Abstract
Background
Healthcare-associated bloodstream infections (BSI) threaten patient safety and are the third most common healthcare-associated infection (HAI) in low- and middle-income countries. An intensive-care-unit (ICU) based HAI surveillance network recording BSIs was started in India in 2017. We evaluated this surveillance network’s ability to detect BSI to identify best practices, challenges, and opportunities in its implementation.
Methods
We conducted a mixed-methods descriptive study from January to May 2022 using the CDC guidelines for evaluation. We focused on hospitals reporting BSI surveillance data to the HAI network from May 2017 to December 2021, and collected data through interviews, surveys, record reviews, and site visits. We integrated quantitative and qualitative results and present mixed methods interpretation.
Results
The HAI surveillance network included 39 hospitals across 22 states of India. We conducted 13 interviews, four site visits, and one focus-group discussion and collected 50 survey responses. Respondents included network coordinators, surveillance staff, data entry operators, and ICU physicians. Among surveyed staff, 83% rated the case definitions simple to use. Case definitions were correctly applied in 280/284 (98%) case reports. Among 21 site records reviewed, 24% reported using paper-based forms for laboratory reporting. Interviewees reported challenges, including funding, limited human resources, lack of digitalization, variable blood culture practices, and inconsistent information sharing.
Conclusion
Implementing a standardized HAI surveillance network reporting BSIs in India has been successful, and the case definitions developed were simple. Allocating personnel, digitalizing medical records, improving culturing practices, establishing feedback mechanisms, and funding commitment are crucial for its sustainability.
Keywords: Healthcare-associated infection (HAI) surveillance in developing countries, Sepsis, Surveillance system, Patient safety, Cross-infection, Nosocomial infections
Introduction
Globally, healthcare-associated infections (HAI) pose a significant threat to patient safety. Bloodstream infections (BSIs) are among the most common HAIs in low- and middle-income countries (LMIC), prolong hospital stays, and increase mortality rates [1–4]. Prospective and active surveillance is associated with reductions in HAI rates by up to 30% in high-income countries when used to measure the disease burden and direct targeted infection prevention and control (IPC) measures [1, 5–7]. Furthermore, HAI surveillance systems can be instrumental in the timely detection of (multidrug-resistant) MDR pathogens in hospitals, especially in tertiary care, which are potential sites for the emergence of MDR pathogens owing to high antimicrobial pressure [8]. However, only 16% (23/147) of LMICs reported having a functional national HAI surveillance system in a survey conducted by the World Health Organization (WHO) in 2010 [2]. There is limited information available from LMICs on the impact of these HAI surveillance systems and the implementation challenges faced.
In 2017, an HAI surveillance network was started in India by the All India Institute of Medical Sciences (AIIMS), New Delhi, with technical coordination by the Indian Council of Medical Research (ICMR) and the National Centre for Disease Control (NCDC), India, and with support from the United States Centers for Disease Control and Prevention (US CDC) [9] to document BSI trends [10]. The BSI surveillance was implemented in intensive-care units (ICUs) in selected tertiary-care hospitals across the country.
After five years, we evaluated BSI surveillance in India’s first HAI surveillance network. We identified best practices, challenges, and opportunities in its implementation to help develop context-specific, cost-effective, and sustainable HAI surveillance systems in limited-resource settings.
Methods
Study design, population, and period
We conducted a descriptive mixed-methods study using a convergent parallel (concurrent) study design from January to May 2022. The evaluation focused on hospitals that reported BSI surveillance data to the HAI surveillance network. The evaluation focused on staff trained to conduct and report active BSI surveillance using the HAI surveillance protocol within each hospital.
Operational definitions
Healthcare-associated BSI was defined in the HAI BSI protocol (available at haisindia.com) for patients admitted for more than two calendar days in a selected hospital ICU participating in the HAI surveillance network. This standard operational definition used for BSI in the HAI network was modified for the Indian setting from the US CDC’s National Healthcare Safety Network (NHSN) case definition [11, 12].
We followed the updated CDC Morbidity and Mortality Weekly Report (MMWR) guidelines 2001 [13] to evaluate BSI surveillance on the following attributes: simplicity, stability, acceptability, representativeness, data quality, timeliness, sensitivity, positive predictive value, and usefulness. We developed operational definitions and monitoring indicators for each of these attributes and created interview and survey questions to score the indicators (Table 1).
Table 1.
HAI Network’s BSI Surveillance system attributes, indicators and data collection method, India, 2022
| Attribute and operational definition | Indicator | Data collection method |
|---|---|---|
| Simplicity: Simplicity of workflow and ease of implementation |
1. Ease of collecting data (staff required and time spent in surveillance activities each day on average) 2. Proportion of surveillance staff who report applying BSI case definition as easy or very easy 3. Proportion of surveillance staff who replied online reporting as easy or very easy 4. Average amount of time spent by surveyed staff in reporting one BSI case in the portal 4. Number of levels of reporting in the system |
Onsite observations Survey of Surveillance staff and Data entry operator (DEO) |
| Stability: System’s reliability, availability, and sustainability |
1. Proportional of surveillance staff trained in BSI surveillance protocols 2. Proportion of sites where denominator data is collected everyday including holidays 3. Proportion of sites with a full-time (24 h) microbiology laboratory 4. Proportion of sites with Laboratory Information System (LIS) 5. Proportion of sites where surveillance staff have access to all positive cultures 6. Proportion of sites who review every positive culture at month-end to capture missing cases 7. Availably of the system since its inception in 2017 8. Proportion of sites with funding support |
Survey of surveillance staff and DEO Review of network data Review of site-visit records |
| Acceptability: Willingness of individuals and institutions to participate in the BSI surveillance network | Number of hospitals participating in surveillance |
Review of HAI network database Review of quarterly reports |
| Representativeness: System’s ability to accurately describe BSIs over time | Proportion of febrile episodes (in patients admitted in surveillance ICUs) where a blood culture is collected | Review of patient case files during on-site visits |
| Data quality: Completeness and validity of the captured data |
1. Data validity: Proportion of CRF with case definition applied correctly 2. Data completeness: Proportion of CRF with 100% mandatory fields filled |
Record review of CRF during site visits Review of site-visit records |
| Timeliness: System’s ability to detect BSI cases and outbreaks in timely fashion |
1. Proportional of febrile episodes (in patients admitted in surveillance ICUs) where a blood culture is collected within 24 h of the febrile episode 2. Proportion of sites reporting BSI data within 10 days of the reporting month 3. Proportion of quarterly reports submitted by the network within one month of the reporting quarter 4. Proportion of ICU physicians who received monthly feedback on their ICU’s BSI rate 5. Proportion of BSI outbreaks detected and controlled while still ongoing |
Review of ICU patient case files Review of HAI network database Review of quarterly reports Survey of physicians Interview of site representatives |
| Sensitivity: System’s ability to detect BSI cases and outbreaks correctly |
1. Proportion of BSI cases reported among all BSI cases detected 2. Proportion of quarterly BSI trend reports submitted in last five years (2017–2021) 3. Number of early warning signals generated in the last one year (Jan-Dec 2021) and last one month (Dec 2021) |
Review of patient case files and laboratory records during site visits Review of network database |
| Positive Predictive Value: Probability that a detected BSI case is true case of BSI |
1. Proportion of true BSI cases among all BSI cases reported 2. Method for confirming true cases |
Review of CRF during site visits |
| Usefulness: Usefulness of the system in achieving its objectives of monitoring BSI trends and using system data to reduce HAI in participating hospitals |
1. Monitor network-based BSI trends over time 2. Number of BSI outbreaks detected using surveillance data 3. Proportion of sites using data to improve IPC practices at their ICUs |
Review of network database Survey of physicians |
Data collection
Data was collected from both the network level and tertiary-care hospitals, which is the reporting level used in document reviews. Data was collected by data extraction from the network database (www.haisindia.com), surveys, semi-structured interviews, focus group discussions, and on-site visits. We developed structured questionnaires for interviews at the network level and the reporting level. We created three separate online surveys targeted at three groups of reporting-level staff involved in BSI surveillance: surveillance staff who validated each BSI case from the intensive care unit (ICU), data entry operators (DEO) who reported each case to the network database from a paper-based case report form (CRF), and ICU physicians (Tables 1 and 2).
Table 2.
Qualitative questions and data collection method used, HAI Network’s BSI Surveillance system evaluation, India, 2022
| Open-ended questions | Probes | Data collection method |
|---|---|---|
| Acceptability: How willing are individuals and institutions to participate in the BSI surveillance network? |
1. How willing were institutions to join this network (is there more acceptance now than before)/ has the agency participation rate increased now than before? 2. Do you see horizontal expansion within hospitals which are already part of the network? 3. Do you see more trust in institutions for reporting their BSI data now than before |
Interview of Project Coordinator and site representatives FGD of technical advisory team |
| Usefulness: Is the system useful in monitoring BSI trends? |
1. How well is this system capturing BSIs? 2. Is the data generated by this system used? 3. Do you feel participation in BSI surveillance benefits the patient or your centre? How? 4. Do clinicians get feedback about their BSI rates 5. Is your feedback linked to IPC activities and QI initiatives- Could you give us a few examples |
Interview: Project Coordinator and site representatives FGD of technical team |
| Best practices: What are the best practices for implementing this surveillance in India? |
1. What are some of the successes of this network according to you? 2. Do you think it has led to QI initiatives/ any IPC practice change? 3. Has the data been used to detect HAI outbreaks? |
Interview of Project Coordinator and site representatives FGD of technical team |
| Challenges: What are some of the challenges encountered during implementation? |
1. What are some of the challenges you encountered during implementation? 2. What are the areas where you wish things could be better- at the network or facility level 3. Where do you think it's not succeeding? Why is this? |
Interview of Project Coordinator and site representatives and FGD |
Network level: We identified key stakeholders who had participated in developing and implementing the HAI surveillance network or were actively overseeing its operations and included them purposively. They included the project coordinator, statistician, and research fellow of the HAI surveillance program placed at AIIMS New Delhi and the technical advisers for the HAI surveillance program from the US CDC. We collected qualitative data from them using semi-structured interviews (network coordinators) and focus group discussions (FGD) (technical advisors) to evaluate the simplicity, stability, acceptability, usefulness, funding and organization of the surveillance system; and to document best practices, opportunities, and challenges during implementation.
We reviewed monthly reporting pattern of reporting units, and time of submission of quarterly reports to evaluate timeliness. We examined reports from routine site visits to document the presence of a 24-h laboratory and access of surveillance staff to all positive culture reports (blood, urine, sputum, pus, etc.), the percentage of laboratories having a laboratory information system (LIS) and monthly reporting pattern of units from 2017 to 2021 to evaluate system stability. We checked CRFs submitted by sites from October-December 2021 to evaluate data quality.
Reporting Unit level: We invited the principal investigators of all the sites (reporting units) enrolled in the network to participate in the evaluation and included the sites who volunteered to participate. We collected data using semi-structured interviews with key stakeholders of these sites to evaluate acceptability and usefulness and to document opportunities and challenges during implementation. Stakeholders who participated in interviews were asked to suggest at least one surveillance staff, DEO, and ICU physician from their site to receive the survey.
We included all suggested site staff and shared the surveys via email or WhatsApp Messenger. Each person could respond only once on the survey link provided. Questions included ease of applying the BSI case definition, the time required for data collection, ease of submitting data online to evaluate simplicity, and whether surveillance feedback was received monthly and used (only for physicians) to assess timeliness, acceptability, and usefulness. Given the BSI case definition required a positive blood culture, understanding blood culture ordering practices in eligible patients at surveillance sites was important to contextualize representativeness. Eligible patients were those admitted for more than two calendar days in a surveillance ICU, had a febrile episode and a potential BSI. During the on-site visits, we reviewed ICU patient files for the two months preceding the visit to look for febrile episodes. For each febrile episode, we searched for a blood culture entry in the corresponding laboratory records and if it was performed within 24 h. This information was used to calculate the percentage of febrile episodes that were cultured to investigate the blood culture ordering practices. We reviewed the list of positive blood cultures (sensitivity) and physical copies of CRF to assess the correct application of the case definition (positive predictive value) from October 2021 to December 2021.
Data analyses
After manually coding the transcribed qualitative data from interviews, we performed a thematic analysis. Quantitative data from monitoring indicators, surveys, and document reviews were calculated using Microsoft Excel, and are reported as counts and percentages. We combined qualitative and quantitative data to create a mixed methods interpretation, which we presented under the domains of best practices, opportunities, and challenges.
Results
Description of the system
The HAI surveillance network started reporting in May 2017 with 20 sites (66 ICUs) and increased to 39 sites (29 public and 10 private) with 131 surveillance units (ICUs) across 22 of the 36 states and union territories of India as of December 2021. Reporting ICUs included 26/131 (20%) medical, 19/131(19%) neonatal, 16/131 (12%) pediatric medical, 14/131 (11%) surgical, 10/131 (8%) COVID-19 ICUs, and others. At the reporting ICU, the BSI event data flow starts once a patient admitted to one of the surveillance units has a positive blood culture (Fig. 1). This patient’s case details are checked to see if they fit the BSI case definition. If yes, then a case report form (CRF) is generated by the surveillance staff, and it is uploaded into the HAIS web portal by the data entry operator after validation by the site principal investigator (PI). Using the BSI case numbers and the denominator data from the respective sites, facility and network level rates are generated and communicated to all stakeholders. To identify concerning BSI trends and outbreaks, the network database’s early warning signal generates an alert to users automatically when an ICU-specific BSI rate exceeds 20 per 1,000 patient days in that reporting unit/ ICU.
Fig. 1.
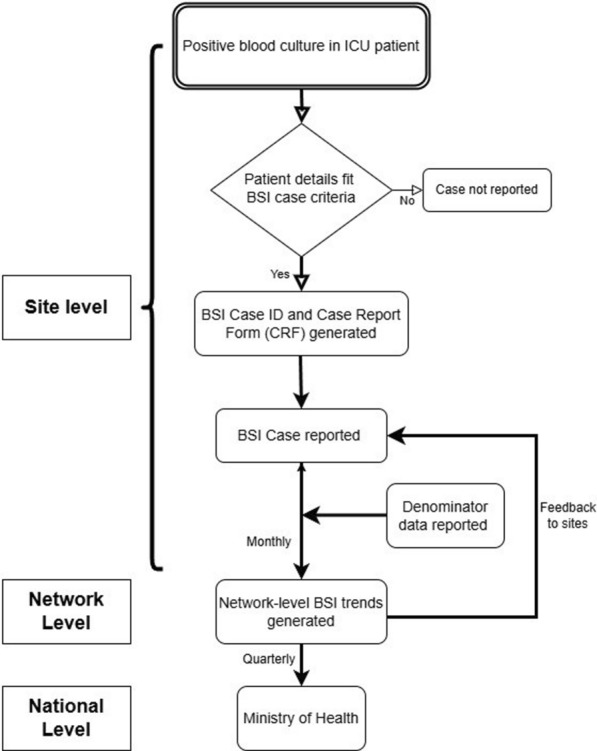
Data flow in HAI Network’s Bloodstream Infection Surveillance (BSI), India, 2022
The network received funding support from the US CDC under a cooperative agreement between 2017–2022 technically coordinated by ICMR. The CDC funding was provided to AIIMS, and AIIMS distributed the funds to the funded sites to hire surveillance staff depending on the units under surveillance. Sites that are not funded by the AIIMS-CDC projects and are part of the surveillance network as voluntary participants receive technical support and reporting platform access. They use internal funds to hire surveillance staff or use existing staff for surveillance activities. Material resources for data collection and any additional human resources required for surveillance expansion are financed through the site’s internal budget.
Evaluation of the system: Quantitative results
At the network level, we reviewed 21 site visit reports, 14 quarterly reports, data reported to the network database from 1st May 2017 to 31st December 2021, and 284 CRF. Ten hospitals agreed to participate in our evaluation. At the reporting level, surveys were distributed to 20 surveillance staff (two from each of the 10 sites), 20 DEOs (two from each of the 10 sites), and 20 physicians (two from each of the 10 sites). Among these, all the surveillance staff, all DEOs and ten physicians responded. Surveillance staff who responded to the surveys included infection control nurses (ICN), laboratory technicians, and research fellows (RF). We visited four (two funded and two non-funded) sites. We reviewed 135 ICU patients clinical case files, 72 positive blood culture reports (reported during the evaluation period) and 26 CRFs (reported during the evaluation period) from six surveillance ICUs in these four sites to evaluate system attributes (Table 3).
Table 3.
Evaluation results of HAI Network’s BSI Surveillance system attributes, India, 2022
| Attribute | Indicator | Evidence collected | Assessment | Overall evaluation |
|---|---|---|---|---|
| Simplicity | Ease of collecting data |
• Minimum 2 full-time surveillance staff • 80% staff spends > 2 h or more every day in collecting the 23 variables and the lab confirmation for case confirmation from paper-based reports |
Time-consuming | Simple |
| Ease of applying case definition | • 15/20 (75%) staff rate it as very easy or easy | Easy | ||
| Ease of online reporting |
• 18/20 (90%) rate it as very easy or easy • 10–15 min to submit one CRF, described as “user-friendly” |
Easy | ||
| Levels of reporting | • Two a) Local (hospital administration), and b) National (AIIMS, New Delhi) | Easy | ||
| Stability | Reliability |
• All 40 staff surveyed are trained in protocol • All 21 sites reviewed collected denominator data on all days including weekends/holidays • All 21 sites reviewed have access to a 24 by 7 working laboratory • 16/21 sites reviewed have access to all positive cultures, required for classifying BSI type • 5/21 sites reviewed reported not having LIS/ HMIS, reported using manual registers • 15/21 (71%) sites review every positive culture at month-end to capture missing cases |
Reliable | Stable if funding is available |
| Availability |
• Available from 2017 including during second wave of COVID-19 in India • Decreased reporting to 84/131 (64%) in ICUs and surveillance stopped in 22/39 (56%) sites seen briefly during April 2020 |
Available | ||
| Sustainability |
• 26/39 (67%) of the sites are funded by US CDC • Reporting decreased to 63/131 (48%) in the quarter 4, 2021 when funding was interrupted |
Funding stability a concern | ||
| Acceptability | Willingness of stakeholders to participate |
• Started with 20 ICUs in 2017 and has increased to 131 ICUs in 2019 • Hospital administration of all 10 hospitals interviewed accepts this system is required to control multi-drug resistant pathogens |
High | Acceptable |
| Proportion of physicians accepting feedback from the surveillance system | • 90% physicians surveyed starting a QI initiative in their ICU based on the feedback received from surveillance | |||
| Representativeness | Population representative of the participating hospital | • 32/58 (55%) of the febrile episodes reviewed had their blood cultured | Not representative | Not representative |
| Data Quality | Data validity | • 280/284 (98%) of CRFs reviewed have correctly applied the case definition | Valid | Data quality is good |
| Data completeness | • 259/284 (91%) of CRFs have data filled in each data va without any missing details | Complete | ||
| Timeliness | Blood collection | • 27/61 (44%) of febrile episodes reviewed were cultured (blood collection) < 24 h of fever | Not timely | Blood culture collection and feedback to ICU physicians is not timely |
| Reporting to network | • 36/39 (92%) sites reported data within 10 days of the reporting month | Timely | ||
| Dissemination to key stakeholders |
• 6/10 (60%) ICU physicians surveyed reported getting consistent monthly feedback • 13/14 (93%) quarterly reports shared by AIIMS with all key stakeholders within one month of the reporting quarter |
Not timely Timely |
||
| Detection of BSI outbreaks |
• Sites not comfortable in sharing information regarding outbreaks detected in their hospitals, hence this information could not be captured • One outbreak of Burkholderia cepacia detected in the network using surveillance data and controlled (retrieved from published data) |
––– | ||
| Sensitivity | True cases detected | • 26/26 (100%) cases reviewed during site visit were correctly identified | Sensitive | Sensitive |
| Monitoring trends | • System can identify BSI trends from May 2017 to December 2021 | Sensitive | ||
| Early warning signals generated |
• System has generated 684 ICU specific BSI rate alerts from May 2017 to December 2021 • Alerts are generated when ICU specific BSI rates are > 20 for that month |
Sensitive | ||
| Positive Predictive Value | Proportion of true cases with confirmed BSI | • 26/26 (100%) cases reported as BSI for the months of November 2021 to March 2022 were reviewed and found to be true cases | Good | Good PPV |
| Usefulness |
Monitoring trends Detecting outbreaks Improving IPC |
• Quarterly trend analysis done for every quarter from Jan 2018 to Dec 2018 • Three outbreaks detected including one outbreak of Burkholderia cepacia using surveillance data and controlled (retrieved from published data) • 12/39 (31%) sites have completed/ongoing QI projects to improve BSI rates • 7/10 (70%) physicians surveyed reported having increased adherence to recommended Central Line insertion and maintenance practices following feedback from the system |
Useful | Useful |
Simplicity
Among the surveyed staff, 83% rated the modified NHSN case definitions as easy to apply and found the online reporting platform user-friendly.
Stability
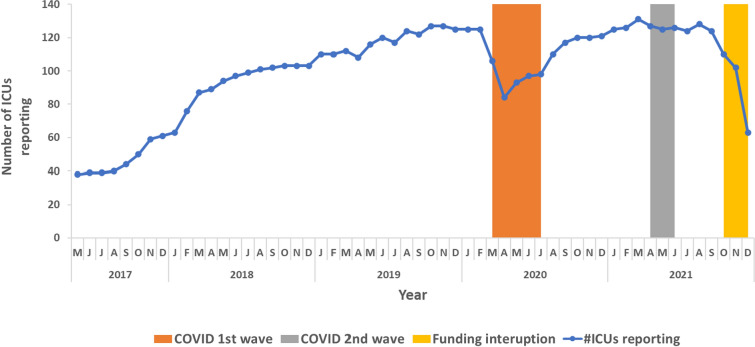
The network functioned throughout these five years (May 2017 to December 2021) with variable reporting. The number of reporting ICUs dropped from 125/131 (95%) in February 2020 to 84/131 (64%) in April 2020 coinciding with the start of the COVID-19 pandemic. Reporting gradually increased to 100% in March 2021 before decreasing again in April 2021 during the second wave of COVID-19. The reporting increased till August 2021(128/131, 98%) before decreasing again to 63/ 131 (48%) in December 2021 when external funding for this project was interrupted (Fig. 2). Despite reduced project funding, 25% of the hospitals continued to provide data to the system with their own dedicated infection prevention and control (IPC) staff.
Fig. 2.
Reporting pattern of HAI Network ICUs reporting BSIs, May 2017 to December 2021
Among the 21 site visit reports reviewed, 76% of sites reported that surveillance staff had access to all positive cultures (cultures taken from other body sites). All sites which reported challenges in surveillance staff accessing all positive culture reports were public hospitals. These hospitals used manual registers for recording and reporting laboratory results and did not have a Laboratory Information System (LIS) or Hospital Management Information System (HMIS).
Representativeness
Among the 135 ICU patient case files reviewed, blood culture was collected within 24 h in 27/61 (44%) febrile episodes identified in these patient files. Two of these hospitals cultured blood based on patient symptoms (with 44% of patients being cultured within 24 h of a febrile episode in both hospitals), and the other two hospitals cultured patients twice a week irrespective of patient symptoms (26% and 62% of patients having a febrile episode being cultured in each hospital respectively). Eight physicians reported sending paired blood cultures from each febrile patient, while five physicians reported culturing up to 80% of febrile patients in their ICU.
Data quality
Among the 284 CRFs reviewed, 91% had complete data, and 98% had correctly applied the BSI case definition.
Timeliness
From 2017, the network submitted 13/14 (93%) quarterly HAI surveillance reports to the Ministry of Health and Family Welfare within one month of the reporting quarter. Of the ten ICU physicians surveyed, 60% reported receiving consistent monthly feedback on BSI rates from their ICUs.
Sensitivity
Among 72 positive blood cultures reports reviewed, 26 positive blood cultures and their corresponding patient case files met the BSI case definition criteria and all 26 were correctly reported as BSIs by the sites to the network database. The 14 quarterly reports reported on pooled network trends mapped for each quarter. The system generated 684 ICU-specific BSI rate alerts from May 2017 to December 2021.
Positive Predictive Value
All 26 CRFs reported from site-level surveillance staff to the network database during site visits met the BSI case definition, with a positive predictive value (PPV) of 100%.
Usefulness
Using data from this network, 12 of the 39 (31%) participating sites had implemented targeted IPC measures to reduce their BSI rates. Three major healthcare-associated BSI outbreaks, including an outbreak caused by Burkholderia cepacia, were detected and controlled [14]. Among surveyed physicians, 70% stated that surveillance data feedback positively impacted care in the ICU by improving documentation and increasing adherence to recommended central-line practices.
Evaluation of the system: Qualitative results
At the network level, we conducted three interviews (the program coordinator, one statistician, and one research fellow of the HAI surveillance program) and one FGD (with three technical advisers from the US CDC). At the site level, we conducted ten interviews (one per site, with one to two staff participating in each). The interviewees included six microbiologists, four ICNs, six RFs, and two DEOs. The qualitative analysis from the interviews yielded ten themes related to implementing the surveillance (Tables 4 and 5).
Table 4.
Summary of Themes from Qualitative Analysis, BSI Surveillance Evaluation, India, 2022
| 1. Developed a context-specific resource-appropriate case definition: Simple case definitions and easy-to-follow SOPs permitted implementation in low-resource settings |
| 2. Established surveillance through a network-based approach: Starting with a few committed, well-resourced sites before gradually expanding to more sites was important |
| 3. Regular IPC training and use of QI to improve IPC: Regular IPC and QI training helped sites use data for targeted IPC interventions to improve patient care |
| 4. Awareness and acceptance of BSI surveillance among participating sites: Site representatives felt joining this network helped them prioritize scarce resources to tackle the threat of HAIs |
| 5. Limited human resources: Limited staff allotted to surveillance impacted data collection and reporting in sites |
| 6. Lack of digitalization of medical and laboratory records: Without hospital and laboratory management systems, it was difficult to track patients outside of ICUs and follow multiple positive cultures for a single patient |
| 7. Variable blood culturing practices: Surveillance protocols were not always followed; instead, some decisions to collect blood cultures were based on the treating physician's judgment, the availability of culture bottles, and the patient's ability to pay |
| 8. Inconsistent information sharing and data use: Analyzed data was not always shared with physicians; even when it was shared, they did not always accept the results |
| 9. Funding and sustainability: Funding commitment was important to maintain and expand the surveillance network and to retain staff |
| 10. Impact of the COVID-19 Pandemic: Surveillance stopped in many sites. Staff were reassigned for COVID-19 duties in other sites, which reduced the number of reporting ICUs |
Table 5.
Themes, codes and representative quotes obtained from interviews, HAI Network’s BSI Surveillance evaluation, India, 2022
| Themes | Codes | Quotes |
|---|---|---|
| Developed a resource-appropriate case definition |
Simple easy to implement case-definition Applicable to Indian hospitals Sensitive case definition |
“The team developed a simple case definition, clear SOP, which helped us train sites well” #ProgramCoordinator “I’m pretty confident that in a given unit, more than 80–90% of the BSIs were being captured, pre-COVID-19.” #TechnicalTeam01 “Modified case definition is sensitive in capturing true CLABSIs: “when you do these focussed QI projects, their CLABSI rates go down significantly, which tells us there’s probably not a huge definitional gap where we are finding BSI and classifying them as CLABSI when they are really secondary BSI” #TechnicalTeam02 |
| Established a representative network-based surveillance to detect trends and investigate outbreaks |
Implementing a surveillance program across different states Representative of different patient populations within ICUs Representation of public and private hospitals Geographically representative Outbreaks detected |
“(we have been successful in helping) how to implement a surveillance and prevention program that can be implemented consistently across a network of 39 + hospitals in a relatively well-resourced setting, it’s a huge success.” #TechnicalTeam01 “Good representation of patient population- medical, surgical, paediatric, neonatal and oncology” #TechnicalTeam01 “When it comes to tertiary care hospitals in the country, we are fairly representative as we have geographical diversity, also a mix of private and public hospitals” #TechnicalTeam02 “We have used data from this surveillance to investigate HAI outbreaks, and control them in time” #TechnicalTeam03 |
| Ensured regular ongoing IPC trainings with Quality Improvement (QI) projects |
Shifted to online training Quick dissemination of COVID-19 IPC measures in network Every infection is accounted for Targeted recommendation to avoid each infection |
“This IPC training and surveillance helped us in correct handling of COVID-19 patients during the pandemic” #ICN02” “With QI initiatives, I can say that, first we were just recording and showing the rates in the HICC (hospital infection control committee) meeting, now we are asking questions for every BSI reported and suggesting ways to avoid that infection.” #ResearchFellow06 |
| Limited human resources |
Limited staff allotted to surveillance Staff attrition |
“Limited staff allotted to surveillance, so we are not expanding surveillance to all ICUs within the hospital” #Microbiologist03 “Difficult to retain trained staff once funding ends, impacts our data collection” #Microbiologist05 “Staff turnover impacts timeliness of data upload” #Microbiologist02 |
| Lack of digitalization of medical and laboratory records |
Patient tracking outside of ICU is difficult Tracking different culture reports is difficult End-of month validation difficult Deciphering handwritten notes is challenging |
“Tracking samples from other body sites is difficult as no common book in ICU which has a list of all cultures sent for that day….it takes a lot of man-hours” #ICN03 “Accessing and deciphering handwritten notes from manual registers is time-consuming and inefficient” #ICN01 “Monthly validation of all blood cultures is not always done, difficult in centres with no LIS” # DEO (02) |
| Variable blood culturing practices |
Lack of availability of culture bottles Patient unable to pay for blood cultures Clinician’s judgement |
“Blood culture is paid, in our hospital social service organizations help poor patients but these are not present in all hospitals” #Microbiologist01 (private hospital) “In our hospital, blood culture is free, but sometimes patients must buy supplies’# Microbiologist03 (public hospital) “This is a tertiary care hospital, patients have already taken a lot of antibiotics and come in very sick, so we directly start on antibiotics empirically, only when patient does not respond, we send cultures.” #Microbiologist03 |
| Inconsistent information sharing and data use |
Staff not analysing data Analyzed data not shared with physicians Physicians may not accept recommendations |
“Even though they have data, they (sites) are not analysing this data for antibiograms, nor sharing it (with their physicians)” # TechnicalTeam02 “Physicians many times do not accept the results of the data, or the IPC measures suggested possibly due to a difference in surveillance and clinical definitions” #TechnicalTeam03 “there’s a kind of hierarchy and defensiveness that can exist between the microbiology department and the clinical side, that’s been a bottleneck to the data actually being used.” # TechnicalTeam02 |
| Funding and sustainability |
Funding affects stability Further expansion of network depends on funding Institutionalization of IPC staff helps stability |
“Difficult to retain trained staff once funding ends, impacts our data collection” #Microbiologist06 “If we have allocated budget either internal or external, we plan to expand the network by creating regional trainers and including tier 2 (secondary care) hospitals in the network.” #ProgramCoordinator “Hospitals with dedicated IPC staff continued surveillance despite drop in funding” #TechnicalTeam03 |
| Impact of the COVID pandemic |
Disrupted regular trainings and expansion Staff attrition Reduced reporting units Limited access of staff to COVID ICUs |
“During the COVID-19 pandemic nobody was willing to support any intervention to improve CLABSI (infection rates) as there was staff shortage, now is not the right time, they said” #ReseachFellow05 “Many ICU were designated COVID ICU and surveillance stopped” # Program Coordinator “Surveillance staff were not allowed in COVID ICU” # ReseachFellow03 |
| Awareness and acceptance of BSI surveillance among participating sites |
Surveillance is useful Sites eager to join network Increase in blood cultures received after joining surveillance |
“For a public hospital, where we have so much patient load, because of this surveillance, we can focus on areas to improve,” #Microbiologist02 “They (ICU physicians) have come to realise that without IPC, (BSI) rates cannot be reduced” #Microbiologist03 “Number of blood culture samples have increased significantly in the last 2 years after our trainings” #ICN02 “Sites are actively soliciting opportunity to participate in surveillance” #TechnicalTeam02 |
Mixed-methods integration: We consolidated the quantitative attributes, their indicators, and the qualitative themes under best practices, challenges, and opportunities (Table 6). Best practices encompassed developing case definitions suitable for the available resources in a diverse health system, establishing network-based surveillance, and IPC training of surveillance staff. Challenges identified included limited human resources, lack of digitalization, variable blood culturing practices, inconsistent information sharing, funding, and the COVID-19 pandemic. Opportunities highlighted the awareness and acceptance of BSI surveillance among participating sites.
Table 6.
Integration of qualitative themes and quantitative indicators, HAI Network’s BSI surveillance evaluation, India, 2022
| Domain | Qualitative themes | Corresponding quantitative indicator result |
|---|---|---|
| Best practices | Developed a resource-appropriate case definition | Simplicity: easy to apply case definition |
| Established a network-based surveillance to detect BSI trends and outbreaks |
Stability: All 21 (100%) sites checked had access to 24 by 7 lab facility Sensitivity and PPV: Checked events had 100% PPV and 100% sensitivity Sensitivity: Sensitive in detecting BSI trends from May 2017 to Dec 2021 One outbreak of Burkholderia cepacia detected in the network using surveillance data |
|
| Ensured regular ongoing IPC trainings with Quality Improvement (QI) projects |
Acceptability: 90% physicians surveyed starting a QI initiative in their ICU based on the feedback received from surveillance Usefulness: 70% reported the feedback and trainings affecting care in the ICU by improving documentation of, and increasing adherence to, recommended central-line practices, 31% sites implemented one or more QI measures to decrease BSI rates |
|
| Challenges | Limited human resources | |
| Lack of digitalization of medical and laboratory records |
Stability: 76% sites had access to all positive cultures, required for classifying BSI type, rest 24% did not have LIS, recorded lab results in manual registers Stability: 71% sites capture missing cases at end of month Simplicity: 80% of surveyed surveillance staff reported spending two hours or more per day collecting data from paper-based reports |
|
| Variable blood culturing practices |
Representativeness: 55% had their blood cultured with 44% cultured within 24 h of a febrile episode Survey: 50% physicians reported culturing 80% of the febrile patients Timeliness: 44% of the febrile episodes reviewed had blood cultured within 24 h |
|
| Inconsistent information sharing and data use | Timeliness: 6/10 (60%) ICU physicians reported getting consistent monthly feedback | |
| Funding and sustainability | Stability: reporting ICUs decreased to 63/131 (48%) and reporting sites to 30/39 (77%) during quarter 4, 2021 when funding was interrupted | |
| Impact of the COVID-19 pandemic | Stability: Surveillance stopped in 22/39 (56%) sites during March–April 2020 as staff were absorbed in COVID-19 duties | |
| Opportunities | Awareness and acceptance of BSI surveillance among participating sites | Acceptability: Acceptable among stakeholders at national and site level |
In all domains, the evidence from surveys, interviews, and document reviews aligned with each other except in blood culturing practices. While the surveyed physicians reported culturing 80% of febrile patients, document reviews indicated a figure of 44%.
Discussion
Our evaluation demonstrates that implementing a standardized BSI surveillance among a diverse resourced network across India has been successful, with lessons learned for other countries interested in initiating similar HAI surveillance networks. The BSI surveillance is simple, acceptable, and sensitive in reporting trends. but there are challenges to sustainability due to limited human resources, lack of digitalization of medical records, variable blood culture practices, limited information sharing among key stakeholders, and funding.
The BSI surveillance conducted by the HAI surveillance network has achieved many successes since its inception. The team has established network-level surveillance of BSI for India by assembling hospitals with varying capacities and from different Indian states on a common platform. They have adapted CDC’s NHSN case definitions for resource-limited settings and trained network sites using a common modified case definition that can track trends at the facility, subnational, and national levels. The surveillance established is an active, prospective surveillance with higher specificity and sensitivity than passive or retrospective surveillance. Beyond detecting BSI rates, this study shows that sites are willing to use surveillance data to improve IPC processes and reduce BSI rates if provided human resources and training. This is a best practice to adopt and is consistent with other studies [15, 16]. While not a primary purpose of the network, interviewed staff felt they benefited from the efficient and timely dissemination of IPC information and guidelines during the COVID-19 pandemic. The use of such networks can be leveraged to quickly disseminate and amplify information in epidemics and pandemics.
Our study highlights the importance of stable, dedicated funding to the stability of a surveillance network, including the impact on staff retention, institutional knowledge, and data reporting. Unreliable funding also limited expansion of surveillance to other intensive care units (ICUs) within these hospitals. We found that external funding partially mitigated the shortage of human resources in funded public hospitals in the short-term. It should be noted that relying solely on external funding may serve as an initial step to initiate work and pilot a surveillance program. Sustainable long-term solutions to address resource limitations should be sought, as demonstrated by funding challenges faced by antimicrobial resistance surveillance programs in LMICs [17, 18] and aligns with WHO guidance to allot dedicated funding to build IPC programs with capacity to conduct HAI surveillance [19].
Our study's findings regarding the impact of a shortage of trained staff on data collection, data use, and surveillance expansion are consistent with previous research conducted in both low- and high-resource settings. These studies have consistently identified inadequate staffing as a common barrier to performing essential IPC activities [20–23]. Our study also showed that the lack of sufficient supplies specific to blood culture and the lack of digital medical records, issues unique to public hospitals, compromise data quality and increase the time required for surveillance activities. Specifically, the challenges highlighted in our study at the facility level align with challenges in IPC core component 6 (monitoring/audit of IPC practices and feedback), and 7 (workload, staffing and bed occupancy) reported in the WHO’s Global IPC report [24]. Considering these findings, and the disruption seen with turnover of staff, we believe that appointing full-time infection control professionals in both public and private hospitals, along with allocating adequate material resources, implementing a robust supply chain management system and digitalization of medical and laboratory records in public hospitals, are fundamental to establishing a successful HAI surveillance program as reported in previous research [25, 26].
Our study highlights the presence of inconsistent culturing practices during febrile episodes and a lack of agreement between actual and reported febrile patients among physicians, which is not exclusive to low-resource settings. Similar deficits in blood culture ordering and adherence to guidelines have also been observed among inpatient care physicians in high-resource settings [27–29]. The underlying reasons for these variations in culturing practices remain unclear but should be studied to provide ways to enhance the detection of BSIs and improve the representativeness of the surveillance system. Contrary to physician opinions in our study suggesting that conducting cultures is too costly, studies conducted in low-resource settings demonstrates investing in laboratory capacity and culturing practices can result in cost savings despite greater upfront investments and lead to improved health outcomes by reducing inappropriate antibiotic use [30].
Several limitations were identified in our study. The participating sites joined the study voluntarily, which might have introduced a potential selection bias as these sites may have had a more favorable opinion towards the network. The onsite visits were conducted in four network hospitals, and blood culture ordering practices documented in these hospitals might not represent the entire network.
Conclusion
An active, prospective BSI surveillance, utilizing a common definition, is feasible in a low-resource settings. Prioritizing allocation of dedicated personnel for surveillance, training them to use data for action, digitalizing medical records, improving blood culturing practices, establishing systematic feedback mechanisms to share data with treating physicians, and long-term funding commitment from policymakers are crucial to make HAI surveillance networks sustainable.
Acknowledgements
We acknowledge all project staff and PI of the “Capacity Building and Strengthening of Hospital Infection Control to Detect and Prevent Antimicrobial Resistance in India” supported by the U.S. Centers for Disease Control and Prevention, Global Health Security Agenda cooperative agreement, including Mr. Sharad Srivastava, Statistician, Dr. Rasna Parveen, Scientist C, Mr. Naresh, Field Investigator, Mr. Pawan Kashik and Infection Control Nurses at AIIMS, New Delhi. We acknowledge the support of Dr Camilla Rodriguez, Head of Department, Microbiology, PD Hinduja Hospital, Mumbai, Ms. Julliah Chelliah, Senior Research Fellow, Dr. Veena Kumari, Head of Department, Microbiology, NIMHANS, Bangalore, and their Infection Control Nurses and Dr. Rajni Gaind, Head of Department, Microbiology, Safdarjung Hospital, New Delhi, Dr. Rushika Saksena, and team. We acknowledge the support of Mathew Hudson, EIS Officer, DHQP, CDC Atlanta, USA, Ms. Dorothy Southern, Scientific Writing Advisor, SAFETYNET, and mentors at EIS Cell, NCDC.
Author contributions
SKV, VAS, DV, PMal, and TD conceived the study design. SKV acquired on-field data. KW and PMath approved the acquisition of data. VAS and PMath supervised the study. SKV, VAS, DV, PMal, AV and TD analyzed the data and interpreted the results. SKV wrote the original manuscript text and prepared the figures and tables. SKV, VAS, DV, PMal, AV, TD, KW and PMath revised and edited the manuscript. All authors reviewed the manuscript, approved the submitted version, and agreed to be personally accountable for the manuscript.
Funding
While there was support from the US-CDC for the surveillance system operated by AIIMS, the evaluation was conducted by an external source affiliated with the National Centre for Disease Control, Government of India and no funding support was received from the CDC or any other source for either the evaluation or writing the manuscript.
Availability of data and materials
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
We conducted this study as part of the monitoring and evaluation of a national public health surveillance project titled “Capacity Building and Strengthening of Hospital Infection Control to Detect and Prevent Antimicrobial Resistance in India”. The project received ethical approval (IEC/NP-386/10.09.2015) from the Institutional Ethics Committee, All India Institute of Medical Sciences (AIIMS), New Delhi, and approval from the Health Ministry Screening Committee (HMSC), India. We obtained permission from the HAI surveillance network’s program coordinators before reaching out to network sites, and study participants provided consent via email for interviews, FGD, and surveys.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. CDC or the U.S. Department of Health and Human Services.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Report on the Burden of Endemic Health Care-Associated Infection Worldwide. World Health Organisation; 2011. Available from: https://www.who.int/publications/i/item/report-on-the-burden-of-endemic-health-care-associated-infection-worldwide. Accessed 27 August 2022
- 2.Global report on the epidemiology and burden of sepsis: current evidence, identifying gaps and future directions. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://www.who.int/publications/i/item/9789240010789. Accessed 3 September 2023
- 3.Rosenthal, V., Bat-Erdene, I et al. (2020). Six-year multicentre study on short-term peripheral venous catheters-related bloodstream infection rates in 727 intensive care units of 268 hospitals in 141 cities of 42 countries of Africa, the Americas, Eastern Mediterranean, Europe, South East Asia, and Western Pacific Regions: International Nosocomial Infection Control Consortium (INICC) findings. Infection Control & Hospital Epidemiology.10.1017/ice.2020.20 [DOI] [PubMed]
- 4.Reimer LG, Wilson ML, Weinstein MP. Update on detection of bacteremia and fungemia. Clin Microbiol Rev. 1997. 10.1128/CMR.10.3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haley RW, et al. The SENIC project study on the efficacy of nosocomial infection control (SENIC Project). summary of study design. Am J Epidem. 1980. 10.1093/oxfordjournals.aje.a112928. [DOI] [PubMed] [Google Scholar]
- 6.Haley RW, Culver DH, White JW, et al. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985. 10.1093/oxfordjournals.aje.a113990. [DOI] [PubMed] [Google Scholar]
- 7.Minimum requirements for infection prevention and control. Geneva: World Health Organization;2019. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://www.who.int/publications/i/item/9789241516945. Accessed 3 September 2023
- 8.Agodi A, Maugeri A, Favara G. SPIN-UTI network, Trends of HAI and AMR in Italian intensive care units: findings from the SPIN-UTI network. Eur J Pub Health. 2023. 10.1093/eurpub/ckad160.554. [Google Scholar]
- 9.Mathur P, Malpiedi P. Indian healthcare associated infection surveillance collaborators. health-care-associated bloodstream and urinary tract infections in a network of hospitals in India: a multicentre, hospital-based, prospective surveillance study. Lancet Global Health. 2022. 10.1016/S2214-109X(22)00274-1. [DOI] [PubMed] [Google Scholar]
- 10.Healthcare-associated Infection Surveillance India, HAIS-India. http://www.haisindia.com. Accessed 2 January 2022
- 11.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008. 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Surveillance For Healthcare-associated Infections (HAI) in Intensive Care Units; Standard Operating Procedures. Prepared by AIIMS, New Delhi; CDC, India and ICMR, New Delhi. Published November 2018:pg-23. https://www.haisindia.com/upload/fileuploads/1543398274_SOP%20updated%20November%202018.pdf. Accessed 2 January 2022
- 13.Center for Diseases Control and Prevention (CDC). MMWR updated guidelines for evaluating public health surveillance systems: recommendations from the guidelines working group, (July 2001). https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5013a1.htm. Accessed 20 October 2021 [PubMed]
- 14.Fomda B, Velayudhan A, Siromany VA, Bashir G, Nazir S, Ali A, et al. An outbreak of Burkholderia cepacia bloodstream infections in a tertiary-care facility in northern India detected by a healthcare-associated infection surveillance network. Infect Control Hosp Epidemiol. 2022. 10.1017/ice.2022.111. [DOI] [PubMed] [Google Scholar]
- 15.Wagenaar BH, Hirschhorn LR, Henley C, Gremu A, Sindano N, Chilengi R. Data-driven quality improvement in low-and middle-income country health systems: lessons from seven years of implementation experience across Mozambique, Rwanda, and Zambia. BMC Health Services Res. 2017. 10.1186/s12913-017-2661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odhus CO, Kapanga RR, Oele E. Barriers to and enablers of quality improvement in primary health care in low- and middle-income countries: a systematic review. PLOS Glob Public Health. 2024. 10.1371/journal.pgph.0002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandra S, Alvarez-Uria G, Turner P, Joshi J, Direk Limmathurotsakul H, van Doorn R. Antimicrobial resistance surveillance in low- and middle-income countries: progress and challenges in eight South Asian and Southeast Asian countries. Clin Microbiol Rev. 2020. 10.1128/CMR.00048-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iskandar K, Molinier L, Hallit S, et al. Surveillance of antimicrobial resistance in low- and middle-income countries: a scattered picture. Antimicrob Resist Infect Control. 2021. 10.1186/s13756-021-00931-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global strategy on infection prevention and control. Geneva: World Health Organization; 2023. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://cdn.who.int/media/docs/default-source/gsipc/who_ipc_global-strategy-for-ipc.pdf?sfvrsn=ebdd8376_4. Accessed 3 December 2023
- 20.Supriadi IR, Haanappel CP, Saptawati L, Widodo NH, Sitohang G, Usman Y, et al. Infection prevention and control in Indonesian hospitals: identification of strengths, gaps, and challenges. Antimicrob Resist Infect Control. 2023. 10.1186/s13756-023-01211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aghdassi SJS, Hansen S, Bischoff P, Behnke M, Gastmeier P. A national survey on the implementation of key infection prevention and control structures in German hospitals: results from 736 hospitals conducting the WHO Infection Prevention and Control Assessment Framework (IPCAF). Antimicrob Resist Infect Control. 2019. 10.1186/s13756-019-0532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azak E, Sertcelik A, Ersoz G, Celebi G, Eser F, Batirel A, et al. Turkish Hospital Infection Research Group. Evaluation of the implementation of WHO infection prevention and control core components in Turkish health care facilities: results from a WHO infection prevention and control assessment framework (IPCAF)-based survey. Antimicrob Resist Infect Control. 2023. 10.1186/s13756-023-01208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harun MGD, Anwar MMU, Sumon SA, Hassan MZ, Haque T, Mah-E-Muneer S, et al. Infection prevention and control in tertiary care hospitals of Bangladesh: results from WHO infection prevention and control assessment framework (IPCAF). Antimicrob Resist Infect Control. 2022. 10.1186/s13756-022-01161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Global report on infection prevention and control. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://iris.who.int/bitstream/handle/10665/354489/9789240051164-eng.pdf?sequence=1. Accessed 3 September 2023
- 25.van Mourik MSM, Perencevich EN, Gastmeier P, Bonten MJ. Designing surveillance of healthcare-associated infections in the era of automation and reporting mandates. Clin Infect Diseases. 2018;66(6):970–6. 10.1093/cid/cix835. [DOI] [PubMed] [Google Scholar]
- 26.Atreja A, Gordon SM, Pollock DA, Olmsted RN, Brennan PJ. Healthcare infection control practices advisory committee opportunities and challenges in utilizing electronic health records for infection surveillance, prevention, and control. Am J Infect Control. 2008. 10.1016/j.ajic.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raupach-Rosin H, Duddeck A, Gehrlich M, et al. Deficits in knowledge, attitude, and practice towards blood culture sampling: results of a nationwide mixed-methods study among inpatient care physicians in Germany. Infection. 2017. 10.1007/s15010-017-0990-7. [DOI] [PubMed] [Google Scholar]
- 28.Dräger S, Giehl C, Søgaard KK, Egli A, de Roche M, Huber LC, Osthoff M. Do we need blood culture stewardship programs? A quality control study and survey to assess the appropriateness of blood culture collection and the knowledge and attitudes among physicians in Swiss hospitals. Eur J Intern Med. 2022. 10.1016/j.ejim.2022.04.028. [DOI] [PubMed] [Google Scholar]
- 29.Yalçinkaya R, Öz FN, Erdoğan G, Kaman A, Aydın Teke T, Yaşar Durmuş S, et al. Turkish pediatric residents’ knowledge, perceptions, and practices of blood culture sampling. Arch Pediatr. 2021. 10.1016/j.arcped.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Gebretekle GB, Mariam DH, Mac S, et al. Cost–utility analysis of antimicrobial stewardship programme at a tertiary teaching hospital in Ethiopia. BMJ Open. 2021. 10.1136/bmjopen-2020-047515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.