Abstract
Aims and objectives
This systematic review and meta-analysis study evaluated the association between mortality due to COVID-19 and coagulative factors.
Methods
A systematic search was conducted on electronic databases including PubMed, Scopus, and the Web of Science from the beginning of the pandemic until October 2024 to identify relevant studies on COVID-19 patients and their laboratory findings related to coagulation markers and mortality outcome. Eligibility criteria were defined based on the PICO framework, and data extraction was performed by two authors independently using a standardized sheet. Statistical analysis was accomplished using the random effects model, and heterogeneity among studies was assessed using the I2 test. R and RStudio were used for statistical analysis and visualization.
Results
Our systematic literature search yielded 6969 studies, with 48 studies meeting the inclusion criteria for our meta-analysis. The mean platelet count was significantly lower in deceased COVID-19 patients compared to survivors (20.58), while activated partial thromboplastin time (aPTT) and fibrinogen levels did not show significant differences. The pooled mean difference of D-Dimer, International Normalized Ratio (INR), and prothrombin time (PT) were significantly lower in survived patients (-2.45, -0.10, and -0.84, respectively). These findings suggest that platelet count, D-Dimer, INR, and PT may serve as potential indicators of mortality in COVID-19 patients.
Conclusion
The results of our systematic review and meta-analysis revealed a significant reduction in the pooled platelet count among deceased individuals when compared to survivors. However, no significant distinctions were observed in the pooled mean activated aPTT and fibrinogen levels between the deceased and survivor groups. On the other hand, there were noticeable variations in the pooled estimated mean of INR, PT, and D-Dimer levels, with significantly higher values in the deceased group compared to those who survived.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10229-y.
Keywords: COVID-19, Mortality, Coagulopathy, D-dimers, Fibrinogen, Prothrombin time, Meta-analysis
Background
The global impact of the COVID-19 pandemic caused by the SARS-CoV-2 virus has been profound, resulting in a significant loss of lives in a relatively short period. SARS-CoV-2 which belongs to the coronavirus family, renowned for its ability to induce respiratory infections. Notably, COVID-19 has been linked to coagulopathy, leading to the development of intravascular thrombi and fibrinogen deposition [1–3]. Extensive research has demonstrated that COVID-19 infection triggers the activation and dysregulation of the coagulation system, leading to abnormalities in various coagulation parameters. Crucially, COVID-19-associated coagulopathy exhibits unique characteristics that distinguish it from other coagulation disorders. Initial reports from Wuhan, China, have highlighted elevated levels of D-dimers and fibrinogen, accompanied by alterations in platelet activation. These findings suggest a state of hypercoagulability in individuals with COVID-19 [4–10].
Severe cases of COVID-19 have been found to fulfil the criteria for disseminated intravascular coagulation (DIC), signifying a serious and potentially life-threatening complication associated with the disease [11–14]. However, recent investigations have revealed that COVID-19-associated coagulopathy differs from coagulopathic disorders caused by bacterial infections and other disorders. Specifically, individuals with COVID-19 often exhibit increased D-dimer and fibrinogen levels, while prothrombin time and platelet count may not exhibit significant changes during the early stages of the disease. Given the observed coagulation abnormalities, the early detection of elevated coagulation biomarkers becomes paramount for risk stratification in COVID-19 patients [15–20]. Thromboembolic complications have emerged as potential causes of clinical deterioration in severe cases, even after patients have been discharged from the hospital. This underscores the significance of vigilant monitoring of coagulation status in individuals with COVID-19 [21–23].
To assess the reliability of risk stratification based on early coagulation parameters and its impact on mortality and disease outcome, we conducted a comprehensive meta-analysis of published research worldwide. By scrutinizing data from various clinical studies, we aim to gain deeper insights into coagulation parameters in individuals with COVID-19.
Methods and materials
The present study was conducted based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline 2020 [24] (see Supplemental Table 1).
Search strategy
A systematic search was accomplished on electronic databases such as Web of Science, Scopus, and PubMed from the beginning of the pandemic until October 2024. The search strategy included a combination of relevant medical subject heading (MeSH) terms and relevant keywords for (“Covid-19” OR “Sars-Cov-2” OR “Coronavirus”) AND (“Laboratory findings” OR “Coagulation” OR “Biochemical markers”) AND (“Mortality”, “Survival”, “Death”, “Outcome”) (see supplemental Table 2).
Eligibility criteria
We defined our eligibility criteria based on the PICO framework: (P) Population: COVID patients (confirmed by serum antibody tests, polymerase chain reaction, or clinical symptoms after close exposure to proven cases of COVID) who deceased (I) Intervention/Exposure: Laboratory and coagulative measures. (C) Control: COVID patients who survived (O) Outcome: Mortality. The exclusion criteria were defined as: absence of laboratory findings, not reporting mortality as outcome, lack of individual data, reporting only patients with confounding conditions such as warfarin usage, pregnant women, cancer, coagulopathy disorder. Furthermore, articles in non-English language were excluded. The kappa statistic was used to measure inter-rater reliability in screening and selecting the eligible studies and the results were interpreted as less than 0.2 represents no agreement; 0.21–0.29 represents minimal agreement; 0.40–0.59 represents weak agreement; 0.60–0.79 represents moderate agreement; 0.80–0.90 represents strong agreement; greater than 0.9 represents almost perfect agreement [25].
Data extraction and outcome measures
The methodological quality evaluation of the included articles was assessed by the Newcastle–Ottawa Scale (NOS) checklist [26]. The quality scores were classified as follows: very good (score of 9–10), good (score of 7–8), satisfactory (score of 5–6), or unsatisfactory (score of 0–5). The data extraction using a standardized sheet was performed by two independent authors. A third-party discussion was done when any disagreement occurred. The standardized sheet included: authors’ name, year of publication, total number of participants, mean age of the participants, design of the study, and country of the study along with mean platelet count and its standard deviation (SD) and total number, mean prothrombin time (PT) and its standard deviation (SD) and total number, mean activated partial thromboplastin time (aPTT) and its standard deviation (SD) and total number, mean D-Dimer level and its standard deviation (SD) and total number, mean fibrinogen level and its standard deviation (SD) and total number, and mean International Normalized Ration (INR) and its standard deviation (SD) and total number. The aforementioned variables were extracted in two groups: the survivors and the deceased.
Statistical analysis and data synthesis
The pooled mean difference was calculated using random effects model and Hedges’ g along with SD estimation. For assessing the heterogeneity of the included studies, the I2 (I square) test was used. Moreover, < 25% considered low, 25–75% considered moderate, and > 75% considered high heterogeneity. Additionally, the leave-one-out sensitivity analysis was conducted to assess robustness of findings. The Mantel–Haenszel method and random effects model was used for pooling the effect sizes and SD was consequently calculated. For testing the overall significance of the random model, z-test was performed Potential publication bias was graphically assessed by creating funnel plots for each of the aforementioned groups. Furthermore, subgroup analysis based on patients' average age (> 60 or ≤ 60 years) and meta-regression was performed to find the source of heterogeneity. Two independent reviewers evaluated the certainty of synthesized evidence using GRADE guidelines [27]. R (R Foundation for Statistical Computing, Vienna, Austria) and RStudio (RStudio, Inc., Boston, MA) were used for the statistical analysis and creating forest and funnel plots.
Results
Study selection and characteristics
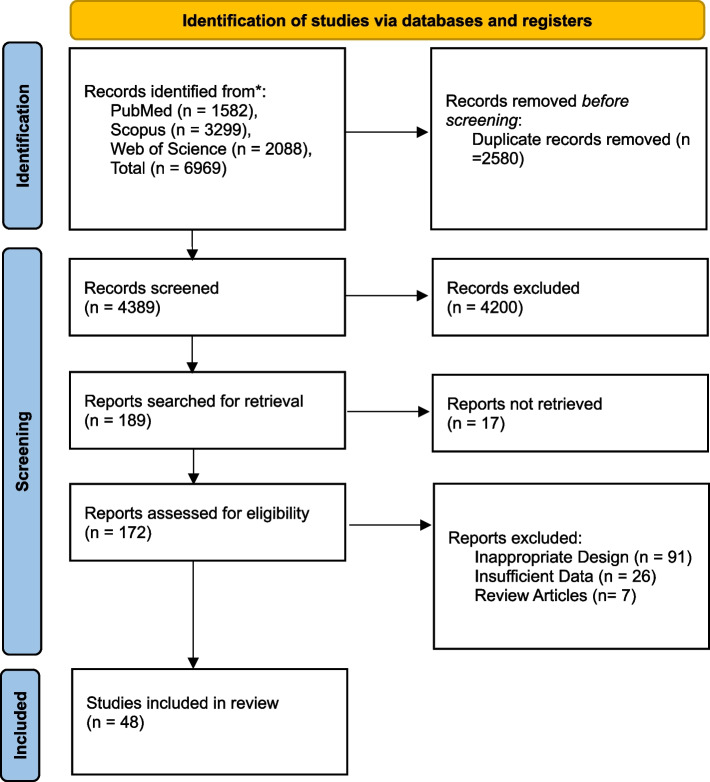
Our systematic search of the literature obtained 6969 studies, primarily. After removing the duplicates (n = 2580), 4389 studies were screened based on their title and abstract. Finally, 189 studies were included for full-text evaluation. Based on our inclusion and exclusion criteria, 48 studies [22, 28–74] were included in our final meta-analysis model (Fig. 1). The agreement between authors was moderate for the screening and selection process (κ = 0.70). The detailed quality assessment of the included studies is presented in supplemental Table 3. Supplemental Tables 4–6 represent characteristics of included articles and exact values of coagulative parameters of survived and deceased cases.
Fig. 1.
PRISMA flow diagram of the systematic search and study selection
Results of syntheses
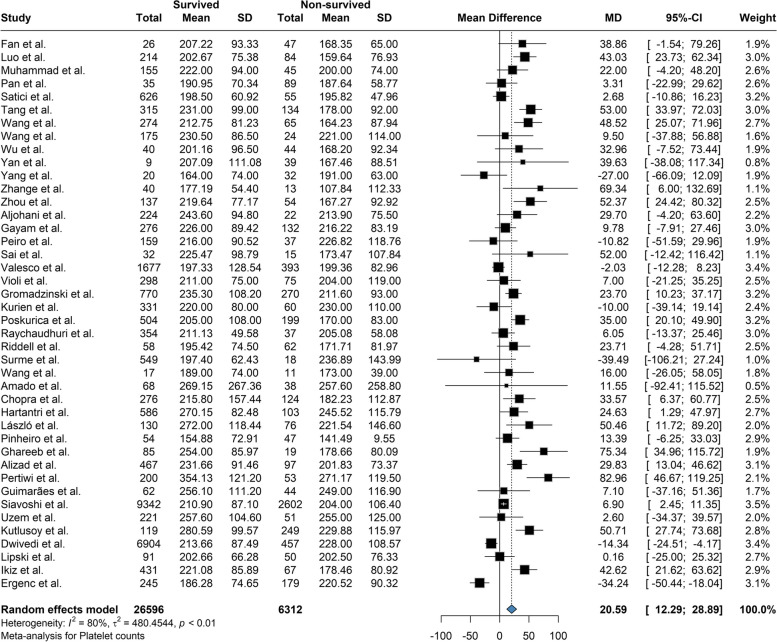
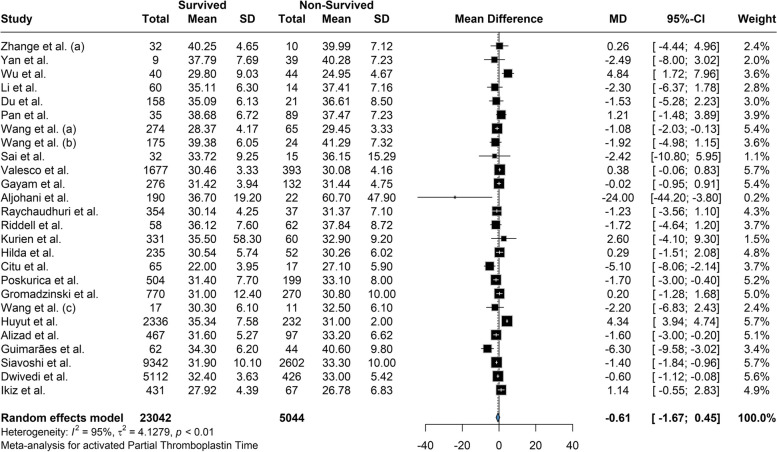
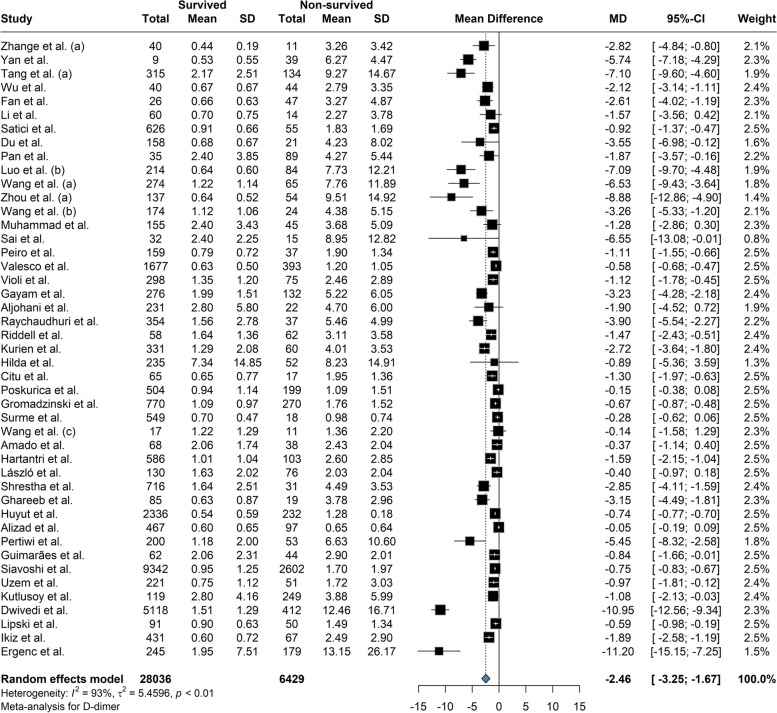
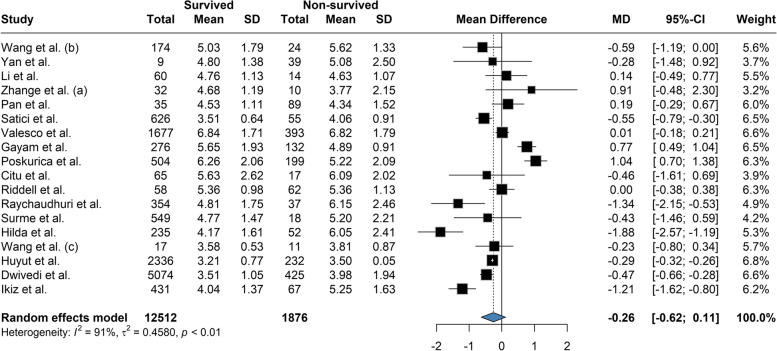
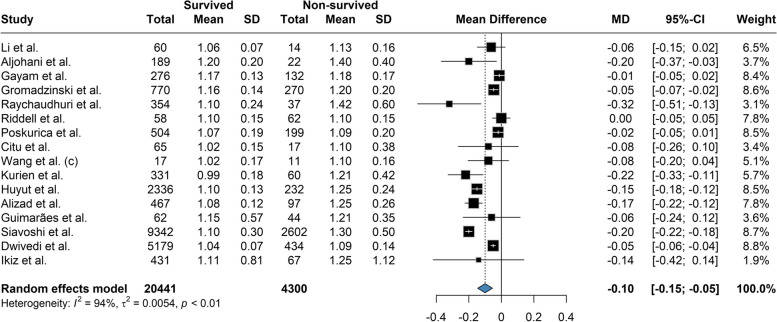
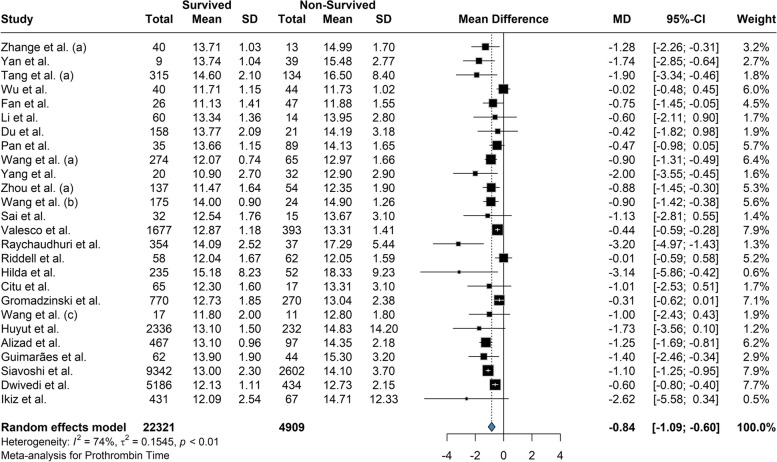
Based on our meta-analysis, the mean difference between Platelet count among the survivors and the deceased was 20.58 (95% CI: 12.28 to 28.88; P = < 0.01) based on the random effects model and was significantly (p-value < 0.01) lower among the deceased (Fig. 2). The between-study heterogeneity was high (I2 = 80%). Results of the Leave-one-out by sensitivity analysis method showed no difference from excluding any studies (Supplemental Fig. 1). Current meta-analysis indicated the mean difference between aPTT count among the survivors and the deceased was not statistically significant (MD: -0.609; 95% CI: -1.66 to 0.45; P = 0.24) based on the random effects model (Fig. 3). High heterogeneity (I2 = 95.3%) was observed between the included studies. Leave-one-out analysis indicated no significant difference by and studies' omission (Supplemental Fig. 2). Meta-analysis of D-dimer levels revealed that the average D-dimer counts were significantly lower in the survived compared to non-survived patients (MD: -2.45; 95% CI: -3.24 to -1.66; P < 0.01) (Fig. 4). The included studies were highly heterogeneous (I2 = 92.5%). The pooled effect and heterogeneity were not statistically changed by removing any studies using the leave-one-out method (Supplemental Fig. 3). Meta-analysis of the difference in fibrinogen levels among survival and deceased showed a non-significant mean difference between the two groups (MD: -0.25; 95% CI: -0.62 to 0.11; P = 0.16) (Fig. 5). The between-study heterogeneity was markedly high (I2 = 91%). Further sensitivity analysis using the leave-one-out method indicated that removing any studies did not significantly alter the polled results (Supplemental Fig. 4). The meta-analysis results based on the random effects showed significantly higher INR count in deceased than in the survived patients (MD: -0.10; 95% CI: -0.14 to -0.05; P < 0.01) (Fig. 6). High between-study heterogeneity (I2 = 93.9%%) in the included studies was observed. Furthermore, the Leave-one-out sensitivity analysis resulted in no significant difference by removing any selected studies (Supplemental Fig. 5). The findings of the meta-analysis indicated significantly lower PT levels in the survived compared to the deceased (MD: -0.84; 95% CI: -1.09 to -0.59; P < 0.01) (Fig. 7). The included articles were moderately heterogeneous (I2 = 73.5%). Sensitivity analysis by the leave-one-out method did not reveal any significant change in pooled estimate results or heterogeneity by any studies' omission (Supplemental Fig. 6).
Fig. 2.
The mean difference between Platelet count among the survivors and the deceased was 20.58 and was significantly higher among the survivors
Fig. 3.
The mean difference between aPTT count among the survivors and the deceased was -0.60 and was not significantly lower among the deceased
Fig. 4.
The mean difference between D-Dimer count among the survivors and the deceased was -2.45 and was significantly lower among the survived
Fig. 5.
The mean difference between Fibrinogen count among the survivors and the deceased was -0.25 and was not significantly lower among the deceased
Fig. 6.
The mean difference between INR count among the survivors and the deceased was -0.10 and was significantly lower among the survived
Fig. 7.
The mean difference between PT count among the survivors and the deceased was -0.84 and was significantly lower among the survived
Subgroup analyses
Subgroup analyses were performed based on age (Figure Supplemental 7–12). The tests of between-group differences were non-significant for all variables except for fibrinogen (P value = 0.02) and INR (P value < 0.01). In both Fibrinogen and INR analyses, only a subgroup of studies with an average age of less than 60 years showed significant mean differences between survived and non-survived cases (Figure Supplemental 10 and 11). Furthermore, the between-study heterogeneity within each group remained in the same category as the overall heterogeneity for all variables.
Meta-regression
Meta-regression analysis with year of publication, sample size, and age was performed for all variables and presented in supplemental Table 4. Findings of platelet, PT, D-dimer, INR and aPTT meta-regression were non-significant. However, the age in fibrinogen analysis significantly accounted for heterogeneity.
Certainty of evidence
Supplemental Table 5 shows a detailed investigation of the quality of polled results. The certainty was low in platelet, aPTT, INR, and PT results and very low in D-dimer and fibrinogen findings.
Risk of publication bias
The risk of publication bias was assessed by visual investigation and statistical tests (Figure Supplemental 13–18). The funnel plots of platelet, aPTT, Fibrinogen, and INR meta-analyses were symmetrical, and statistical tests did not demonstrate a significant risk of publication bias. In contrast, funnel plots of DD and PT analyses were asymmetrical, representing probable risk of publication bias confirmed by the Begg test (P values < 0.05).
Discussion
According to the results of our systematic review and meta-analysis study, the estimated pooled platelet count was significantly lower among the deceased compared to the survivors. Also, the pooled mean aPTT and fibrinogen levels did not show any significant difference among the deceased and the survivors. The pooled estimated mean of INR, PT, and D-Dimer was significantly higher among the deceased compared to those who survived.
Platelets play a critical role in maintaining haemostasis and contribute to thrombo-inflammatory processes during viral infections. Changes in platelet production or destruction at various stages of viral infection can lead to coagulation imbalances, resulting in pro-thrombotic events or platelet dysfunction and bleeding risks. Thrombocytopenia (low platelet count) has emerged as a significant marker of COVID-19 severity and mortality [75–78]. Thrombocytopenia has diverse reasons and may include early suppression of platelet production, damage to bone marrow, and hemo-phagocytosis [79–83].
In the convalescent phase of COVID-19, some patients may experience reactive thrombocytosis following initial thrombocytopenia. The increase in immature reticulated platelets observed in COVID-19 patients may impact the effectiveness of anti-platelet therapies [84–88]. Changes in platelet parameters and reactivity have been associated with severe COVID-19 infections. Moreover, studies have reported dysfunction of megakaryocytes (platelet progenitors) in COVID-19 patients, with elevated and abnormal megakaryocytes found in various organs, including the lungs, heart, brain, and bone marrow. The lungs appear to play a significant role in platelet biogenesis, and hypoxia-induced thrombocytopenia is linked to reduced lung megakaryocytes and impaired platelet generation in the lungs [89–95].
The SARS-CoV-2 virus has been implicated in up-regulating megakaryocyte progenitors and elevating circulating megakaryocytes in severe COVID-19, possibly through infection of early megakaryocyte progenitors. This suggests the possibility of viral RNA transfer from megakaryocytes to platelets and circulating plasma extracellular vehicles (EVs) [96–100]. Notably, plasma SARS-CoV-2 RNA has been strongly associated with increased mortality in COVID-19 patients. However, there is a gap in our knowledge of platelet non-canonical function and regeneration, so other research is essential to comprehensively grasp COVID-19 pathogenesis and identify increased thromboembolic risk [96–106].
Publication bias and variability in cut-off values for D-dimer (DD) measurement across studies may influence reported outcomes. Haemostasis tests, including D-dimers, may have limited usefulness because there is some differences in methodologies, antibody origin, detection methods, calibrators, and diagnostic thresholds among different laboratories.
Autopsy findings have shown that increased DD levels can be linked to fibrin deposits in the pulmonary extravascular space and alveoli. However, these elevations may not always be specific to intravascular fibrin formation. Elevated DD levels, along with increased neutrophil counts, have been identified as predictors of pulmonary embolism in COVID-19 patients. DD and fibrinogen (FIB) levels are elevated in both COVID-19 and thromboembolism, making them non-specific and unhelpful as single tests to distinguish between these conditions [107–110]. The lack of specificity might be attributed to the involvement of various systems, such as endothelial cells, complement activation, and hypo-fibrinolysis, in the abnormal coagulation processes during COVID-19, leading to changes that routine tests may not capture. Initial reports from China indicated a decreased activated partial thromboplastin time (APTT) as a marker of hypercoagulation [111–114]. However, later studies reported prolonged APTT, which could suggest deficiencies in clotting factors or the presence of inhibitors, such as heparin therapy. Another explanation could be the presence of antiphospholipid antibodies (aPL) or lupus anticoagulant (LA) which is observed in patients affected by COVID-19. Prolonged APTT may also be influenced by increased heparin therapy usage, especially in severe cases of COVID-19 [55, 115–117].
There are conflicting reports regarding fibrinogen levels and COVID-19 severity. Some studies show high fibrinogen levels in COVID-19 patients, while others report alternative findings. Severe COVID-19 infection may induce fibrinolysis shutdown, contributing to high levels of D-dimers and fibrinogen. However, other reports suggest increased plasmin-antiplasmin complexes and mild consumption coagulopathy in COVID-19 patients [118–123]. Tissue-type plasminogen activator elevation has also been found in severe COVID-19 cases. Fibrinogen plays a crucial role in linking coagulation, complement system, and inflammation in COVID-19. Recent post-mortem studies have identified microvascular thrombi in multiple organs, indicating a hypercoagulable state with impaired fibrinolysis. However, the association between abnormal visco-elastometric testing (VET) patterns and clinical outcomes has not been demonstrated in patients affected by COVID-19 [124–128].
Routine coagulation tests which are taken at the time of admission can be helpful in differentiating severe and non-severe COVID-19 patients. The mechanisms of fibrinolysis shutdown may involve reduced fibrinolysis factors (plasminogen) and elevated inhibitors of fibrinolysis (such as α2-antiplasmin and plasminogen activator inhibitor PAI-1). Longitudinal observational studies are crucial for understanding COVID-19 infection dynamics and disease outcomes. Daily changes in relevant haemostasis parameters, including D-dimers and PT, have been observed in severe COVID-19 patients. However, it's essential to consider the variability in disease progression and admission timepoints when interpreting study results.
The current study has certain constraints. Included studies may have used different methods and kits for performing coagulation tests. Furthermore, the timing of tests could vary from study to study, while coagulation parameters may change throughout the disease. Additionally, the vaccination may affect the coagulation tests [129], and since study publications varied from 2020 to 2023, some of the differences may have contributed to vaccinations. Likewise, several underlying conditions, such as cancers, pregnancy, and receiving anticoagulants, may alter the results of coagulation tests. Unfortunately, detailed analyses on each condition were not allowed due to the incomplete reports of patients' conditions in the included articles, and readers should interpret our findings with caution. Further investigation, considering all the confounding factors mentioned, should be necessary to evaluate the findings of the current study.
Conclusion
The outcomes of our systematic review and meta-analysis indicate a significant decrease in the estimated pooled platelet count in deceased individuals compared to survivors. However, no significant differences were found in the pooled mean aPTT and fibrinogen levels between the deceased and survivor groups. Conversely, there were notable variations in the pooled estimated mean of INR, PT, and D-Dimer levels, which were significantly higher in the deceased group compared to those who survived.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- DIC
Disseminated intravascular coagulation
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- MeSH
Medical subject heading
- PICO
Population, Intervention, Comparison, Outcome
- SD
Standard deviation
- aPTT
Activated partial thromboplastin time
- PT
Prothrombin time
- INR
International Normalized Ration
- DD
D-dimer
- EVs
Extracellular vehicles
- FIB
Fibrinogen
- aPL
Antiphospholipid antibodies
- LA
Lupus anticoagulant
- VET
Visco-elastometric testing
Authors’ contributions
S.N. and Z.P. designed the study and participated in the search and screening sections. A.A., S.H.I., and S.M.P. extracted and analysed data. S.S. and S.M.P. wrote the manuscript. All authors revised and modified the final manuscript.
Funding
Not received.
Data availability
Data would be available based upon an eligible request to the corresponding author.
Declarations
Ethics approval and consent to participate
The protocol for this systematic review was approved by the ethics committee at IKHC ethics committee (Ethics code: IR.TUMS.IKHC.REC.1399.102).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wolny M, Dittrich M, Knabbe C, Birschmann I. Immature platelets in COVID-19. Platelets. 2023;34(1):2184183. [DOI] [PubMed] [Google Scholar]
- 2.Russell L, Weihe S, Madsen EK, Hvas CL, Leistner JW, Michelsen J, et al. Thromboembolic and bleeding events in ICU patients with COVID-19: a nationwide, observational study. Acta Anaesthesiol Scand. 2023;67(1):76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rurua M, Ratiani L, Sanikidze T, Machvariani K, Pachkoria E, Ormocadze G, et al. Impact of the angiotensin-converting enzyme (ACE) inhibitors on the course of the septic shock developed during covid-19 and other severe respiratory infections in presence of hyperferritinemia. Georgian Med News. 2023;337:110–7. [PubMed] [Google Scholar]
- 4.Shoaee S, Rezaie F, Payab M, Bakhtiari F, Heydari M-H. Experiences from the management of COVID-19 pandemic in a nursing home in Iran (March–April, 2020). J Diabetes Metab Disord. 2022;21(1):1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu G, Modepalli S, Anand M, Li H. Computational modeling of hypercoagulability in COVID-19. Comput Methods Biomech Biomed Engin. 2023;26(3):338–49. [DOI] [PubMed] [Google Scholar]
- 6.Zaghloul MS, Jammeh M, Gibson A, Luo S, Chadwick-Mansker K, Liu Q, et al. Chronic anti-coagulation therapy reduced mortality in patients with high cardiovascular risk early in COVID-19 pandemic. Thromb J. 2023;21(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing Y, Li Y, Feng L, Huo R, Ma X, Dong Y, et al. Predictors of COVID-19 Severity in Elderly Patients Infected by Omicron in China, 18 December 2022-5 February 2023. Infect Drug Resist. 2023;16:4505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuculeanu G, Barbu EC, Lazar M, Chitu-Tisu CE, Moisa E, Negoita SI, Ion DA. Coagulation disorders in sepsis and COVID-19-two sides of the same coin? a review of inflammation-coagulation crosstalk in bacterial sepsis and COVID-19. J Clin Med. 2023;12(2):601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smail SW, Babaei E, Amin K. Hematological, inflammatory, coagulation, and oxidative/antioxidant biomarkers as predictors for severity and mortality in COVID-19: a prospective cohort-study. Int J Gen Med. 2023;16:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smadja DM, Gendron N, Philippe A, Diehl JL, Ochat N, Bory O, et al. Fibrin monomers evaluation during hospitalization for COVID-19 is a predictive marker of in-hospital mortality. Front Cardiovasc Med. 2023;10:1001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moscucci F, Gallina S, Bucciarelli V, Aimo A, Pelà G, Cadeddu-Dessalvi C, et al. Impact of COVID-19 on the cardiovascular health of women: a review by the Italian Society of Cardiology Working Group on ‘gender cardiovascular diseases.’ J Cardiovasc Med (Hagerstown). 2023;24(Suppl 1):e15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayne ES, George JA, Louw S. Assessing biomarkers in viral infection. Adv Exp Med Biol. 2023;1412:159–73. [DOI] [PubMed] [Google Scholar]
- 13.Malkoc A, GnanaDev R, Botea L, Jeney A, Glover K, Retamozo M, et al. A Comparative analysis of critical limb ischemia in the intensive care unit since the COVID-19 pandemic. Ann Vasc Surg. 2023;90:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippi G, Mullier F, Favaloro EJ. D-dimer: old dogmas, new (COVID-19) tricks. Clin Chem Lab Med. 2023;61(5):841–50. [DOI] [PubMed] [Google Scholar]
- 15.Mortazavi H, Sadeghian A, Hazrati P, Heydari M-H, Madihi S. Oral hemorrhagic blister and its possible related factors: analyzes of reported cases in the literature. J Oral and Maxillofac Surg Med Pathol. 2023;35(4):358–67. [Google Scholar]
- 16.Krinsky N, Sizikov S, Nissim S, Dror A, Sas A, Prinz H, et al. NETosis induction reflects COVID-19 severity and long COVID: insights from a 2-center patient cohort study in Israel. J Thromb Haemost. 2023;21(9):2569–84. [DOI] [PMC free article] [PubMed]
- 17.Korb VG, Schultz IC, Beckenkamp LR, Wink MR. A systematic review of the role of purinergic signalling pathway in the treatment of COVID-19. Int J Mol Sci. 2023;24(9):7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kole C, Stefanou Ε, Karvelas N, Schizas D, Toutouzas KP. Acute and post-acute COVID-19 cardiovascular complications: a comprehensive review. Cardiovasc Drugs Ther. 2024;38(5):1017-32. [DOI] [PMC free article] [PubMed]
- 19.Khreefa Z, Barbier MT, Koksal AR, Love G, Del Valle L. Pathogenesis and Mechanisms of SARS-CoV-2 Infection in the Intestine, Liver, and Pancreas. Cells. 2023;12(2):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khatib Y, Samele R, Chaudhari J, Khare M, Gupte P, Sable Y, Shah V. Hematological and other laboratory parameter changes in COVID-19 patients. J Assoc Phys India. 2023;71(4):11–2. [DOI] [PubMed] [Google Scholar]
- 21.Idrissi A, Lekfif A, Amrani A, Yacoubi A, Yahyaoui A, Belmahi S, et al. Biomarkers predicting poor prognosis in covid-19 patients: a survival analysis. Cureus. 2023;15(1):e33921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huyut MT, Huyut Z. Effect of ferritin, INR, and D-dimer immunological parameters levels as predictors of COVID-19 mortality: a strong prediction with the decision trees. Heliyon. 2023;9(3):e14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hojker M, Tršan J, Tršan U, Gale A, Jerman A, Košuta D. Predictive value of inflammatory and coagulation biomarkers for venous thromboembolism in COVID-19 patients. Clin Hemorheol Microcirc. 2023;83(4):387–95. [DOI] [PubMed] [Google Scholar]
- 24.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276–82. [PMC free article] [PubMed] [Google Scholar]
- 26.GA Wells BS, D O'Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 27.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. [DOI] [PubMed] [Google Scholar]
- 28.Aljohani FD, Khattab A, Elbadawy HM, Alhaddad A, Alahmadey Z, Alahmadi Y, et al. Prognostic factors for predicting severity and mortality in hospitalized COVID-19 patients. J Clin Lab Anal. 2022;36(3):e24216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amado LA, Coelho WLDCNP, Alves ADR, Carneiro VCDS, Moreira ODC, de Paula VS, et al. Clinical profile and risk factors for severe COVID-19 in hospitalized patients from rio de janeiro, brazil: comparison between the first and second pandemic waves. J Clin Med. 2023;12(7):2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chopra P, Sehgal T, Yadav R, Meena S, Maitra S, Soni KD, et al. A combination of inflammatory and hematological markers is strongly associated with the risk of death in both mild and severe initial disease in unvaccinated individuals with COVID-19 infection. Electron J Int Federation Clin Chem Lab Med. 2023;34(1):42–56. [PMC free article] [PubMed] [Google Scholar]
- 31.Citu C, Burlea B, Gorun F, Motoc A, Gorun OM, Malita D, et al. predictive value of blood coagulation parameters in poor outcomes in covid-19 patients: a retrospective observational study in Romania. J Clin Med. 2022;11(10):2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan H, Zhang L, Huang B, Zhu M, Zhou Y, Zhang H, et al. Cardiac injuries in patients with coronavirus disease 2019: Not to be ignored. Int J Infect Dis. 2020;96:294–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gayam V, Chobufo MD, Merghani MA, Lamichhane S, Garlapati PR, Adler MK. Clinical characteristics and predictors of mortality in African-Americans with COVID-19 from an inner-city community teaching hospital in New York. J Med Virol. 2021;93(2):812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gromadzinski L, Zechowicz M, Moczulska B, Kasprzak M, Grzelakowska K, Nowek P, et al. Clinical characteristics and predictors of in-hospital mortality of patients hospitalized with COVID-19 infection. J Clin Med. 2023;12(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartantri Y, Debora J, Widyatmoko L, Giwangkancana G, Suryadinata H, Susandi E, et al. Clinical and treatment factors associated with the mortality of COVID-19 patients admitted to a referral hospital in Indonesia. Lancet Reg Health Southeast Asia. 2023;11(C7):100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hilda F, Liana P, Nurtjahyo A, Hudari H, Sari NP, Umar TP, et al. D-dimer as a sensitive biomarker of survival rate in patients with COVID-19. Eurasian J Med. 2022;54(3):219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurien SS, David RS, Chellappan AK, Varma RP, Pillai PR, Yadev I. Clinical profile and determinants of mortality in patients with COVID-19: a retrospective analytical cross-sectional study in a tertiary care center in South India. Cureus. 2022;14(3):e23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.László I, Berhés M, Tisza K, Miltenyi Z, Balázsfalvi N, Vaskó A, et al. The prognostic value of laboratory parameters referring to hemopoietic stress in patients with COVID-19-a single center experience. Signa Vitae. 2022;19(3):36–43.
- 40.Li J, Xu G, Yu H, Peng X, Luo Y, Cao C. Clinical characteristics and outcomes of 74 patients with severe or critical COVID-19. Am J Med Sci. 2020;360(3):229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo X, Zhou W, Yan X, Guo T, Wang B, Xia H, et al. Prognostic value of C-reactive protein in patients with coronavirus 2019. Clin Infect Dis. 2020;71(16):2174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muhammad R, Ogunti R, Ahmad B, Munawar M, Donaldson S, Sumon M, et al. Clinical characteristics and predictors of mortality in minority patients hospitalized with COVID-19 infection. J Racial Ethn Health Disparities. 2022;9(1):335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan F, Yang L, Li Y, Liang B, Li L, Ye T, et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int J Med Sci. 2020;17(9):1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peiró ÓM, Carrasquer A, Sánchez-Gimenez R, Lal-Trehan N, Del-Moral-Ronda V, Bonet G, et al. Biomarkers and short-term prognosis in COVID-19. Biomarkers. 2021;26(2):119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinheiro FD, Lopes LW, Dórea R, Araújo GRL, da Silva FAF, de Brito BB, et al. Epidemiological and clinical characteristics of COVID-19 in a Brazilian public hospital. World J Clin Cases. 2023;11(8):1761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poskurica M, Stevanović Đ, Zdravković V, Čekerevac I, Ćupurdija V, Zdravković N, et al. Admission predictors of mortality in hospitalized COVID-19 patients-a Serbian cohort study. J Clin Med. 2022;11(20):6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raychaudhuri S, Pujani M, Menia R, Verma N, Singh M, Chauhan V, et al. COVID-19 associated coagulopathy in an indian scenario: a correlation with disease severity and survival status. Indian J Hematol Blood Transfus. 2022;38(2):341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riddell A, Chowdary P, Davenport A. The effect of SARS-Co-V2 infection on prothrombotic and anticoagulant factors in dialysis patients. Artif Organs. 2022;46(7):1328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sai F, Liu X, Li L, Ye Y, Zhu C, Hang Y, et al. Clinical characteristics and risk factors for mortality in patients with coronavirus disease 2019 in intensive care unit: a single- center, retrospective, observational study in China. Ann Palliat Med. 2021;10(3):2859–68. [DOI] [PubMed] [Google Scholar]
- 50.Satici C, Demirkol MA, Sargin Altunok E, Gursoy B, Alkan M, Kamat S, et al. Performance of pneumonia severity index and CURB-65 in predicting 30-day mortality in patients with COVID-19. Int J Infect Dis. 2020;98:84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surme S, Tuncer G, Bayramlar OF, Takak H, Copur B, Yazla M, et al. Risk factors and predictors of 1-year overall mortality in patients with COVID-19. Haseki Tip Bulteni. 2022;60(5):439–46. [Google Scholar]
- 52.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Velasco-Rodríguez D, Alonso-Dominguez JM, Vidal Laso R, Lainez-González D, García-Raso A, Martín-Herrero S, et al. Development and validation of a predictive model of in-hospital mortality in COVID-19 patients. PLoS ONE. 2021;16(3):e0247676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Violi F, Ceccarelli G, Cangemi R, Cipollone F, D’Ardes D, Oliva A, et al. Arterial and venous thrombosis in coronavirus 2019 disease (Covid-19): relationship with mortality. Intern Emerg Med. 2021;16(5):1231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, Sun B, Li X, Wang Y, Yang Z. Clinical analysis of severe COVID-19 patients. Technol Health Care. 2022;30(S1):225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Zhang H, Qiao R, Ge Q, Zhang S, Zhao Z, et al. Thrombo-inflammatory features predicting mortality in patients with COVID-19: The FAD-85 score. J Int Med Res. 2020;48(9):300060520955037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China JAMA Intern Med. 2020;180(7):934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang X, Li ZF, Wang BB, Pan YB, Jiang CY, Zhang XG, et al. Prognosis and antibody profiles in survivors of critical illness from COVID-19: a prospective multicentre cohort study. Br J Anaesth. 2022;128(3):491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang F, Xiong Y, Wei Y, Hu Y, Wang F, Li G, et al. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J Med Virol. 2020;92(11):2536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shrestha MR, Basnet A, Tamang B, Khadka S, Maharjan R, Maharjan R, et al. Analysis of altered level of blood-based biomarkers in prognosis of COVID-19 patients. PLoS ONE. 2023;18(8):e0287117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lipski D, Radziemski A, Wasiliew S, Wyrwa M, Szczepaniak-Chicheł L, Stryczyński Ł, et al. Assessment of COVID-19 risk factors of early and long-term mortality with prediction models of clinical and laboratory variables. BMC Infect Dis. 2024;24(1):685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghareeb AAaA, Sazan Moffaq. Clinical & Laboratory markers as predictors, for severity and mortality in COVID-19. Polytechn J. 2023;13(1 A). 10.59341/2707-7799.1746.D.
- 66.Alizad G, Ayatollahi AA, Shariati Samani A, Samadizadeh S, Aghcheli B, Rajabi A, et al. Hematological and biochemical laboratory parameters in COVID-19 patients: a retrospective modeling study of severity and mortality predictors. Biomed Res Int. 2023;2023:7753631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pertiwi D, Nisa M, Aulia AP, Rahayu. Hematological and Biochemical Parameters at Admission as Predictors for Mortality in Patients with Moderate to Severe COVID-19. Ethiop J Health Sci. 2023;33(2):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabriel M. C. Guimarães RF, Any C. Oliveira, Lilian S. Alves, Fabiana R. Carvalho, Katia L. Baptista,, Karina Y. Yaginuma HHKS, Jorge R. Almeida, Thalia Medeiros, Andrea A. Silva. Hematological and coagulation parameters as predictors of death by Coronavirus disease in hospitalized patients: a Brazilian follow-up study. Br J Pharmaceutical Sci. 2023;59:e21798.
- 69.Ikiz F, Ak A. Investigation of the relationship between coagulation parameters and mortality in COVID-19 infection. Blood Sci. 2024;6(2):e00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siavoshi F, Safavi-Naini SAA, Shirzadeh Barough S, Azizmohammad Looha M, Hatamabadi H, Ommi D, et al. On-admission and dynamic trend of laboratory profiles as prognostic biomarkers in COVID-19 inpatients. Sci Rep. 2023;13(1):6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uzum Y, Turkkan E. Predictivity of CRP, albumin, and CRP to albumin ratio on the development of intensive care requirement, mortality, and disease severity in COVID-19. Cureus. 2023;15(1):e33600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kutlusoy S, Koca E. Routine laboratory parameters in estimating mortality and morbidity in COVID-19 diagnosed cases followed in the intensive care unit. Eur Rev Med Pharmacol Sci. 2023;27(12):5885–92. [DOI] [PubMed] [Google Scholar]
- 73.Ergenc Z, Ergenc H, Öztürk A, Kaya T, Nalbant A, Karacaer C, et al. The effect of thrombosis-related laboratory values on mortality in COVID-19 infection. Eur Rev Med Pharmacol Sci. 2023;27(6):2699–705. [DOI] [PubMed] [Google Scholar]
- 74.Dwivedi T, Raj A, Das N, Gupta R, Gupta N, Tiwari P, et al. The Evaluation of laboratory parameters as predictors of disease severity and mortality in COVID-19 patients: a retrospective study from a tertiary care hospital in India. Cureus. 2023;15(6):e40273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harky A, Ala’Aldeen A, Butt S, Duric B, Roy S, Zeinah M. COVID-19 and multiorgan response: the long-term impact. Curr Probl Cardiol. 2023;48(9):101756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hakoshima M, Kitakaze K, Adachi H, Katsuyama H, Yanai H. Clinical, hematological, biochemical and radiological characteristics for patients with splenic infarction: case series with literature review. J Clin Med Res. 2023;15(1):38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta V, Acharya S, Keerti A. Common coagulopathies associated With COVID-19 patients. Cureus. 2023;15(4):e38067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta B, Chandrakar S, Gupta N, Jain G. Nebulized heparin to reduce COVID-19-induced acute lung injury: a prospective observational study. Indian J Crit Care Med. 2023;27(3):222–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Girard TJ, Antunes L, Zhang N, Amrute JM, Subramanian R, Eldem I, et al. Peripheral blood mononuclear cell tissue factor (F3 gene) transcript levels and circulating extracellular vesicles are elevated in severe coronavirus 2019 (COVID-19) disease. J Thromb Haemost. 2023;21(3):629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghanem HB, Elderdery AY, Alnassar HN, Aldandan HA, Alkhaldi WH, Alfuhygy KS, et al. Study of coagulation disorders and the prevalence of their related symptoms among COVID-19 patients in Al-Jouf Region, Saudi Arabia during the COVID-19 pandemic. Diagnostics (Basel). 2023;13(6):1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghanbari EP, Jakobs K, Puccini M, Reinshagen L, Friebel J, Haghikia A, et al. The role of NETosis and complement activation in COVID-19-associated coagulopathies. Biomedicines. 2023;11(5):1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gashimova NR, Pankratyeva LL, Bitsadze VO, Khizroeva JK, Tretyakova MV, Grigoreva KN, et al. Inflammation and Immune Reactions in the Fetus as a Response to COVID-19 in the Mother. J Clin Med. 2023;12(13):4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gardinassi LG, Servian CDP, Lima GDS, Dos Anjos DCC, Gomes Junior AR, Guilarde AO, et al. Integrated metabolic and inflammatory signatures associated with severity of, fatality of, and recovery from COVID-19. Microbiol Spectr. 2023;11(2):e0219422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fanning JP, Weaver N, Fanning RB, Griffee MJ, Cho SM, Panigada M, et al. Hemorrhage, disseminated intravascular coagulopathy, and thrombosis complications among critically Ill patients with COVID-19: an international COVID-19 critical care consortium study. Crit Care Med. 2023;51(5):619–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El Mawla Z, El Saddik G, Zeineddine M, Hassoun M, El Hajj T. Cerebrovascular disease in patients with COVID-19 infection: a case series from Lebanon. Ann Med Surg (Lond). 2023;85(7):3701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eichhorn T, Huber S, Weiss R, Ebeyer-Masotta M, Lauková L, Emprechtinger R, et al. Infection with SARS-CoV-2 is associated with elevated levels of IP-10, MCP-1, and IL-13 in sepsis patients. Diagnostics (Basel). 2023;13(6):1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dong W, Wang J, Tian L, Zhang J, Settles EW, Qin C, et al. Factor Xa cleaves SARS-CoV-2 spike protein to block viral entry and infection. Nat Commun. 2023;14(1):1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Devaux CA, Camoin-Jau L. Molecular mimicry of the viral spike in the SARS-CoV-2 vaccine possibly triggers transient dysregulation of ace2, leading to vascular and coagulation dysfunction similar to SARS-CoV-2 infection. Viruses. 2023;15(5):1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sofi-Mahmudi A, Masinaei M, Shamsoddin E, Tovani-Palone MR, Heydari M-H, Shoaee S, et al. Global, regional, and national burden and quality of care index (QCI) of lip and oral cavity cancer: a systematic analysis of the Global Burden of Disease Study 1990–2017. BMC Oral Health. 2021;21(1):558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen YE, Ren FL, Gu X, Zhang HJ, Li WJ, Yang H, Shang FQ. Clinical value of platelets and coagulation parameters in predicting the severity of delta variant SARS-CoV-2. Pathobiology. 2023;90(4):241–50. [DOI] [PMC free article] [PubMed]
- 91.Ceriz T, Lagarteira J, Alves SR, Carrascal A, Terras AR. Disseminated intravascular coagulation in COVID-19 setting: a clinical case description. Cureus. 2023;15(6):e39941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Busch MH, Timmermans S, Van Kuijk SMJ, Aendekerk JP, Ysermans R, Van Doorn DPC, et al. Thrombin formation via the intrinsic coagulation pathway and von Willebrand factor reflect disease severity in COVID-19. Haematologica. 2023;108(5):1417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boswell MT, Maimela T, Hameiri-Bowen D, Riley G, Malan A, Steyn N, et al. COVID-19 severity and in-hospital mortality in an area with high HIV prevalence. South Afr J HIV Med. 2023;24(1):1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Borulu F, Erkut B, Unlu Y. SARS-CoV-2 infection-associated thoraco-abdomino-iliac thrombosis in a patient without cardiac and systemic co-morbidity. Cardiovasc J Afr. 2023;34(2):114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bolek T, Samoš M, Jurica J, Stančiaková L, Péč MJ, Škorňová I, et al. COVID-19 and the response to antiplatelet therapy. J Clin Med. 2023;12(5):2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balaji Easwaran V, Satarker S, TVG, John J, Veedu AP, George KT, et al. Expediting molecular translational approach of mesenchymal stem cells in COVID-19 treatment. Curr Stem Cell Res Ther. 2023;18(5):653–75. [DOI] [PubMed] [Google Scholar]
- 97.Badaras I, Laučytė-Cibulskienė A. Vascular aging and COVID-19. Angiology. 2023;74(4):308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Antoniello A, Brophy A, Opsha Y. Evaluation of hospitalized patient outcomes in COVID-19 infection for continued versus discontinued use of preadmission antiplatelet regimen. J Pharm Pract. 2023;36(3):508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amatya I, Regmi PR, Adhikari G, Pokhrel B, Baniya A, Dangol A. Pulmonary embolism at CT pulmonary angiography in patients with COVID-19 at a tertiary care center in Nepal: a cross-sectional study. Ann Med Surg (Lond). 2023;85(5):1661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aliani C, Rossi E, Luchini M, Calamai I, Deodati R, Spina R, et al. Automatic COVID-19 severity assessment from HRV. Sci Rep. 2023;13(1):1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shoaee S, Saeedi Moghaddam S, Masinaei M, Sofi-Mahmudi A, Hessari H, Heydari M-H, et al. Trends in dental caries of deciduous teeth in Iran: a systematic analysis of the national and sub-national data from 1990 to 2017. BMC Oral Health. 2022;22(1):634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abdelaal A, Abu-Elfatth A, Bakkar LM, El-Azeem HGA, Hetta HF, Badawy ER. Assessment of COVID -19 associated coagulopathy and multiple hemostatic markers: a single center study in Egypt. Infection. 2023;51(3):655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu FF, Gu BB, Jin YJ, Yao L, Zhou L, Zou D, et al. Risk factors for radiological progression within admissive one week in the hospitalized COVID-19 omicron variant-infected patients. Infect Drug Resist. 2022;15:7127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu A, Zakusilo G, Lee MS, Kim J, Kim H, Ying X, et al. Laboratory parameters and outcomes in hospitalized adults with COVID-19: a scoping review. Infection. 2022;50(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Z, Lin F, Liu F, Li Q, Li Y, Zhu Z, et al. Proteomic profiling reveals a distinctive molecular signature for critically ill COVID-19 patients compared with asthma and chronic obstructive pulmonary disease. Int J Infect Dis. 2022;116:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zendehdel A, Jamalimoghadamsiahkal S, Arshadi M, Godarzi F, S SH, Hekmat H, et al. Survival analysis of COVID-19 patients based on different levels of D-dimer and coagulation factors. Biomed Environ Sci. 2022;35(10):957–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yulistiani, Neldi V, Suprapti B, Nur RA. Efficacy and safety of anticoagulants for COVID-19 patients in the intensive care unit: a systematic review and meta-analysis. J Pharm Pharm Sci. 2022;25:274–84. [DOI] [PubMed] [Google Scholar]
- 108.Yuan Y, Wang G, Chen X, Ye XL, Li XK, Li R, et al. Thrombocytopenia and increased risk of adverse outcome in COVID-19 patients. PeerJ. 2022;10:e13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yoo EH, Chang SH, Song DY, Lee CH, Cheong GY, Park S, et al. Comprehensive laboratory data analysis to predict the clinical severity of coronavirus disease 2019 in 1,952 patients in Daegu. Korea Ann Lab Med. 2022;42(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yıldırım Ayaz E, Coşkun Z, Kaplan M, Bulut AS, Yeşildal M, Ankaralı H, et al. Comparison of initial CT findings and CO-RADS stage in COVID-19 patients with PCR, inflammation and coagulation parameters in diagnostic and prognostic perspectives. J Belg Soc Radiol. 2022;106(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu Z, Zhang Y, Zhang C, Xiong F, Zhang J, Xiong J. Clinical features and outcomes of COVID-19 patients with acute kidney injury and acute kidney injury on chronic kidney disease. Aging Dis. 2022;13(3):884–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu X, Feng Y, Jia Y, Zhang X, Li L, Bai X, Jiao L. Prognostic value of von Willebrand factor and ADAMTS13 in patients with COVID-19: a systematic review and meta-analysis. Thromb Res. 2022;218:83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu S, Xu Y, Zhang J, Ran X, Jia X, Wang J, et al. Longitudinal serum proteome characterization of COVID-19 patients with different severities revealed potential therapeutic strategies. Front Immunol. 2022;13:893943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu M, Zou ZY, Chen YH, Wang CL, Feng YW, Liu ZF. Severe COVID-19-associated sepsis is different from classical sepsis induced by pulmonary infection with carbapenem-resistant klebsiella pneumonia (CrKP). Chin J Traumatol. 2022;25(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wen C, Shi G, Liu W, Zhang H, Lin G, Chen H. COVID-19 in a child with transposition of the great arteries S/P fontan palliation: a case report and literature review. Front Cardiovasc Med. 2022;9:937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang J, Lu Z, Jin M, Wang Y, Tian K, Xiao J, et al. Clinical characteristics and risk factors of COVID-19 patients with chronic hepatitis B: a multi-center retrospective cohort study. Front Med. 2022;16(1):111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang J, Choy KW, Lim HY, Ho P. Impaired fibrinolytic potential predicts oxygen requirement in COVID-19. J Pers Med. 2022;12(10):1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Volod O, Wegner J. Viscoelastic testing in the management of adult patients on mechanical circulatory support devices with focus on extracorporeal membrane oxygenation. Semin Thromb Hemost. 2022;48(7):814–27. [DOI] [PubMed] [Google Scholar]
- 119.Vatansev H, Karaselek MA, Yılmaz R, Küççüktürk S, Topal A, Yosunkaya Ş, et al. Evaluation of coagulation with TEG in patients diagnosed COVID-19. Turk J Med Sci. 2022;52(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vahdat S. A review of pathophysiological mechanism, diagnosis, and treatment of thrombosis risk associated with COVID-19 infection. Int J Cardiol Heart Vasc. 2022;41:101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ueland T, Hausberg I, Mørtberg TV, Dahl TB, Lerum TV, Michelsen A, et al. Anti-PF4/polyanion antibodies in COVID-19 patients are associated with disease severity and pulmonary pathology. Platelets. 2022;33(4):640–4. [DOI] [PubMed] [Google Scholar]
- 122.Tiwari L, Gupta P, N Y, Banerjee A, Kumar Y, Singh PK, et al. Clinicodemographic profile and predictors of poor outcome in hospitalised COVID-19 patients: a single-centre, retrospective cohort study from India. BMJ Open. 2022;12(6):e056464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Theofilis P, Vordoni A, Kalaitzidis RG. COVID-19 and kidney disease: a clinical perspective. Curr Vasc Pharmacol. 2022;20(4):321–5. [DOI] [PubMed] [Google Scholar]
- 124.Nugroho J, Wardhana A, Rachmi DA, Mulia EPB, A’yun MQ, et al. Simple coagulation profile as predictor of mortality in adults admitted with COVID-19: a meta-analysis. Arch Clin Infect Dis. 2021;16(5):e115442. [Google Scholar]
- 125.Len P, Iskakova G, Sautbayeva Z, Kussanova A, Tauekelova AT, Sugralimova MM, et al. Meta-analysis and systematic review of coagulation disbalances in COVID-19: 41 studies and 17,601 patients. Front Cardiovasc Med. 2022;9:794092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang A, Leng Y, Zhang Y, Wu K, Ji Y, Lei S, Xia Z. Meta-analysis of coagulation parameters associated with disease severity and poor prognosis of COVID-19. Int J Infect Dis. 2020;100:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Teimury A, Khameneh MT, Khaledi EM. Major coagulation disorders and parameters in COVID-19 patients. Eur J Med Res. 2022;27(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin J, Yan H, Chen H, He C, Lin C, He H, et al. COVID-19 and coagulation dysfunction in adults: a systematic review and meta-analysis. J Med Virol. 2021;93(2):934–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sekulovski M, Mileva N, Vasilev GV, Miteva D, Gulinac M, Peshevska-Sekulovska M, et al. Blood coagulation and thrombotic disorders following SARS-CoV-2 infection and COVID-19 vaccination. Biomedicines. 2023;11(10):2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data would be available based upon an eligible request to the corresponding author.