Abstract
Background
A significant portion of total knee and hip arthroplasty (TKA/THA) patients experience chronic postsurgical pain (CPSP). The prevalence of afflicted individuals ranges from 10 to 34%. CPSP is the main cause of postoperative dissatisfaction. For prevention purposes it is essential to know which preoperative factors are predictive for CPSP. It is unknown whether neuropathic-like symptoms add predictive value to known predictors for CPSP and dissatisfaction after TKA/THA.
Methods
A prospective cohort study including 453 TKA/THA patients (TKA 208, THA 245) was conducted. Pain intensity (numeric rating scale [NRS]) and neuropathic-like symptoms (modified-painDETECT questionnaire [mPDQ]; score ≥ 13) were obtained preoperatively. One year postoperatively, CPSP and dissatisfaction (single NRS item (0–10); dissatisfied: ≤ 5) were captured: CPSP by means of the Oxford Knee/Hip Score (moderate or severe pain on question 1) as well by pain intensity at rest and with movement (NRS ≥ 1). Multivariate logistic regression modeling was used to determine the additive predictive value of preoperative neuropathic-like symptoms (mPDQ ≥ 13) on experiencing CPSP and dissatisfaction for the total group and for knee and hip patients separately.
Results
Preoperative neuropathic-like symptoms (m-PDQ ≥ 13) had an additional value for experiencing CPSP after one year, with odds ratios (p < 0.05) ranging from 2.16 (total group) to 4.15 (hip patients). Neuropathic-like symptoms had no additional value for predicting CPSP in knee patients or for predicting dissatisfaction.
Conclusion
The results of this study showed that neuropathic-like symptoms (m-PDQ ≥ 13) have an additional predictive value over known predictors, especially in hip patients. Patients with neuropathic-like symptoms have over twice the odds of suffering from CPSP one year after TKA/THA. Neuropathic-like symptoms had no additional value for predicting dissatisfaction.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-024-08129-y.
Keywords: Neuropathic pain, Residual pain, PainDETECT questionnaire, Total joint replacement, Total knee prosthesis, Total hip prosthesis
Background
Osteoarthritic pain is one of the most frequent causes of chronic pain [1]. As there is no cure, osteoarthritis (OA) treatment is mostly aimed at pain alleviation to restore physical function and quality of life [2]. When pain alleviation is no longer sufficient, arthroplasty is inevitable [2]. Despite the success of arthroplasty, a significant portion of patients experience chronic postsurgical pain (CPSP) [3]. The proportion of patients suffering from an unfavorable postoperative pain outcome runs in the 10–34% range after total knee arthroplasty (TKA) and 7–23% after total hip arthroplasty (THA) [4, 5]. CPSP seems to be the main cause of postoperative dissatisfaction in addition to mental health issues like anxiety and depression and pain in other joints. CPSP is defined as pain that persists longer than 2 months after a surgical procedure, has no identifiable cause, is not a continuation of a preexisting pain problem, and is not related to a postoperative complication [4]. Reports show CPSP percentages of 20% and 7% dissatisfaction after TKA and THA, respectively [5, 6]. The diagnosis should be established as early as possible to optimize the chances of improvement. The treatment of CPSP can combine a number of perioperative prophylactic strategies and the treatment of chronic neuropathic pain. Local treatments can consist of transcutaneous electrical nerve stimulation and lidocaine patches combined with tramadol. When this treatment is inadequately effective, an antidepressant or anticonvulsant can be added. A capsaicin patch is the third-line treatment, and step III opioids are the last option. Rehabilitation therapy and physical exercises are considered beneficial. Last, psychological counseling and/or cognitive behavioral therapy should be offered, if indicated [3].
Due to the high proportion of CPSP, it is essential to know which preoperative patient factors are predictive. Some factors are already known to be predictors for experiencing CPSP and dissatisfaction. Predictors such as female gender, elevated body mass index (BMI) and advanced age (specifically observed in hip osteoarthritis patients) have been identified as factors associated with CPSP [7–9]. Patients with high preoperative scores for knee pain (at rest) and prolonged preoperative pain are also at increased risk of CPSP after TKA [9–11]. For dissatisfaction it is known that a lower radiographic grade of OA and pain/arthritis in other joints are significant predictors [12–15]. Moreover, patients with less preoperative pain seem to improve less after arthroplasty [7, 9–11] and are hypothesized as more likely to be dissatisfied. This could be explained by their relatively low initial pain scores, which leaves less room for significant improvement. Consequently, lower satisfaction can be expected.
It remains unclear whether neuropathic-like symptoms predict long-term CPSP in patients undergoing TKA and THA. These symptoms, such as numbness, paresthesia, and heightened sensitivity to mechanical and thermal stimuli [2], likely stem from osteoarthritic joint processes affecting the peripheral nervous system and potentially leading to central sensitization (CS) [2]. Studies suggest that 20–67% of knee OA patients and 20% of hip OA patients experience these symptoms [16–23]. There is a possibility that these symptoms are not adequately recognized or treated before surgery, potentially contributing to persistent pain after arthroplasty (CPSP). It is hypothesized that an altered preoperative pain system (central sensitization) may persist post-arthroplasty, even after removal of the articular nerve fibers that initially induced sensitization [24].
To our knowledge, only two studies in knee OA patients have investigated whether preoperative neuropathic-like symptoms correlate with CPSP [25, 26]. Results were hard to interpret due to a limited number of patients experiencing these symptoms (n = 6) [25] and/or because it was not investigated whether the symptoms were predictive of long-term CPSP (only six months postoperatively) [26]. Aim of this study is therefore to examine whether preoperative neuropathic-like symptoms add predictive value to known predictors of long-term CPSP and dissatisfaction one year after TKA and THA.
Material and methods
Study participants and procedure
Patients scheduled for primary arthroplasty of the hip or knee (inclusion criterion) were invited by mail (physical format) to participate in this longitudinal cohort study. Out of 655 invited patients, 453 (69%) ended up participating. Reasons for non-participation are reported in Fig. 1. Patients underwent arthroplasty at University Medical Center Groningen, Martini Hospital Groningen or Medical Center Leeuwarden, all in the Netherlands, between October 2015 and November 2016. Exclusion criteria included secondary OA (e.g. inflammatory arthritis, fracture) and/or cognitive impairments that made it impossible to complete questionnaires. Patient-reported outcome measures (PROMs) were completed preoperatively and one year postoperatively. The study was approved by the local medical ethics committee of University Medical Center Groningen (no. METc2014/381) and conducted in accordance with the principles of the Declaration of Helsinki (64th, 2013). Informed consent was obtained from all the participants involved in the study. Informed consent was considered as obtained if the patient granted our request to participate in the study by returning the completed questionnaire. Patients were informed of this way of obtaining consent by the invitation letter.
Fig. 1.
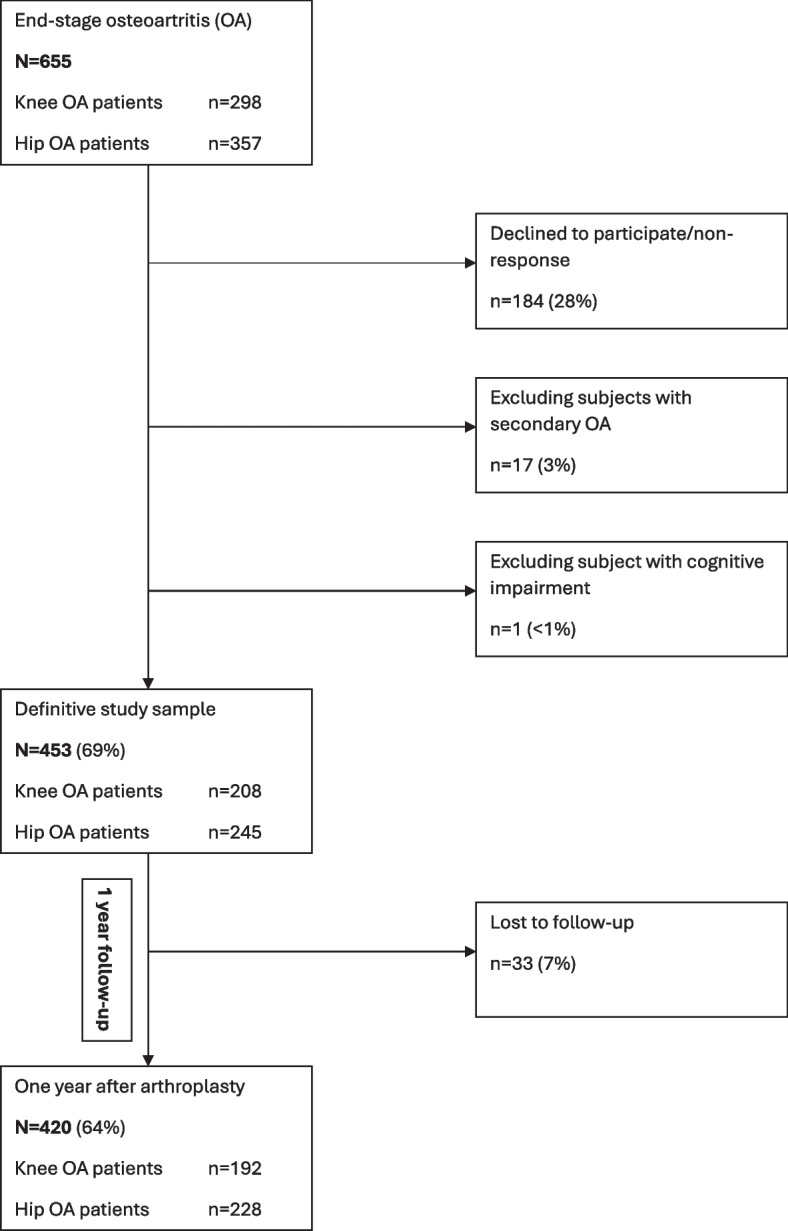
Flowchart of the study
Measures
Patient characteristics
Age (at time of arthroplasty), gender, BMI (kg/m2), cohabitation status (living alone), educational level, ASA (American Society of Anesthesiologists) classification [27] (assessed by anesthesiologist: I, II or III/IV), Charnley Classification (A: single joint arthroplasty; B1: one other joint in need of arthroplasty or B2: contralateral joint has been treated with arthroplasty; C: multiple joints in need of arthroplasty, multiple failing arthroplasties or an illness that significantly impairs the patient’s ability to walk) [28]. Duration of preoperative pain (months) was also assessed, as was number of painful regions/joints on most days of the month preceding arthroplasty (body diagram; 20 regions: head, neck, shoulders bilaterally, elbows bilaterally, wrists bilaterally, hands bilaterally, upper spine, lower spine, hips bilaterally, knees bilaterally, ankles bilaterally, feet bilaterally).
Radiographic assessment
Radiographic severity was determined from the most recent anteroposterior radiograph; the radiograph was only included if it was taken not more than one year before the date of surgery. These radiographs were all taken as part of the patient’s usual care. Anteroposterior radiographs were rated by the same observer (TB) using the Kellgren and Lawrence (KL) grade classification (I-IV) [29]. Rating was done in one session and the rater was blinded to the clinical status of the patient.
Neuropathic-like symptoms
The Dutch-language version of the modified-painDETECT Questionnaire (mPDQ) was used to assess neuropathic-like symptoms [30]. It is composed of nine items evaluating pain quality (seven items), pain pattern (one item), and pain radiation (one item). Total score ranges from −1 to 38. The 12-point cutoff point was used to discriminate unlikely neuropathic phenotype patients (nociceptive pain, mPDQ ≤ 12) from possible neuropathic phenotype patients (neuropathic-like symptoms, mPDQ ≥ 13). The Dutch mPDQ knee/hip proved to be reliable [30] and valid [31].
Pain
Two outcome measures were used to assess CPSP. These two measures were based on outcome measures used in studies included in a systematic review by Beswick et al. [6] investigating long-term pain after THA/TKA. That review included studies that selected patients with an unfavorable long-term pain outcome, i.e. “those with moderate-to-severe pain or for whom surgery had not relieved pain”. The first measure used was item 1 from the Oxford Hip/Knee Score (OHS/OKS): “During the past 4 weeks: How would you describe the pain you usually have in your hip/knee?” If the answer was moderate or severe, it was considered an unfavorable long-term pain outcome (CPSP present). Both the OHS and OKS are proven to be reliable and valid [32, 33]. The second outcome measure used was the numeric rating scale (NRS) of pain at rest and with movement. An 11-point NRS was used, with 0 representing “no pain” and 10 representing “pain as bad as you can imagine”. If there was pain at rest and with movement (NRS ≥ 1), it was considered an unfavorable pain outcome (CPSP present). The NRS showed to be reliable and valid [34, 35].
Satisfaction
Satisfaction was obtained by one single question: “How satisfied are you (in general) about the result of your operation?” The single-answer modality entailed an 11-point NRS (0–10) ranging from “very dissatisfied” (0) to “very satisfied” (10). Dissatisfaction was coded as a score ≤ 5.
Statistical methods
Analyses were performed for the total group, as well for knee and hip patients separately (when numbers allowed it). Fisher’s exact test was given to check for gender differences between respondents and non-respondents. The Student t-test (normality checked by histogram) was administered to compare respondents with non-respondents on age. To investigate if neuropathic-like symptoms (mPDQ ≥ 13) were an additional predictor – next to the known ones – for CPSP and dissatisfaction, multivariate logistic regression modelling (MLRM) was used. Before performing MLRM, univariate odds ratios (ORs) were calculated for all predictors. Multicollinearity was checked by calculating the variance inflation factor. Complete-case approach was used to handle missing data. The dependent variables used in the MLRM were for CPSP “moderate or severe pain (OHS/OKS)” and “pain at rest and with movement (NRS ≥ 1)”. First a basic model was built with the known predictors of age, sex, BMI, pain intensity and pain duration, and subsequently an added model was built with the neuropathic-like symptoms variable added to the known predictors of the basic model. Afterwards these two models (basic vs. added) were compared using Nagelkerke’s R2. For limited cases, only the best predictive univariate variables were added (see Appendix Table A.1). To predict dissatisfaction (satisfaction score ≤ 5 as dependent variable) an identical procedure was followed, yet due to the limited cases it was not possible to do separate analyses for knee and hip patients. In the basic model, the MLRM incorporated the variables KL-grade 2 (as representative of a lower radiographic grade of OA), Charnley classification (as representative of pain/arthritis in other joints) and pain intensity. In the added model neuropathic-like symptoms were added. Afterwards these two models (basic vs. added) were compared using Nagelkerke’s R2. IBM SPSS (V.23) was used for all analyses. P-values < 0.05 were considered statistically significant.
Results
Respondents versus non-respondents
Age and gender were compared between respondents (n = 471) and non-respondents (n = 184). This was done for the total group as well as for knee and hip patients separately. No statistically significant differences were observed for age. For gender, the total non-respondent group had statistically significantly more females compared to respondents – 76% vs. 67%, respectively (p = 0.030). No statistically significant differences were seen when the total group was split into knee patients (p = 0.105) and hip patients (p = 0.186).
Participants
The characteristics of the study participants are displayed in Table 1. Participants were on average 70 years old, overweight and predominantly female. Median preoperative NRS joint pain intensity was 7 and median preoperative joint pain duration was at least two years. Around half of the participants experienced preoperative neuropathic-like symptoms.
Table 1.
Characteristics of study participantsa
| Characteristics | Number (total/hip/knee) |
Total group | Knee group | Hip group |
|---|---|---|---|---|
| Age (years), mean ± SD | 453/208/245 | 70.0 ± 9.1 | 69.6 ± 8.5 | 70.4 ± 9.7 |
| Female | 453/208/245 | 66.2 (300) | 62.0 (129) | 69.8 (171) |
| BMI (kg/m2), mean ± SD | 453/208/245 | 29.0 ± 4.8 | 30.0 ± 4.7 | 28.1 ± 4.7 |
| Cohabitation | 448/205/243 | 72.8 (326) | 72.2 (148) | 73.3 (178) |
| Educational level | 434/200/234 | |||
| Higher | 26.0 (113) | 24.5 (49) | 27.4 (64) | |
| Secondary | 60.1 (261) | 62.5 (125) | 58.1 (136) | |
| Lower | 13.8 (60) | 13.0 (26) | 14.5 (34) | |
| ASA Classification | 453/208/245 | |||
| I | 14.3 (65) | 10.6 (22) | 17.6 (43) | |
| II | 57.6 (261) | 56.7 (118) | 58.4 (143) | |
| III/IV | 28.0 (127) | 32.7 (68) | 24.1 (59) | |
| Charnley Classification | 453/208/245 | |||
| A | 44.6 (202) | 52.9 (110) | 37.6 (92) | |
| B1 | 30.5 (138) | 24.5 (51) | 35.5 (87) | |
| B2 | 21.6 (98) | 19.2 (40) | 23.7 (58) | |
| C | 3.3 (15) | 3.4 (7) | 3.3 (8) | |
| Preoperative pain duration (months), median (Q1;Q3) | 445/204/241 | 30 (18;60) | 36 (24;90) | 24 (15;48) |
| Preoperative number of painful body regions/joints (/20), median (Q1;Q3) | 449/206/243 | 2 (1;3) | 1 (1;2) | 2 (1;3) |
| KL grade | 450/207/243 | |||
| KL grade II | 16.0 (72) | 14.5 (30) | 17.3 (42) | |
| KL grade III | 58.4 (263) | 62.8 (130) | 54.7 (133) | |
| KL grade IV | 25.6 (115) | 22.7 (47) | 28.0 (68) | |
| Preoperative mPDQ score, mean ± SD | 453/208/245 | 13.1 ± 6.3 | 13.9 ± 6.2 | 12.4 ± 6.3 |
| nociceptive pain | 50.6 (229) | 45.2 (94) | 55.1 (135) | |
| neuropathic-like symptoms | 49.5 (224) | 54.8 (114) | 44.9 (110) | |
| NRS preoperative average pain intensity, median (Q1;Q3) | 445/205/240 | 7 (6;8) | 7 (6;8) | 7 (6;8) |
BMI body mass index, ASA American Society of Anesthesiologists; KL Kellgren & Lawrence, mPDQ modified-painDETECT questionnaire, NRS numeric rating scale
aExcept where indicated otherwise, values are presented as % (n)
Chronic postsurgical pain and dissatisfaction
One year after arthroplasty, CPSP ranged between 11.2% and 36%. It was more commonly reported by knee patients (16.7% with moderate or severe pain (OHS/OKS) and 45.5% with pain at rest and with movement (NRS ≥ 1)). Around 11% of patients was dissatisfied with the arthroplasty, and more dissatisfaction was observed in the knee group (16.3%). CPSP and dissatisfaction were seen most commonly in patients that had preoperative neuropathic-like symptoms (60.5%). See Table 2 for detailed figures.
Table 2.
Pain and dissatisfaction one year postoperativelya
| Characteristics |
Number (total/hip/knee) |
Total group | Knee group | Hip group |
|---|---|---|---|---|
| mPDQ score, median (Q1;Q3) | 412/190/222 | 3 (0;8) | 5 (2;10) | 1 (0;5.3) |
| nociceptive pain | 89.1 (367) | 82.6 (157) | 94.6 (210) | |
| neuropathic-like symptoms | 10.9 (45) | 17.4 (33) | 5.4 (12) | |
| NRS average pain intensity, median (Q1;Q3) | 404/186/218 | 1 (0;30) | 1 (0;3.25) | 0 (0;2) |
| Moderate or severe pain (OHS/OKS) | 418/192/226 | 11.2 (47) | 16.7 (32) | 6.6 (15) |
| patients with neuropathic-like symptoms preoperatively | 72.3 (34) | 68.8 (22) | 80.0 (12) | |
| Pain at rest and with movement (NRS ≥ 1) | 419/191/228 | 36.0 (151) | 45.5 (87) | 28.1 (64) |
| patients with neuropathic-like symptoms preoperatively | 61.6 (93) | 62.1 (54) | 60.9 (39) | |
| Dissatisfied with prosthesis | 398/184/214 | 10.8 (43) | 16.3 (30) | 6.1 (13) |
| patients with neuropathic-like symptoms preoperatively | 60.5 (26) | 56.7 (17) | 69.2 (9) |
mPDQ: modified-painDETECT questionnaire, NRS numeric rating scale, OHS Oxford hip score, OKS Oxford knee score
aExcept where indicated otherwise, values are presented as % (n)
Predicting an unfavorable pain outcome
In the total group and the hip group, preoperative neuropathic-like symptoms were a statistically significant additive predictor for an unfavorable long-term pain outcome (Table 3). The adjusted odds of experiencing such an outcome were 2.16 and 4.15, respectively, for patients that experienced neuropathic-like symptoms preoperatively. In both unfavorable pain outcome measures and in all groups Nagelkerke’s R-squared rose, indicating an improved goodness-of-fit when adding the preoperative neuropathic-like symptoms variable to the known preoperative predictors (Table 3). When it wasn’t possible to incorporate all variables into the multivariate regression analyses, only the best predicting univariate variables were added (see Table 3 and Appendix Table A.1).
Table 3.
Multivariate logistic regression: unfavorable pain outcome one year postoperatively
| Preoperative variables | Total-basic | Total-added | Knee-basic | Knee-added | Hip-basic | Hip-added |
|---|---|---|---|---|---|---|
| Dependent variable: Moderate or severe pain on OKS or OHS | ||||||
| Age (years) | 0.99 (0.96–1.03) | 0.99 (0.96–1.03) | N.A | N.A | 0.97 (0.92–1.02) | - |
| Female gender | 1.19 (0.60–2.35) | 1.12 (0.6–2.2) | 1.66 (0.69–4.00) | - | - | |
| BMI (kg/m2) | 1.09 (1.03–1.17)* | 1.09 (1.0–1.2)* | 1.07 (0.98–1.16) | 1.07 (0.99–1.17) | - | |
| Pain intensity (NRS) | 1.36 (1.10–1.68)* | 1.24 (1.0–1.6)* | 1.22 (0.96–1.56) | 1.19 (0.92–1.56) | 1.63 (1.1–2.5)* | 1.47 (0.9–2.3) |
| Pain duration (months) | 1.00 (0.99–1.01) | - | - | - | ||
| Neuropathic-like symptoms | 2.16 (1.06–4.40)* | 1.48 (0.61–3.59) | 3.91 (1.0–14.7)* | |||
| Nagelkerke R2 | 0.090 | 0.110 | 0.127 | 0.184 | 0.092 | 0.131 |
| Dependent variable: Pain at rest and with movement (NRS ≥ 1) | ||||||
| Age (years) | 0.99 (0.97–1.03) | 0.99 (0.97–1.02) | N.A | N.A | 0.97 (0.92–1.03) | 0.98 (0.92–1.04) |
| Female gender | 1.51 (0.97–2.36) | 1.48 (0.94–2.30) | 1.72 (0.71–4.17) | 1.68 (0.69–4.07) | 0.71 (0.23–2.17) | 0.68 (0.22–2.11) |
| BMI (kg/m2) | 1.01 (0.97–1.06) | 1.01 (0.96–1.05) | 1.07 (0.98–1.17) | 1.07 (0.98–1.16) | 1.09 (0.98–1.20) | 1.10 (0.99–1.22) |
| Pain intensity (NRS) | 1.11 (0.99–1.24) | 1.02 (0.91–1.15) | 1.22 (0.96–1.56) | 1.18 (0.91–1.53) | 1.61 (1.05–2.46)* | 1.49 (0.94–2.37) |
| Pain duration (months) | 1.00 (0.99–1.00) | 1.00 (0.99–1.00) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 1.00 (0.98–1.01) | 1.00 (0.98–1.01) |
| Neuropathic-like symptoms | 2.18 (1.39–3.44)* | 1.39 (0.57–3.42) | 4.15 (1.09–15.86)* | |||
| Nagelkerke R2 | 0.028 | 0.066 | 0.077 | 0.081 | 0.127 | 0.184 |
Values are presented as adjusted odds ratios with their 95% confidence interval: adjusted OR (95%CI)
N.A. not applicable, refers to the Age variable, which is not predictive in knee patients, BMI body mass index, NRS numeric rating scale (0–10)
*p-value < 0.05 (statistically significant); basic: multivariate logistic regression model with the known potential predictors for an unfavorable pain outcome; added: multivariate logistic regression model with the neuropathic-like symptoms variable added to the previous basic model
Predicting dissatisfaction
Neuropathic-like symptoms did not have an additive predictive value on dissatisfaction in the multivariate logistical model (Table 4). Goodness-of-fit did improve though, from 0.020 to 0.024. Due to limited cases in the knee and hip group – 17 and 9, respectively – we were not able to perform any multivariate modelling in those groups separately.
Table 4.
Logistic regression results for dissatisfactiona
| | Dissatisfied-basic | Dissatisfied-added |
|---|---|---|
| KL-grade II (yes) | 0.98 (0.41–2.32) | 0.90 (0.40–2.26) |
| Charnley classification | 0.80 (0.44–1.44) | 0.79 (0.44–1.43) |
| Pain intensity (NRS) | 1.20 (0.98–1.45) | 1.16 (0.94–1.42) |
| Neuropathic-like symptoms | 1.36 (0.68–2.74) | |
| Nagelkerke R2 | 0.020 | 0.024 |
aDependent variable: dissatisfied (yes); values are presented as adjusted odds ratios with their 95% confidence interval: adjusted OR (95%CI); basic: multivariate logistic regression model with the known potential predictors for dissatisfaction; added: multivariate logistic regression model with the neuropathic-like symptoms variable added to the previous basic model; KL: Kellgren & Lawrence; NRS: numeric rating scale (0–10)
Discussion
Based on the results it can be concluded that preoperative neuropathic-like symptoms could serve as an additive predictor for CPSP. In the total group, patients with preoperative neuropathic-like symptoms had over twice-higher odds of experiencing CPSP one year after arthroplasty. Separate analyses revealed that only hip patients with preoperative neuropathic-like symptoms had statistically significantly higher odds of experiencing CPSP, so results for the total group were probably explained by the hip patients in the study group. To our knowledge, our findings are unique with respect to using neuropathic-like symptoms in a multivariate model, i.e. looking at the additional value while adjusting for relevant known predictors for CPSP. The impact and importance of adjusting is clearly visible in the results of the hip group. In line with previous research [11, 36], the basic multivariate model showed initially that higher preoperative pain intensity was a strong significant predictor for CPSP, although this effect became non-significant when adding neuropathic-like symptoms to the model. This suggests that neuropathic-like symptoms (pain quality) are a different concept and a better predictor for CPSP than pain intensity. This can be explained by the fact that neuropathic-like symptoms are likely an expression of a central sensitized state that could still be present after arthroplasty, even in a situation where the peripheral articular nerves that induce sensitization are removed [24].
Available literature describing the association between preoperative neuropathic-like symptoms and CPSP is scarce. Only three studies in knee patients were available for comparison [25, 26, 37]; this was hampered by the limited number of preoperative patients with neuropathic-like symptoms in one study [25], a shorter postoperative time period in the other two [26, 37], and the use of a different neuropathic pain-screening tool in one of the studies [26]. Two out of the three studies found no correlation between preoperative neuropathic-like symptoms and postoperative CPSP [25, 26]. The third study [37] found little if any correlation between preoperative neuropathic-like symptoms and postoperative pain (r = 0.397). These results are in line with our non-significant adjusted ORs found in the knee group, which suggests no association between neuropathic-like symptoms and CPSP. Comparisons were even harder to make for the hip group. There was only one study available, and it was aimed solely at the early postoperative period (two days after THA), thus not at CPSP [38]. Still, the study did share our finding that preoperative neuropathic-like symptoms are associated with higher postoperative pain scores. Hence experiencing preoperative neuropathic-like symptoms seems only predictive for CPSP in hip patients and not in knee patients.
With respect to dissatisfaction, only one knee study reported that preoperative neuropathic-like symptoms were not predictive for dissatisfaction [25]. To our knowledge, no such studies have been conducted among hip patients. The reported knee study results seem to be in line with ours, as we found that preoperative neuropathic-like symptoms were not associated with dissatisfaction in the total group. Given that our analysis was restricted to the total group, future joint-specific research seems warranted.
As the odds for experiencing CPSP one year after surgery are relatively high, it could be suggested that orthopedic surgeons should screen OA patients, and especially hip OA patients, more frequently on their pain phenotype – especially considering the association between neuropathic-like symptoms, lower joint-specific function and quality of life [39]. Customized treatment for their predominant and/or accompanying pain phenotype (nociceptive vs. neuropathic) could lengthen the conservative treatment phase, postponing or even aborting an arthroplasty. Furthermore, if an arthroplasty is performed, treating neuropathic-like symptoms preoperatively could lower a patient’s odds of experiencing CPSP. Further research into the effectiveness of these treatments is warranted.
Strengths of this study include a generous sample size, a prospective longitudinal design up to one year postoperatively, and its aim to investigate whether neuropathic-like symptoms – next to known predictors – are an additive predictor for CPSP and dissatisfaction. As hip studies are lacking, this study is the first to elucidate an association between preoperative neuropathic-like symptoms and CPSP. Another strength is that we used the OKS/OHS, which next to the Western Ontario and McMaster Universities Arthritis Index (WOMAC) is the recommended multi-item questionnaire to capture CPSP [40]. Despite the sample size, there were a limited number of cases of moderate-to-severe pain on the OKS/OHS, especially for the hip group, which made it possible to adjust for only a few variables. The limited number of cases also hampered the joint-specific analyses to predict dissatisfaction. As a result, multivariate logistic regression modelling was only possible for the total group. Another limitation of our study could be that we did not include some important potential predictors, like comorbidities and psychological factors. For instance, pain catastrophizing seems to be a significant predictor for CPSP in knee patients [11]. Future studies with bigger sample sizes would be beneficial, as they can deal with more preoperative predictors. Larger studies will also help perform the needed joint-specific analyses for dissatisfaction after arthroplasty.
Conclusions
The results of this study show that experiencing preoperative neuropathic-like symptoms, which are highly prevalent and likely an expression of a disturbance in the peripheral and/or central nervous system, at least doubles the odds of CPSP one year after TKA/THA. Joint-specific sub-analyses revealed that this was only the case in hip patients. Experiencing preoperative neuropathic-like symptoms does not seem to predict dissatisfaction one year after arthroplasty. Starting enriched enrolment trials for OA patients with neuropathic-like symptoms can be taken into consideration [23] to observe whether neuropathic-like symptoms could be modified, enhancing joint-specific function and health-related quality of life preoperatively. Additionally, it should be investigated whether customized pain phenotype treatment reduces CPSP and dissatisfaction postoperatively.
Supplementary Information
Abbreviations
- ASA
American Society of Anesthesiologists
- BMI
Body mass index
- CS
Central sensitization
- CPSP
Chronic postsurgical pain
- KL
Kellgren and Lawrence
- mPDQ
Modified-painDETECT questionnaire
- MLRM
Multivariate logistic regression modelling
- NRS
Numeric rating scale
- OA
Osteoarthritis
- OHS/OKS
Oxford Hip/Knee Score
- PROMS
Patient-reported outcome measures
- THA
Total hip arthroplasty
- TKA
Total knee arthroplasty
- WOMAC
Western Ontario and McMaster Universities Arthritis Index
Authors’ contributions
TB is the Principal Investigator of the study. IvdA-S and MS managed the progress of the study. TB, IvdA-S, MS and SKB participated in the design of the study. TMvR and BD contributed to the recruitment of patients for the study
Funding
This work is supported and financed by the Dutch Arthritis Society (project number BP 12–3–401). The Society had no role in the design, completion or reporting of this work.
Data availability
The datasets is available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the local medical ethics committee of University Medical Center Groningen (no. METc2014/381). Informed consent was obtained from all the participants involved in the study. Informed consent was considered as obtained if the patient granted our request to participate in the study by returning the completed questionnaire. Patients were informed of this way of obtaining consent by the invitation letter.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–287. 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Fu K, Robbins SR, McDougall JJ. Osteoarthritis: the genesis of pain. Rheumatology. 2017. 10.1093/rheumatology/kex419. [DOI] [PubMed] [Google Scholar]
- 3.Vergne-Salle P. Management of neuropathic pain after knee surgery. Joint Bone Spine. 2016;83:657–63. 10.1016/j.jbspin.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Macrae WA. Chronic post-surgical pain: 10 years on. British J Anaesth. 2008;101:77–86. 10.1093/bja/aen099. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-de-las-Peñas C, Florencio LL, de-la-Llave-Rincón AI, Ortega-Santiago R, Cigarán-Méndez M, Fuensalida-Novo S, et al. Prognostic Factors for Postoperative Chronic Pain after Knee or Hip Replacement in Patients with Knee or Hip Osteoarthritis: An Umbrella Review. J Clin Med. 2023;12:6624. 10.3390/jcm12206624. [DOI] [PMC free article] [PubMed]
- 6.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2. 10.1136/bmjopen-2011-000435. [DOI] [PMC free article] [PubMed]
- 7.Hofstede SN, Gademan MG, Vlieland TPV, Nelissen RG, Marang-van de Mheen, Perla J. Preoperative predictors for outcomes after total hip replacement in patients with osteoarthritis: a systematic review. BMC Musculoskelet Disord. 2016;17:212. 10.1186/s12891-016-1070-3. [DOI] [PMC free article] [PubMed]
- 8.Puolakka PA, Rorarius MG, Roviola M, Puolakka TJ, Nordhausen K, Lindgren L. Persistent pain following knee arthroplasty. Eur J Anaesthesiol. 2010;27:455–60. 10.1097/EJA.0b013e328335b31c. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B, Rao S, Mekkawy KL, Rahman R, Sarfraz A, Hollifield L, et al. Risk factors for pain after total hip arthroplasty: a systematic review. Arthroplasty. 2023;5(1):19). 10.1186/s42836-023-00172-9. [DOI] [PMC free article] [PubMed]
- 10.Lundblad H, Kreicbergs A, Jansson K. Prediction of persistent pain after total knee replacement for osteoarthritis. J Bone Joint Surg Br. 2008;90:166–71. 10.1302/0301-620X.90B2.19640. [DOI] [PubMed] [Google Scholar]
- 11.Lewis G, Rice D, McNair P, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. 2014;114:551–61. 10.1093/bja/aeu441. [DOI] [PubMed] [Google Scholar]
- 12.Scott C, Howie C, MacDonald D, Biant L. Predicting dissatisfaction following total knee replacement a prospective study of 1217 patients. J Bone Joint Surg Br. 2010;92:1253–8. 10.1302/0301-620X.92B9.24394. [DOI] [PubMed] [Google Scholar]
- 13.Anakwe RE, Jenkins PJ, Moran M. Predicting dissatisfaction after total hip arthroplasty: a study of 850 patients. J Arthroplasty. 2011;26:209–13. 10.1016/j.arth.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Scott CE, Oliver WM, MacDonald D, Wade FA, Moran M, Breusch SJ. Predicting dissatisfaction following total knee arthroplasty in patients under 55 years of age. Bone Joint J. 2016;98-B: 1625–1634. 10.1302/0301-620X.98B12.BJJ-2016-0375.R1. [DOI] [PubMed]
- 15.Warner SC, Richardson H, Jenkins W, Kurien T, Doherty M, Valdes AM. Neuropathic pain-like symptoms and pre-surgery radiographic severity contribute to patient satisfaction 4.8 years post-total joint replacement. World J Orthop. 2017;8:761. 10.5312/wjo.v8.i10.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shigemura T, Ohtori S, Kishida S, Nakamura J, Takeshita M, Takazawa M, et al. Neuropathic pain in patients with osteoarthritis of hip joint. European Orthop Traumatol. 2011;2:73–77. 10.1007/s12570-011-0070-x.
- 17.Ohtori S, Orita S, Yamashita M, Ishikawa T, Ito T, Shigemura T, et al. Existence of a neuropathic pain component in patients with osteoarthritis of the knee. Yonsei Med J. 2012;53:801–5. 10.3349/ymj.2012.53.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdes AM, Doherty SA, Zhang W, Muir KR, Maciewicz RA, Doherty M. Inverse relationship between preoperative radiographic severity and postoperative pain in patients with osteoarthritis who have undergone total joint arthroplasty. Semin Arthritis Rheum. 2012;41:568–75. 10.1016/j.semarthrit.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Hochman J, Davis A, Elkayam J, Gagliese L, Hawker G. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1236–42. 10.1016/j.joca.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Hochman J, Gagliese L, Davis A, Hawker G. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage. 2011;19:647–54. 10.1016/j.joca.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Hochman JR, French MR, Bermingham SL, Hawker GA. The nerve of osteoarthritis pain. Arthritis Care Res. 2010;62:1019–23. 10.1002/acr.20142. [DOI] [PubMed] [Google Scholar]
- 22.Aşkın A, Özkan A, Tosun A, Demirdal ÜS, İsnaç F. Quality of life and functional capacity are adversely affected in osteoarthritis patients with neuropathic pain. Kaohsiung J Med Sci. 2017;33:152–8. 10.1016/j.kjms.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Moss P, Benson HAE, Will R, Wright A. Patients With Knee Osteoarthritis Who Score Highly on the PainDETECT Questionnaire Present With Multimodality Hyperalgesia, Increased Pain, and Impaired Physical Function. Clin J Pain. 2018;34:15–21. 10.1097/AJP.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thakur M, Dickenson AH, Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol. 2014;10:374–80. 10.1038/nrrheum.2014.47. [DOI] [PubMed] [Google Scholar]
- 25.Phillips JR, Hopwood B, Arthur C, Stroud R, Toms AD. The natural history of pain and neuropathic pain after knee replacement: a prospective cohort study of the point prevalence of pain and neuropathic pain to a minimum three-year follow-up. Bone Joint J. 2014;96-B:1227–1233. 10.1302/0301-620X.96B9.33756. [DOI] [PubMed]
- 26.Fitzsimmons M, Carr E, Woodhouse L, Bostick GP. Development and Persistence of Suspected Neuropathic Pain After Total Knee Arthroplasty in Individuals With Osteoarthritis. PM&R. 2018. 10.1016/j.pmrj.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2:281–4. [Google Scholar]
- 28.Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11:203–9. 10.1007/s10195-010-0115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rienstra W, Blikman T, Mensink FB, van Raay JJ, Dijkstra B, Bulstra SK, et al. The Modified painDETECT Questionnaire for Patients with Hip or Knee Osteoarthritis: Translation into Dutch, Cross-Cultural Adaptation and Reliability Assessment. PLoS ONE. 2015;10: e0146117. 10.1371/journal.pone.0146117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rienstra W, Blikman T, Dijkstra B, van Raay J, Slager G, Bulstra S, et al. Validity of the Dutch modified painDETECT questionnaire for patients with hip or knee osteoarthritis. Disabil Rehabil. 2017:1–7. 10.1080/09638288.2017.1413429. [DOI] [PubMed]
- 32.Haverkamp D, Breugem SJ, Sierevelt IN, Blankevoort L, Dijk CNv. Translation and validation of the Dutch version of the Oxford 12-item knee questionnaire for knee arthroplasty. Acta orthopaedica. 2005;76:347–352. 10.1080/00016470510030814. [PubMed]
- 33.Gosens T, Hoefnagels NH, de Vet RC, Dhert WJ, van Langelaan EJ, Bulstra SK, et al. The “Oxford Heup Score” The translation and validation of a questionnaire into Dutch to evaluate the results of total hip arthroplasty. Acta Orthop. 2005;76:204–11. 10.1080/00016470510030580. [DOI] [PubMed] [Google Scholar]
- 34.Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–81. 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferraz MB, Quaresma M, Aquino L, Atra E, Tugwell P, Goldsmith C. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J Rheumatol. 1990;17:1022–4. [PubMed] [Google Scholar]
- 36.Pinto PR, McIntyre T, Ferrero R, Almeida A, Araújo-Soares V. Risk factors for moderate and severe persistent pain in patients undergoing total knee and hip arthroplasty: a prospective predictive study. PLoS ONE. 2013;8:e73917. 10.1371/journal.pone.0073917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurien T, Arendt-Nielsen L, Petersen KK, Graven-Nielsen T, Scammell BE. Preoperative Neuropathic Pain Like Symptoms and Central Pain Mechanisms in Knee Osteoarthritis Predicts Poor Outcome 6 Months After Total Knee Replacement Surgery. J Pain. 2018. 10.1016/j.jpain.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 38.den Hartog YM, Hannink G, van Dasselaar NT, Mathijssen NM, Vehmeijer SB. Which patient-specific and surgical characteristics influence postoperative pain after THA in a fast-track setting? BMC Musculoskelet Disord. 2017;18:363. 10.1186/s12891-017-1725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blikman T, Rienstra W, van Raay JJ, Dijkstra B, Bulstra SK, Stevens M, et al. Neuropathic-like symptoms and the association with joint-specific function and quality of life in patients with hip and knee osteoarthritis. PLoS ONE. 2018;13: e0199165. 10.1371/journal.pone.0199165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wylde V, Bruce J, Beswick A, Elvers K, Gooberman-Hill R. Assessment of chronic postsurgical pain after knee replacement: a systematic review. Arthritis Care Res. 2013;65:1795–803. 10.1002/acr.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets is available from the corresponding author on reasonable request.


