.02), triggers domain (ρ=0.14) and sensations (ρ=−0.23), all p<0.001. The CHQ total scores were slightly higher in females compared with males; median (IQR) 10 (6–13) versus 8 (6–11) (p=0.014). The frequencies of CHQ triggers are presented in table 3; the top three triggers were “cold air”, “dry air” and “smoke”.
TABLE 3.
Prevalence of cough triggers in participants with chronic cough (Cohort 2, n=535)
| Triggers | Participants, % |
|---|---|
| Cold air | 75 |
| Dry air | 60 |
| Smoke | 56 |
| Sputum | 56 |
| Postural change | 48 |
| Talking | 47 |
| Post-nasal drip | 37 |
| Perfumes | 32 |
| Exercise | 28 |
| Eating | 25 |
| Laughing | 22 |
| Damp | 21 |
| Hot air | 21 |
| Brushing teeth | 19 |
| Heartburn | 19 |
| Indigestion | 17 |
Cognitive debriefing (Cohort 3)
Cognitive debrief interviews to assess the face validity of the CHQ are summarised in supplementary document E1. In brief, 13 participants with RCC completed the CHQ in a median (IQR) of 64 (51–75) s with a total score of 12 (11–17). Each item was reported by at least three participants and no items were reported by everyone. The majority of items (n=19, 86%) were considered relevant by all participants. Half the items (n=11) were considered easy to answer by all participants. All participants reported that the CHQ was easy to fill in, with easy-to-understand instructions, was relevant to all patients with chronic cough, and the length was “about right”, whereas 11 (85%) considered it complete. The binary scoring system was considered appropriate by 86% participants and the total scoring system by 100%.
Discussion
We developed the CHQ by interviewing patients with RCC to elicit important concepts that trigger their cough. The CHQ had a good fit to a unidimensional model as demonstrated through Rasch analysis and had good internal reliability. Cognitive debrief and face validity was consistent with the CHQ being simple to complete and highly relevant to patients with chronic cough.
RCC is associated with numerous laryngeal sensations and cough triggers, which can be demonstrated in our study of RCC using the CHQ [17]. The sensations and triggers revealed by CHQ are consistent with symptoms of neurally mediated cough hypersensitivity, namely laryngeal paraesthesia (e.g. throat tickle), allotussia (e.g. cough triggered by talking) and hypertussia (e.g. excessive cough to smoke trigger) [9]. Multiple triggers were a common feature in RCC, with median total CHQ scores ranging from 9–15, depending on the population studied. Consistent with known sex differences in chronic cough and cough hypersensitivity [3, 8], female participants had significantly higher CHQ scores compared with males at 10 (6–13) versus 8 (6–11), respectively (p=0.01).
The CHQ is a simple tool that takes approximately 1 min to complete. The checklist of cough sensations and triggers can alert clinicians to the possibility of cough hypersensitivity, possibly at an earlier stage during patient investigation and in future, may guide management options. Clinical experience in chronic cough suggests that patients often do not realise the presence or relevance of cough triggers and sensations, and may therefore need prompting about them. However, in a busy clinic with limited time for consultation, it is difficult to assess the large range of potential triggers. The CHQ may therefore provide information that will aid consultation and could quickly be completed online before the clinic or in a clinic waiting room. For patients, recognition of specific triggers may help them to instigate lifestyle measures aimed at trigger reduction, avoidance or modification, thus reducing the burden of cough. The CHQ may also prove useful in research settings. The systematic nature by which the CHQ identifies sensations and triggers allows for standardised evaluation. The CHQ may therefore be used to study both the presence of cough hypersensitivity symptoms and the response to cough hypersensitivity pharmacotherapy.
Further studies with the CHQ are needed to establish the range of cough sensations and triggers in healthy controls, in a larger cohort of RCC and in chronic lung diseases such as asthma or interstitial lung disease. The potential of using CHQ thresholds to identify RCC at an earlier stage in clinical evaluation needs further study. The repeatability of the CHQ in large cohorts needs to be established. Finally, studies investigating whether the CHQ can predict responsiveness to novel antitussives, such as P2X3 receptor antagonists, are needed.
There are other tools that can investigate the presence of cough hypersensitivity. The Hull Airway Reflux Questionnaire (HARQ) is a validated 14-item self-administered tool utilising Likert-style response scales, which investigates the presence of laryngeal reflux and cough hypersensitivity [23]. The HARQ enquires about five cough triggers (position change, eating certain foods, getting out of bed, singing or speaking) and four sensations, one of which may relate to laryngeal paraesthesia (throat tickle). The HARQ has been used in cough clinical trials [24] and can differentiate between RCC and healthy controls [23]. The Newcastle Laryngeal Hypersensitivity Questionnaire (NLHQ) is a 14-item self-administered questionnaire that uses Likert-style questions to measure the extent of laryngeal paraesthesia in laryngeal conditions such as chronic cough [15]. Cough triggers are not assessed, and all items relate to laryngeal sensations across three domains: obstruction, pain/thermal and irritation. Both the HARQ and NLHQ scores were associated with response to gabapentin [14, 25]. The HARQ was developed with a specific focus on laryngeal or airway reflux, and the NLHQ on laryngeal conditions, such as inducible laryngeal obstruction. Both questionnaires are applicable to cough in those setting. The CHQ is, however, designed to be applicable to chronic cough in a greater range of patients regardless of context and associated treatable traits. The Sensation Provoking Cough Questionnaire (TOPIC), in development, currently consists of 49 items covering cough impact, severity, sensations and triggers [26]. For assessing cough hypersensitivity, the utility of a multidimensional tool, compared with the pure assessment of sensations and triggers in the CHQ, is yet to be elucidated.
There are limitations with our study. Due to the detailed qualitative nature of the investigations, the UK studies used small population samples. However, the interviews provided rich datasets from a well-characterised group of patients with RCC, and this was the basis for developing the CHQ. It is unlikely that larger samples would have significantly changed the results. While the majority of CHQ items were elicited spontaneously by at least one participant, a small number of items were prompted and retained as they were considered important by the MDT review. Therefore, there may have been a potential for bias in item selection [17]. We did not evaluate the CHQ against other (objective) measures of cough hypersensitivity. Currently, there is no gold standard to diagnose cough hypersensitivity. Objective cough reflex sensitivity testing using inhaled tussive challenges, typically with capsaicin or citric acid, has limitations as it does not distinguish RCC well from healthy controls and does not predict response to antitussive treatment [27, 28]. In a previous study of 32 subjects with sarcoidosis, a modest-to-weak correlation was observed between CHQ scores and minimum inhaled capsaicin concentrations required to provoke five coughs (ρ=−0.36, p=0.045) [29]. Alternative tussive challenge end-points to C5 (such as Emax) have shown promise in distinguishing healthy controls from RCC [30]; their association with cough triggers needs further investigation. Furthermore, recent studies have shown poor correlation between capsaicin cough reflex sensitivity and cough triggers in chronic cough [31]. This may relate to differing aetiologies or endotypes of cough, each triggered by a different profile of stimuli [32]. Therefore, cough challenge tests to one stimulus, such as capsaicin, may not correspond with clinical end-points nor recognise all pathological cough hypersensitivity. Nevertheless, future studies should evaluate the CHQ in comparison with cough reflex sensitivity to a range of agents, including capsaicin, ATP, citric acid and hypotonic saline. While the CHQ was developed in a UK cohort with RCC, our study did not reveal any significant cultural differences in the performance of the CHQ between the United Kingdom and South Korea, and the CHQ was also unidimensional in scale in a large population of patients with chronic cough; however, this should be evaluated further in a larger population with patients from other countries. The CHQ was developed with a binary yes/no scale, as opposed to the Likert scale. Likert scales were evaluated in CHQ development; however, both the MDT and patients felt the binary scale provides a simpler user interface, improves the ease and speed of completing the CHQ, reduces ambiguous answers and allows for quick score calculation. Nevertheless, further studies and clinical experience are needed to determine whether a binary or Likert scale is more advantageous for research or clinical use.
We have developed the CHQ to provide a structured and systematic evaluation of symptoms of cough hypersensitivity. The CHQ is simple to administer and score in RCC, and is designed for use in clinics, research and clinical trials. Further studies are needed to evaluate the CHQ in a larger patient population, varied cultural settings and respiratory diseases and to establish its repeatability and responsiveness.
Acknowledgements
We acknowledge BSc students Jonathan La-Crette, Aish Sinha and Ashley Solomon (King's College London, UK), who helped significantly with data collection in preparation of this manuscript.
Provenance: Submitted article, peer reviewed.
Ethics statement: The study was approved by the East London and the City Research Ethics Committee (REC reference 10/H0703/6) and the participating institutions in South Korea (H-1602-024-739).
Author contributions: Conception and design: B. Hirons, S.S. Birring, W.-J. Song and P.S.P. Cho. Drafting manuscript: B. Hirons, S.S. Birring, W-J. Song, P.S.P. Cho, R.J. Siegert, C. Krägeloh, R. Turner, H.-K. Won and J.-Y. Kim. Revised manuscript: B. Hirons, S.S. Birring, W.-J. Song, P.S.P. Cho, R.J. Siegert, C. Krägeloh, R. Turner, H.-K. Won, J.-Y. Kim, K. Rhatigan, H. Kesavan and E. Mackay.
Conflict of interest: R. Turner is an associate editor and W.-J. Song is the current Chief Editor of ERJ Open Research. The remaining authors have nothing to disclose.
References
- 1.Song W-J, Chang Y-S, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015; 45: 1479–1481. doi: 10.1183/09031936.00218714 [DOI] [PubMed] [Google Scholar]
- 2.Dicpinigaitis PV, Tso R, Banauch G. Prevalence of depressive symptoms among patients with chronic cough. Chest 2006; 130: 1839–1843. doi: 10.1378/chest.130.6.1839 [DOI] [PubMed] [Google Scholar]
- 3.Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020; 55: 1901136. doi: 10.1183/13993003.01136-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGarvey LP, Carton C, Gamble L, et al. Prevalence of psychomorbidity among patients with chronic cough. Cough 2006; 2: 1–6. doi: 10.1186/1745-9974-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung KF, McGarvey L, Song WJ, et al. Cough hypersensitivity and chronic cough. Nat Rev Dis Primers 2022; 8: 45. doi: 10.1038/s41572-022-00370-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ando A, Smallwood D, McMahon M, et al. Neural correlates of cough hypersensitivity in humans: evidence for central sensitisation and dysfunctional inhibitory control. Thorax 2016; 71: 323–329. doi: 10.1136/thoraxjnl-2015-207425 [DOI] [PubMed] [Google Scholar]
- 7.Shapiro CO, Proskocil BJ, Oppegard LJ, et al. Airway sensory nerve density is increased in chronic cough. Am J Respir Crit Care Med 2021; 203: 348–355. doi: 10.1164/rccm.201912-2347OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koskela HO, Nurmi HM, Birring SS. Utility of cough provocation tests in chronic cough and respiratory diseases: a comprehensive review and introduction of new reference ranges for the capsaicin test. Allergy Asthma Immunol Res 2021; 13: 833–849. doi: 10.4168/aair.2021.13.6.833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vertigan AE, Gibson PG. Chronic refractory cough as a sensory neuropathy: Evidence from a reinterpretation of cough triggers. J Voice 2011; 25: 596–601. doi: 10.1016/j.jvoice.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 10.Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 2014; 13: 924–935. doi: 10.1016/S1474-4422(14)70102-4 [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto H, Tabuena RP, Niimi A, et al. Cough triggers and their pathophysiology in patients with prolonged or chronic cough. Allergol Int 2012; 61: 123–132. doi: 10.2332/allergolint.10-OA-0295 [DOI] [PubMed] [Google Scholar]
- 12.Song WJ, Dupont L, Birring SS, et al. Consensus goals and standards for specialist cough clinics: the NEUROCOUGH international Delphi study. ERJ Open Res 2023; 9: 00618-2023. doi: 10.1183/23120541.00618-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morice AH, Millqvist E, Belvisi MG, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J 2014; 44: 1132–1148. doi: 10.1183/09031936.00218613 [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Chen Q, Dong R, et al. Prediction of therapeutic efficacy of gabapentin by Hull Airway Reflux Questionnaire in chronic refractory cough. Ther Adv Chronic Dis 2020; 11: 2040622320982463. doi: 10.1177/2040622320982463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vertigan AE, Bone SL, Gibson PG. Development and validation of the Newcastle laryngeal hypersensitivity questionnaire. Cough 2014; 10: 1. doi: 10.1186/1745-9974-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker SM, Smith JA, Birring SS, et al. British Thoracic Society Clinical Statement on chronic cough in adults. Thorax; 2023; 78: s3–s19. doi: 10.1136/thorax-2023-220592 [DOI] [PubMed] [Google Scholar]
- 17.Hirons B, Rhatigan K, Kesavan H, et al. Qualitative assessment of sensations and triggers in chronic cough. ERJ Open Res 2023; 10: 00923–2023. doi: 10.1183/23120541.00923-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res 1995; 4: 293–307. doi: 10.1007/BF01593882 [DOI] [PubMed] [Google Scholar]
- 19.Rattray J, Jones MC. Essential elements of questionnaire design and development. J Clin Nurs 2007; 16: 234–243. doi: 10.1111/j.1365-2702.2006.01573.x [DOI] [PubMed] [Google Scholar]
- 20.Tennant A, McKenna SP, Hagell P. Application of Rasch analysis in the development and application of quality of life instruments. Value Health 2004; 7: Suppl. 1, S22–S26. doi: 10.1111/j.1524-4733.2004.7s106.x [DOI] [PubMed] [Google Scholar]
- 21.Andrich D, Sheridan B, Luo G. RUMM 2030. Perth, Australia: RUMM Laboratory. Date last accessed: 5 March 2024. Date last updated: 2009. https://www.rummlab.com.au/rumm-2030.
- 22.Smith EV. Detecting and evaluating the impact of multidimensionality using item fit statistics and principal component analysis of residuals. J Appl Meas 2002; 3: 205–231. [PubMed] [Google Scholar]
- 23.Morice AH, Faruqi S, Wright CE, et al. Cough hypersensitivity syndrome: A distinct clinical entity. Lung 2011; 189: 73–79. doi: 10.1007/s00408-010-9272-1 [DOI] [PubMed] [Google Scholar]
- 24.Morice A, Birring S, Dicpinigaitis P, et al. Cough triggers and symptoms among patients with refractory or unexplained chronic cough in two phase 3 trials of the P2X3 receptor antagonist gefapixant (COUGH-1 and COUGH-2). J Allergy Clin Immunol 2021; 147: AB61. doi: 10.1016/j.jaci.2020.12.242 [DOI] [Google Scholar]
- 25.Gibson PG, Vertigan AE. Gabapentin in chronic cough. Pulm Pharmacol Ther 2015; 35: 145–148. doi: 10.1016/j.pupt.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 26.Huong Y, Yuille D, Caress A, et al. P102 The sensations provoking cough: quantitative study. Thorax 2017; 72: A138–A139. doi: 10.1136/thoraxjnl-2017-210983.244 [DOI] [Google Scholar]
- 27.Prudon B, Birring SS, Vara DD, et al. Cough and glottic-stop reflex sensitivity in health and disease. Chest 2005; 127: 550–557. doi: 10.1378/chest.127.2.550 [DOI] [PubMed] [Google Scholar]
- 28.Belvisi MG, Birrell MA, Wortley MA, et al. XEN-D0501, a novel transient receptor potential vanilloid 1 antagonist, does not reduce cough in patients with refractory cough. Am J Respir Crit Care Med 2017; 196: 1255–1263. doi: 10.1164/rccm.201704-0769OC [DOI] [PubMed] [Google Scholar]
- 29.Sinha A, Lee KK, Rafferty GF, et al. Predictors of objective cough frequency in pulmonary sarcoidosis. Eur Respir J 2016; 47: 1461–1471. doi: 10.1183/13993003.01369-2015 [DOI] [PubMed] [Google Scholar]
- 30.Holt KJ, Belcher J, Smith JA. Novel capsaicin cough endpoints effectively discriminate between healthy controls and patients with refractory chronic cough. Respir Med 2023; 208: 107142. doi: 10.1016/j.rmed.2023.107142 [DOI] [PubMed] [Google Scholar]
- 31.Xu T, Chen Z, Zhan C, et al. Profile of cough triggers and their relationship with capsaicin cough sensitivity in chronic cough. Ther Adv Respir Dis 2024; 18: 17534666231225562. doi: 10.1177/17534666231225562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belvisi MG, Birrell MA, Khalid S, et al. Neurophenotypes in airway diseases: Insights from translational cough studies. Am J Respir Crit Care Med 2016; 193: 1364–1372. doi: 10.1164/rccm.201508-1602OC [DOI] [PMC free article] [PubMed] [Google Scholar]