Abstract
Background
Continuous glucose monitoring (CGM) is increasingly popular for managing Type 2 Diabetes Mellitus (T2DM). Many systematic reviews have reported on CGM's effectiveness, but with heterogeneous methodologies and objectives. We aim to conduct an umbrella review (UR) to consolidate a most contemporaneous and comprehensive evidence base comparing CGM with self-monitoring of blood glucose or usual care (SMBG/UC).
Methods
Ovid MEDLINE, Ovid Embase, Cochrane Database of Systematic Reviews, CINAHL, Epistemonikos, SCOPUS, Web of Science and PubMed were searched from their dates of inception to 28th June 2024. Systematic reviews (SR) with or without meta-analyses comparing the use of CGM with SMBG or usual care (UC) for T2DM management in patients treated with or without insulin were included. Narrative synthesis of HbA1c, glycemic variability metrics and other physical measurements were done. Corrected covered area (CCA) was calculated to assess suitability of meta-meta-analysis.
Results
31 SRs were included in this UR. There was high overlap within meta-analyses of HbA1c, time-in-range (TIR), time-above-range (TAR) and time-below-range (TBR). A primary study-level meta-analysis demonstrated that compared to SMBG/UC, CGM was associated with significantly greater HbA1c decrease (n = 11,494, MD = -0.40% [95% CI: -0.54 to -0.25]), TIR increase (n = 1452, MD = 6.00% [95%CI: 3.13 to 8.88]) and TAR decrease (n = 1113, MD = -4.33% [95%CI: -8.37 to -0.28]).These findings were invariant with CGM modality, study funding, pre-existing insulin treatment and risk-of-bias. Meta-analysis of patient reported outcome measures (PROMs) demonstrated insignificant differences in PROMs with CGM use compared to SMBG/UC.
Conclusion
CGM could lead to better clinical outcomes than SMBG/UC and was of moderate evidence certainty (GRADE), while its effect on PROMs remains inconclusive. We recommend the introduction of CGM into standard care alongside SMBG for T2DM and further research exploring patient experience and acceptability of CGM use.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13690-024-01459-2.
Keywords: Type 2 Diabetes, Continuous Glucose Monitoring, Chronic Disease Management, Umbrella Review, Systematic Review, Meta-Analysis
| Text box 1. Contributions to the literature |
|---|
| • The effectiveness of the use of continuous glucose monitoring (CGM) devices for management of type 2 diabetes mellitus (T2DM) has been evaluated in a number of ways across multiple studies, but the totality of the evidence has not been consolidated into a single review. |
| • CGM is better than self-monitoring of blood glucose or usual care in reducing glycated hemoglobin levels and increasing time-in-range but no significant differences were found in patient-reported outcome measure changes. |
| • CGM is a promising alternative for T2DM management, although more studies on patient usability and experience is needed. |
Introduction
Type 2 Diabetes Mellitus (T2DM) contributes significantly to global disease burden, with a projected prevalence of 7079 per 100,000 by 2030 [1]. Amongst T2DM patients, poor glycemic control has been consistently associated with vascular complications [2, 3] and overall increased mortality [4].
Technological innovations have enabled close monitoring of the patient’s blood glucose profile through the continuous glucose monitoring (CGM) device [5], which is a subcutaneous device left on the patient that samples the interstitial fluid to estimate blood glucose levels. Unlike self-monitoring of blood glucose (SMBG), CGM generates a continuous glycemic profile of the individual across the day, empowering behavioral modifications to improve glycemic control [6]. Furthermore, as SMBG involves pricking oneself to obtain blood for a glucometer to estimate the blood sugar, there are barriers to this arising from needle-related fear, pain and inconvenience [7, 8], which CGM was designed to overcome. Initially indicated for patients with Type 1 Diabetes Mellitus (T1DM) due to its synergy with insulin pumps [9] and the high risk of dysglycemia [10] in T1DM, CGM is an increasingly common intervention for patients with T2DM for self-education and behavioral modification [11].
One of the most common outcomes evaluated in T2DM trials is that of glycated hemoglobin (HbA1c). HbA1c demonstrates strong prognostication of end-stage complications, including coronary heart disease [12], renal and cardiovascular complications [13], neuropathy [14], depression [15] and all-cause mortality [16]. As such, appropriate HbA1c management has the potential to improve quality and quantity of life amongst T2DM patients. However, a shortcoming of HbA1c is that it represents the average glycemic control of a T2DM patient over the previous two to three months but not day-to-day glycemic variability (GV) [17]. Recent findings have demonstrated an association between high GV and hypoglycemia [18, 19], which can cause distress and poor treatment adherence. A popular GV metric is that of time-in-range (TIR) of blood glucose within 70–180 mg/dL [20, 21], and has shown strong association with diabetes-related complications such as vasculopathy and retinopathy [22, 23]. As such, both TIR and HbA1c are important endpoints to be evaluated in therapeutic trials of T2DM.
CGM is becoming increasingly popular due to its comfort, ease-of-use and self-education on their diabetes management [24, 25]. Numerous systematic reviews (SRs) with or without meta-analyses have demonstrated that CGM is associated with greater HbA1c reduction than SMBG [26], and increase patient awareness and management of their glycemic excursions [27]. However, it was noted that the present SRs had differing research questions and methodologies, leading to heterogeneity in outcomes synthesis and reporting. For example, some SRs restricted their inclusion criteria to studies investigating flash glucose monitoring (FGM), otherwise known as intermittently-scanned CGM (isCGM), only [28–33] while other SRs included only studies investigating real-time CGM (rtCGM) [34, 35]. In addition, some SRs only included randomized controlled trials (RCTs) [27, 29, 34, 36–40], while others focused primarily on glycemic control rather than HbA1c reduction [27, 31, 40–42].
Goals of this investigation
Thus, we conducted an umbrella review (UR) of SRs on CGM in T2DM patients to address the following: (1) consolidate the most contemporaneous evidence on the effectiveness of CGM in reducing HbA1c and improving GV metrics amongst patients with T2DM as compared to SMBG or usual care (UC), and (2) compare other outcomes, such as patient reported outcome measures (PROMs), between patients using CGM and SMBG/UC.
Methods
This umbrella review was registered in PROSPERO (ID: CRD42023447844) and reported according to the Preferred Reporting Items for Overview of Reviews (PRIOR) guidelines [43], with reference to methodological guidelines for umbrella reviews in the Cochrane Handbook for Systematic Reviews of Interventions [44].
Search strategy
Seven databases, namely the Cochrane Database of Systematic Reviews, CINAHL, Epistemonikos, SCOPUS, Web of Science, Medline and Embase, were searched from their respective dates of inception to 28th June 2024, including ahead-of-print and in-process publications and indexed citations. Bibliographies of included studies were hand-searched to identify more studies. A medical information specialist was consulted to construct the strategy on OvidSP, a search platform for both Embase and MEDLINE, comprising Medical Subject Headings (MeSH) and text terms relating to T2DM and CGM. The strategy was then translated as befitting the syntax of the remaining databases, presented in eMethods 1.
Study selection
The PICOTS framework was used to formulate the research question [45]. The population was limited to adult patients with T2DM who were either insulin-treated or non-insulin-treated, with no limitation by age or country. The intervention of interest was any modality of CGM, including professional CGM, retrospective CGM (rCGM), rtCGM and FGM. The comparison of interest was SMBG/UC. The primary outcomes were mean difference or standardized mean difference changes in both endpoint and pre-post change in HbA1c values, Time-In-Range (TIR), Time-Above-Range (TAR), Time-Below-Range (TBR) and patient reported outcome measures (PROMs) between the CGM and SMBG/UC groups. For the time frame of interest, meta-analyses of studies with a follow-up duration of three months or more were the primary focus of analysis. This is because HbA1c changes are likely seen only after three months, given that it is a measure of average blood sugar level over three months. However, for completeness, we also considered meta-analyses which included studies with a duration of three months or less. If a meta-analysis of meta-analyses were conducted, such meta-analyses would be excluded in the sensitivity analysis. Likewise, primary studies with a follow-up length of three months or less would be excluded in sensitivity analysis if a meta-analysis of primary studies was conducted. The setting was limited to peer-reviewed systematic reviews, with or without meta-analyses of RCTs and or prospective studies investigating CGM in the community or outpatient setting. Narrative reviews, systematic reviews of qualitative studies and SRs investigating exclusively T1DM studies were excluded. Gray literature was excluded due to a lack of peer review, which reduces its certainty of evidence and methodological quality.
Data collection
Titles and abstracts of publications retrieved from the above databases were uploaded onto Covidence, a systematic review management platform. Two investigators (amongst Y.Y., E.H. and S.B.S.) screened the title-abstracts against the inclusion and exclusion criteria independently. Full-texts for relevant abstracts were then retrieved and assessed by two investigators independently for inclusion in the UR. Data of each included SR was extracted by one investigator and verified by the other on a piloted data extraction sheet. It was prospectively planned that disagreements between the investigators at the screening, extraction and quality assessment stages would be reconciled in a discussion, failing which, the senior author (S.T.) would adjudicate.
The following information was extracted from each SR: study characteristics, systematic review methodology, quality assessment outcomes, and narrative synthesis and/or meta-analysis findings of the following outcomes: (1) HbA1c measurements, (2) TIR, TAR and TBR values, (3) PROMs, (4) GV measures, and (5) other physiological measurements.
No missing data from any publication was reported during data extraction.
Quality assessment
Risk-of-bias of each systematic review was assessed by two investigators independently using the A MeaSurement Tool To Assess systematic Reviews 2 (AMSTAR-2) instrument [46], with conflicts resolved via a three-way consensus-making discussion between the senior author and the investigators.
Quality assessment findings informed the discussion of narrative synthesis outcomes and sensitivity analysis subsequently, whereby the impact of high-risk-of-bias SRs on the outcomes were explored and discussed. Risk-of-bias findings were visualised on a traffic-light plot [47].
Risks-of-biases for primary studies were extracted from the SRs using the Cochrane Risk-of-Bias Tool 2 (RoB2) [48], and the Newcastle–Ottawa Scale [49] (NOS) or the Cochrane Risk Of Bias In Non-randomised Studies of Interventions [50] (ROBINS-I). If a single primary study was assessed for risk-of-bias by more than one SR, we conservatively picked the assessment that graded the primary study with a higher risk-of-bias.
Overlap analysis
Overlap of primary studies amongst included meta-analyses in a UR is a methodological problem [51]. To assess primary study overlap, the Graphical Representation of Overlap for Overviews [52] (GROOVE) was used to evaluate the corrected covered area (CCA), a statistical measure of overlap in primary studies between SRs [51]. Structural missingness refers to the impossibility of a SR including a primary study published after its publication date. CCA was adjusted for structural missingness to avoid underestimation [52]. The following CCA thresholds were described by Bracchiglione et al.:
CCA < 5%: Slight overlap
5% < CCA < 10: Moderate overlap
10% < CCA < 15%: High overlap
CCA > 15%: Very High overlap
The interpretation and application of described CCA thresholds for the current UR were based on existing literature [53]. Specifically, if the CCA of all studies was less than 10%, a meta-meta-analysis of endpoints was done using the summary effect sizes reported by the included meta-analyses. Between studies with “high” or “very high” overlap as assessed by the GROOVE tool, preference for inclusion in meta-meta-analysis would be given to the study that i) was of higher study quality, ii) included more studies with larger sample size, iii) was published more recently. Both studies would be retained otherwise.
If the CCA of all included meta-analyses was more than 10%, all unique primary studies included across these meta-analyses would be retrieved instead, and a subsequent round of data extraction from these primary studies was done. This was done by using the primary study statistics reported by the SRs, or extracting from the primary studies, to provide an accurate summary effect estimate.
Statistical analysis and heterogeneity assessment
Narrative synthesis of findings reported by included SRs of each primary and secondary endpoint was attempted and reported. Data analysis was performed using R version 4.3.1. (The R Foundation) [54], using the metafor [55] and meta [56] packages. Recovery of studies for meta-analysis with incomplete outcome reporting, such as missing variances or pre-post within-group differences, was done by contacting the corresponding authors for information. If authors were uncontactable, reasonable statistical inference and data imputation methods were employed, as recommended by the Cochrane Handbook (vers 5.1.0) [57].
Meta-analysis for a particular endpoint was conducted if at least two studies, be it meta-analyses or primary studies, reported it with consistent outcome measurement and effect size estimation. The methods described subsequently would apply to both meta-meta-analysis, or meta-analysis of primary studies, contingent on the extent of primary study overlap as quantified by GROOVE. A random-effects model with inverse-variance weighting was fitted to pool (1) mean difference in pre-post within-group differences of HbA1c between CGM and control groups and (2) standardized mean difference (SMD) in endpoint PROM scores between CGM and control groups. A priori determined subgrouping by CGM modality, presence of study funding and presence of insulin therapy was done. Random effects model was used to account for study heterogeneity arising from community setting, geographic distribution and possible co-interventions alongside CGM and SMBG. The results were visualized as forest plots. SMD was chosen for PROM meta-analysis, considering the heterogeneity in PROM instruments used across different studies. Heterogeneity was estimated using the restricted maximum likelihood estimator (REML), as it has the least bias compared to other estimators regardless of sample size [58]. Subgroup differences were assessed using Cochran’s Q. Publication bias was assessed using funnel plots and Egger’s regression test [59] for meta-analyses including 10 studies or more. The significance level was set at p < 0.05 for all hypothesis testing.
Sensitivity and subgroup analyses
Sensitivity analysis by varying correlation coefficient from 0 to 1 was done to ensure the robustness of variance imputation via correlation. Then, the random-effects model was fitted only on RCTs as such a study design minimizes bias and confounding, thus best establishing causality [60]. A Baujat plot [61] was produced for each meta-analysis to identify (1) studies disproportionately contributing to the summary effect estimate and (2) studies with significantly greater variance. Such outliers were excluded from a post-hoc Baujat-informed meta-analysis. Meta-regression was fitted on the dataset based on a priori determined variables, such as publication year, study region and baseline characteristics for meta-analyses with more than eight studies to ensure interpretability of results [62].
Evidence certainty assessment
Evidence certainty was assessed for the primary and secondary endpoints using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework [63] by the two investigators, with conflicts reconciled by the senior author.
Results
The title-abstracts of 989 studies were screened against the inclusion and exclusion criteria, of which 938 studies were excluded, and the full-text articles of the remaining 51 studies were retrieved for evaluation. The full-text articles of 13 studies identified from citation searching and keyword searching of the included studies were also retrieved. 33 articles were excluded from the 64 articles from which full-texts were retrieved, leaving 31 articles for inclusion into the review [26–42, 64–77] (eFigure 1).
Amongst the studies, 2 were health technology assessments with systematic review methodology, of which 1 further conducted meta-analysis, 9 were SRs without meta-analysis, and the remaining 20 were SRs with meta-analyses. The publication year range was 2011 to 2024. 4 of the SRs [26, 36, 70, 75] searched only one database (Table 1). The corrected cover area (CCA) of included T2DM studies across included SRs was 10.12% after correcting for structural missingness (eFigure 3a), indicating high overlap.
Table 1.
Summary of characteristics of included systematic reviews of studies evaluating the effectiveness of continuous glucose monitoring from 2011 to 2024
| Author Year | Meta Analysis Conducted? | Funding Sources and COI | T2D Studies included | Commercial Funding in Primary Studies | Risk of bias tool(s) used | Narrative synthesis endpoints | Meta-analysis endpoints |
|---|---|---|---|---|---|---|---|
| Gandhi 2011 [41] | Yes |
Funding not reported COIs not reported |
3 RCTs | Not reported | Investigator's own checklist | Patient QoL/Satisfaction | HbA1c (%) |
| Hoeks 2011 [35] | No |
Funding not reported No COIs |
1 RCT | Not reported | Others |
HbA1c (%) Hypo/Hyperglycemia TIR Adverse events CGM compliance Glycemic Control |
Not reported |
| Meade 2012 [73] | No |
Funding not reported COIs not reported |
3 RCTs 2 PCSs |
Not reported | No quality assessment done | Hypo/Hyperglycemia | Not reported |
| Poolsup 2013 [64] | Yes |
Funding not reported No COIs |
4 RCTs | Not reported | Maastricht—Amsterdam | HbA1c (%) | HbA1c (%) |
| Bidonde 2017 [67] | Yes |
Funding not reported COIs not reported |
1 RCT | Not reported | RoB2 | Not reported | HbA1c (%)a |
| Garcia-Lorenzo 2018 [69] | Yes |
Funded COIs not reported |
5 RCTs | Not reported | RoB2 | Not reported |
HbA1c (%) Health economics |
| Mattishent 2018 [72] | No |
Funded COIs not reported |
1 CS 1 PCS 1 RCT |
Not reported | No quality assessment done |
Hypo/Hyperglycemia Patient QoL/Satisfaction Adverse events |
Not reported |
| Park 2018 [74] | Yes |
Not funded No COIs |
3 RCSs 7 RCTs |
Not reported | RoB2,NOS |
Adverse events Physical activity Blood pressure Body weight/BMI |
HbA1c (%) |
| Dicembrini 2019 [36] | Yes |
Not funded COIs not reported |
5 RCTs | Not reported | Jadad |
Hypo/Hyperglycemia Insulin dose Patient QoL/Satisfaction |
HbA1c (%) |
| Ida 2019 [34] | Yes |
Not funded No COIs |
7 RCTs | Not reported | RoB2 | Patient QoL/Satisfaction |
HbA1c (%) Body weight/BMI Hypo/Hyperglycemia Hypo/Hyperglycemia Blood pressure |
| Janapala 2019 [70] | Yes |
Not funded No COIs |
5 RCTs | Not reported | RoB2 | HbA1c (%) | HbA1c (%) |
| Ontario 2019 [32] | No |
Funding not reported COIs not reported |
1 PCS 1 RCT |
Not reported | RoB2,ROBINS-I |
TIR HbA1c (%) Glycemic Control Hypo/Hyperglycemia Patient QoL/Satisfaction Adverse events |
Not reported |
| Pleus 2019 [75] | No |
Funded COIs present |
10 RCTs 2 PCSs |
Not reported | No quality assessment done | Adverse events | Not reported |
| Ang 2020 [28] | No |
Not funded No COIs |
2 RCTs | Not reported | No quality assessment done |
TIR Patient QoL/Satisfaction Adverse events HbA1c (%) |
Not reported |
| Asarani 2020 [33] | No |
Funded No COIs |
2 RCTs 1 PCS |
Not reported | No quality assessment done |
Patient QoL/Satisfaction Adverse events |
Not reported |
| Azhar 2020 [66] | No |
Not funded No COIs |
1 RCS 1 CS 2 PCSs |
Not reported | No quality assessment done |
Patient QoL/Satisfaction Care provider QoL/Satisfaction HbA1c (%) |
Not reported |
| Castellana 2020 [68] | Yes |
Funding not reported COIs present |
2 RCTs | 4 | RoB2,NHLBI | Patient QoL/Satisfaction |
HbA1c (%) TIR, TBR, TAR Hypoglycemia Insulin dose SMBG readings |
| Cowart 2020 [29] | No |
Not funded No COIs |
3 RCTs | 6; 2 unknown | RoB2 |
HbA1c (%) Hypo/Hyperglycemia TIR Patient QoL/Satisfaction |
Not reported |
| Evans 2020 [30] | Yes |
Funded COIs present |
2 RCTs | Not reported | No quality assessment done | Not reported | HbA1c (%) |
| Maiorino 2020 [27] | Yes |
Funding not reported COIs present |
2 RCTs | 13 | RoB2 | Not reported |
HbA1c (%) TIR Hypo/Hyperglycemia |
| Aggarwal 2022 [65] | No |
Not funded No COIs |
5 RCTs 1 RCS |
Not reported | Jadad,NOS |
HbA1c (%) TIR Health economics |
Not reported |
| Gao 2022 [31] | Yes |
Funded No COIs |
10 RCTs | Not reported | RoB2 | Not reported | HbA1c (%) |
| DiMolfetta 2023 [26] | Yes |
Funded COIs present |
2 NRCTs 4 RCSs 7 RCTs |
13 | RoB2,NHLBI | Not reported |
HbA1c (%) TIR Hypo/Hyperglycemia |
| Kieu 2023 [71] | No |
Not funded No COIs |
1 NRCT 1 PCS 3 RCTs |
6 | NHLBI,NHLBI |
HbA1c (%) TIR |
Not reported |
| Roldan 2023 [76] | Yes |
Funded No COIs |
6 RCTs | Not reported | No quality assessment done | Not reported |
HbA1c (%) Hypo/Hyperglycemia |
| Seidu 2023 [39] | Yes |
Funded COIs present |
26 RCTs | Not reported | RoB2 | Not reported |
HbA1c (%) TIR Hypo/Hyperglycemia Glycemic control Patient QoL/Satisfaction Body weight/BMI Blood pressure Lipids Adverse events Lifestyle habits |
| Uhl 2023 [40] | Yes |
Not funded COIs not reported |
14 RCTs | 10 | RoB2 | Not reporteed |
HbA1c (%) TIR Hypo/Hyperglycemia |
| Ferreira 2024 [38] | Yes |
Not funded No COIs |
6 RCTs | Not reported | RoB2 | HbA1c (%) |
HbA1c (%) TIR Hypo/Hyperglycemia Glycemic Control Patient QoL/Satisfaction |
| Jancev 2024 [77] | Yes |
Not funded No COIs |
12 RCTs | Not reported | RoB2 | Not reporteed |
HbA1c (%) TIR Hypo/Hyperglycemia Glycemic Control Adverse events |
| Kong 2024 [42] | Yes |
Not funded No COIs |
17 RCTs | 16 | JBI—RCT | Not reporteed |
HbA1c (%) TIR Hypo/Hyperglycemia Patient QoL/Satisfaction Body weight/BMI Blood pressure Lipids |
| Lu 2024 [37] | Yes |
Funded No COIs |
11 RCTs | Not reported | RoB2 | Not reported |
HbA1c (%) TIR Hypo/Hyperglycemia |
aBidonde 2017 included only 1 RCT in meta-analysis of CGM efficacy outcomes, thus findings may not be reliable
COI Conflict of interest, JBI Joanna-Briggs Institute, NHLBI National Heart, Lung and Blood Institute, NOS Newcastle–Ottawa Scale, PCS prospective cohort study, RCS Retrospective cohort study, RoB2 Cochrane Risk-of-Bias Tool 2, ROBINS-I Risk-Of-Bias In Non-randomized Studies of Intervention
8 SRs [27, 32, 35, 41, 64, 67, 68, 73] did not declare their funding sources, or absence thereof, while 4 SRs were commercially funded [30, 37, 39, 75]. The study quality was visualized in eFigure 2a-b. Most papers did not provide a rationale for the study designs included (27/31), report funding sources of primary studies (18/31), or sufficiently interpret the risk-of-bias assessments and their impact on the review conclusions (24/31). There was poor reporting of methodology for study selection (8/31) and data extraction (10/31) amongst some studies as well.
From data reported by the 25 SRs [26, 27, 29, 31, 32, 34–42, 42, 64–72, 74] which conducted quality assessment, the majority of studies [26, 27, 29, 31, 32, 34–41, 64, 65, 67–71, 74, 77] used the Cochrane Risk-of-Bias 2 Tool (RoB2) to assess study quality of RCTs. Generally, SRs highlighted poor documentation of allocation concealment [78–94], and incomplete outcome reporting [82, 84, 88, 95–99]. As such, all RCTs were at either moderate or high risk-of-bias. A number of SRs [26, 27, 31, 36, 39, 68] highlighted the absence of participant and assessor blinding to exposure and outcome assessment was highlighted as a risk-of-bias. However, it was noted that the intervention (i.e. introducing a subcutaneous device) was difficult to conceal to both participants and assessors [68], and thus risk-of-bias might be overestimated in some SRs. 7 studies assessed risk-of-bias in Non-Randomised Studies of Interventions (NRSI) [26, 32, 65, 66, 68, 71, 74], with a majority using the National Heart, Blood, Lung Institute (NHLBI) risk-of-bias instrument. Generally, SRs highlighted issues in sample size calculation and loss to follow-up.
HbA1c
Narrative synthesis
19 included SRs conducted a meta-analysis on HbA1c outcomes [26, 27, 30, 31, 34, 36–42, 64, 68–70, 74, 76, 77], while eight SRs [28, 29, 32, 35, 64–66, 71] conducted a narrative synthesis of the same. Amongst SRs which narratively synthesized HbA1c outcomes, one SR concluded that more studies on CGM use amongst a T2DM-specific cohort were needed to sufficiently assess CGM’s effectiveness [66]. The remainder [28, 32, 35, 64, 71] either suggested that current trial outcomes significantly improved T2DM outcomes when CGM is prescribed over SMBG or that no significant difference is seen between the two [29, 66].
Amongst the 19 SRs conducting meta-analysis, 15 studies concluded a significant improvement in HbA1c reduction in CGM patients over UC with effect sizes between −0.74% to −0.20%, ranging from low to high heterogeneity (I2 = 0% to 89%) [26, 31, 34, 36–42, 64, 69, 70, 76, 77]. Two SRs concluded a significant reduction in HbA1c for real-time and professional CGM patients but not for flash CGM [37, 74]. Three SRs demonstrated that HbA1c reduction was invariant with CGM modality through meta-regression or subgroup analysis [38–40]. Two SRs concluded an insignificant difference in HbA1c reduction between CGM and UC patients with high heterogeneity (I2 > 80%) [27, 68].
There was methodological heterogeneity across the URs which conducted meta-analysis, for example, either pooling differences in follow-up HbA1c values between intervention and control [27, 34, 36, 41, 64, 70, 76], or differences in HbA1c reduction from baseline to follow-up between intervention and control [26, 30, 31, 37–40, 42, 68, 69, 74, 77]. Some meta-analyses opted for a fixed-effect model [64, 70], citing low between-study heterogeneity as a rationale, while most opted for random effects. One SR pooled weighted mean difference [38] and one SR pooled standardized mean difference using Hedge’s G values [42].
The corrected cover area calculated after adjusting for structural zeros was 20.49% (eFigure 3b), indicating a very high overlap in included studies for meta-analyses of HbA1c outcomes, thus motivating a primary study level data extraction.
Meta-analysis of primary studies
Data were extracted for 41 studies [78–118], of which the variances of 15 studies were imputed using correlation. A correlation coefficient of 0.3 was elected to arrive at a more conservative pooled estimate. A meta-analysis of 34 studies (Fig. 2b), comprising 28 RCTs [78–91, 93–99, 103, 108, 111, 112, 114, 115, 117], four retrospective NRSIs [102, 105, 106, 109] and two non-randomised controlled trials (NRCT) [104, 107] involving 11,494 patients demonstrated that CGM use was significantly associated with greater HbA1c reduction over SMBG/UC (MD = −0.40% [95% CI: −0.54 to −0.25]), with significant heterogeneity (I2 = 81%, t2 = 0.100). This was invariant with study type and sensitivity analyses using different coefficients to re-impute missing variances(eFigure 5a-c). A post-hoc sensitivity analysis (eFigure 5d) excluding outliers [82, 83, 86, 90, 99, 108] identified using a Baujat plot (eFigure 11a), showed a reduction in heterogeneity (I2 = 26%) and an overall effect significantly favoring CGM over SMBG/UC. Funnel plot (eFigure 4a) and Egger’s test (p = 0.105) (Supplementary Table 2) were insignificant for publication bias.
Fig. 2.
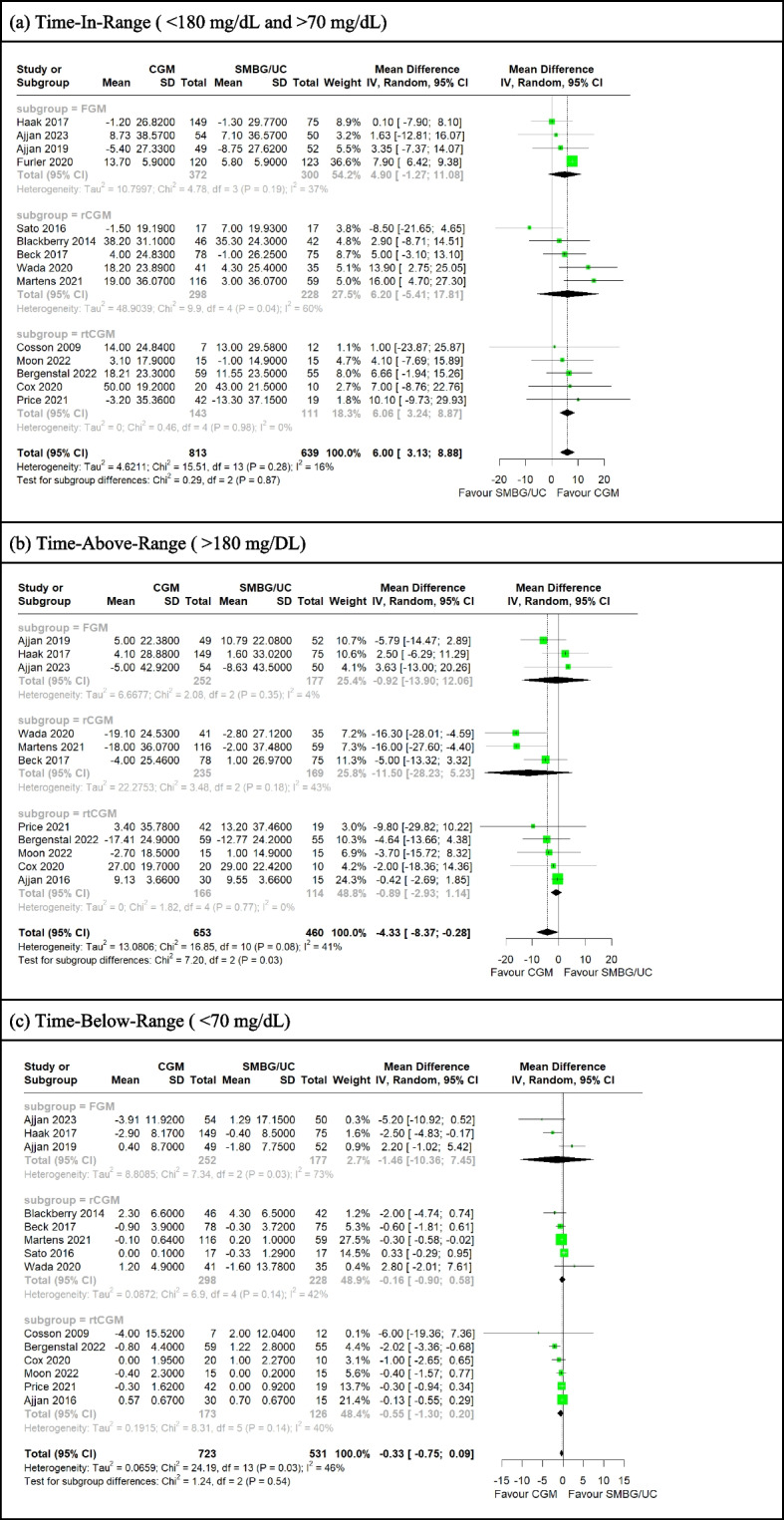
a-c Forest plot for meta-analysis of primary studies comparing CGM with SMBG from 2009 to 2023 by pooling the mean difference in pre-post change of (a) TIR, (b) TAR and (c) TBR between CGM and SMBG/UC participants. CGM significantly associated with greater TIR increase (MD= 6.00 [95%CI: 3.13 to 8.88]) and greater TAR decrease (MD= −4.33 [95%CI: −8.37 to −0.28]), and insignificantly associated with greater TBR decrease (MD= −0.33 [95%CI: −0.75 to 0.09]) over SMBG/UC participants. TIR and TBR were invariant with CGM modality (p = 0.87, p = 0.54) but not TAR (p = 0.03)
A meta-analysis of 28 RCTs (Fig. 1b) involving 2695 patients, subgrouped by CGM modality, demonstrated a significantly greater reduction in HbA1c in patients on CGM compared to UC (MD = −0.42 [95% CI: −0.60 to −0.24]), invariant with CGM modality, with high heterogeneity (I2 = 91%, t2 = 0.14). Of note, rCGM and rtCGM subgroups were not heterogeneous, while the FGM group was highly heterogeneous. Outlier exclusion sensitivity analysis (eFigure 5e) similarly demonstrated significantly greater HbA1c reduction with CGM use over SMBG/UC, invariant with CGM modality. The heterogeneity in FGM significantly improved (I2 = 33%), as a majority of outliers were FGM studies.
Fig. 1.
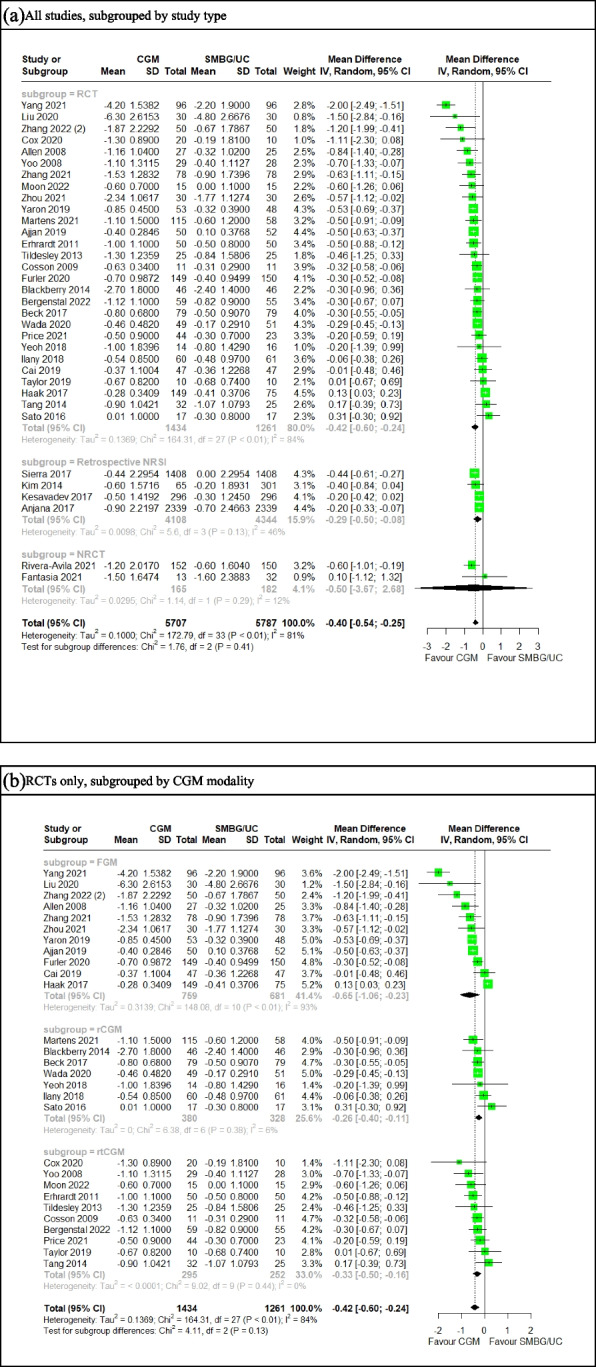
a-b Forest plot for meta-analysis of primary studies comparing CGM with SMBG from 2008 to 2022 by pooling the mean difference in pre-post HbA1c (%) change between CGM and SMBG users (a) across all studies, subgrouped by study type and (b) across RCTs only subgrouped by CGM modality. Correlation coefficient for imputation set at 0.30. CGM significantly associated with greater HbA1c decrease over SMBG (a) (MD = −0.40, 95% CI: −0.54 to −0.25) (b) (MD = −0.42, 95% CI: −0.60 to −0.24)
Subgroup analysis demonstrated that studies without commercial funding showed an insignificantly greater HbA1c reduction in CGM patients over SMBG/UC patients (eFigure 5f). Otherwise, magnitude of pre-post HbA1c reduction was invariant with funding status of studies, insulin treatment and risk-of-bias (eFigure 5f-h).
Average baseline HbA1c value (%) and duration of diabetes in years prior to study onset were significantly associated with pre-post HbA1c change on meta-regression, with a beta-coefficient of −0.35 (p = 0.001) and 0.030 (p = 0.040) respectively. Thus, it might be inferred from the included primary studies that each 1% increase in baseline HbA1c could lead to a 0.35% greater HbA1c reduction and each additional year in diabetes duration prior to CGM initiation could lead to 0.030% lesser HbA1c reduction (Supplementary Table 1a).
Funnel plots and Egger’s regression test were insignificant for publication bias for all studies, each subgroup of CGM modality, and each subgroup of funding status (eFigure 4a-c, Supplementary Table 2).
Sensitivity analysis was done by pooling for the mean difference in follow-up HbA1c between the CGM and control groups. CGM remains strongly associated with greater HbA1c reduction over SMBG/UC. This was invariant with CGM modality, study funding, insulin treatment and risk-of-bias and after Baujat plot-informed outlier removal (eFigure 6a-f, 11c). Further sensitivity analyses were done for the meta-analyses of pre-post HbA1c change for all studies and RCTs as well as endpoint HbA1c change for all studies, by including only studies with a follow-up duration of three months of more. In all analyses, CGM still remained significantly associated with greater HbA1c reduction over SMBG/UC (eFigure 5i – j, 6 h). This demonstrated the robustness of our findings even when restricted to a clinically meaningful follow-up period.
Glycemic variability
Narrative synthesis
Six SRs [28, 29, 34, 41, 71, 76] reported time-inr-range (TIR), time-below-range (TBR) and time-above-range (TAR) outcomes for T2DM exclusive studies, with one SR reporting similar outcomes for mixed T1DM and T2DM studies. Generally, TIR, TAR and TBR was defined as a percentage of the day when blood glucose stayed between 70 mg/dL to 180 mg/dL, above 180 mg/dL and below 70 mg/dL respectively.
For TIR, six SRs [28, 29, 34, 41, 71, 76] conducted a narrative synthesis, with all SRs concluding nonsignificant or low evidence certainty of CGM patients having greater TIR than SMBG/UC patients. Seven SRs [26, 37–40, 42, 77] conducted a meta-analysis on pre-post change in TIR, with summary effect sizes ranging from an increase of 2.52% to 11.06% in the CGM arm over SMBG/UC and five SRs concluding significantly greater increases in TIR in the CGM arm [26, 37, 38, 40, 77]. Heterogeneity ranged between 0% to 91.3%. One SR conducted a meta-analysis on follow-up TIR values rather than pre-post change, reporting a significant increase in TIR by 78.11 min in the CGM group over SMBG/UC [27].
For hypoglycemia, six studies conducted a narrative synthesis, with three SRs [29, 32, 73] concluding that CGM led to lower time spent in hypoglycemia than SMBG/UC, presumably due to increased patient awareness of hypoglycemia trends, while the remaining three concluded [35, 36, 72] nonsignificant effects on time spent in hypoglycemia between CGM and SMBG/UC. One SR noted that hypoglycemia awareness was increased amongst the participants, especially the elderly, following the implementation of CGM during the study period. Nine studies conducted meta-analysis on TBR, with two concluding [34, 38, 39, 42, 76, 77] significant reductions in TBR amongst CGM patients over SMBG/UC, and three SRs [26, 27, 40] concluding no significant difference. Seven SRs conducted meta-analysis on TAR, with three SRs concluding significant reductions in TAR amongst CGM patients over SMBG/UC [38, 39, 77] and four SRs concluding no significant difference [27, 34, 40, 42].
Three SRs reported glycemic variability (GV) measures apart from TIR, TBR and TAR. One SR [32] stated that results were inconsistent due to nonstandard reporting of GV scales, while another concluded significant improvement in GV [35] with CGM use, although specific measures were not mentioned. One SR included a study [36] which reported no significant difference in the coefficient of variation (CV) between CGM and SMBG/UC users at follow-up.
The corrected cover area calculated for TIR, TAR and TBR after adjusting for structural zeros were 33.33%, 41.38%, 29.82% respectively (eFigure 3c-d), indicating very high overlap in included studies for meta-analyses of glycemic variability outcomes. Thus, primary study level data extraction and meta-analysis was conducted.
Meta-analysis of primary studies
Meta-analysis of pre-post change in TIR of 14 studies involving 1452 participants showed that CGM use was significantly associated with greater pre-post TIR increase over SMBG/UC (MD = 6.00% [95%CI: 3.13 to 8.88]) (Fig. 2a ). This was invariant with outlier inclusion sensitivity analysis (eFigure 7a, eFigure 11d), CGM modality, study funding, insulin treatment and risk-of-bias (Fig. 2a, eFigure 7b-d). On further subgroup analysis, studies utilizing rCGM, studies without commercial funding, studies recruiting only insulin-treated patients and low risk-of-bias studies had insignificant differences in pre-post TIR change between CGM and SMBG/UC. Study trials recruiting both insulin-treated and non-insulin-treated participants were associated with greater pre-post increase in TIR amongst CGM participants compared to study trials recruiting only insulin-treated participants (B = 4.40, p = 0.045) on meta-regression (Supplementary Table 1b).
Meta-analysis of pre-post change in TAR of 11 studies involving 1113 participants showed that CGM use was significantly associated with greater pre-post TAR decrease over SMBG/UC (MD = −4.33% [95%CI: −8.37 to −0.28]) (Fig. 2b). This was invariant with outlier inclusion sensitivity analysis (eFigure 8a, eFigure 11e), CGM modality, study funding, insulin treatment and risk-of-bias (Fig. 2b, eFigure 8b-d). Study trials utilising rCGM over FGM (B = −9.90, p = 0.029) and studies conducted in Europe over Asia (B = 9.66, p = 0.029) were associated with a decrease and increase in pre-post TAR respectively on meta-regression (Supplementary Table 1c).
Meta-analysis of pre-post change in TBR of 14 studies involving 1254 participants showed no significant difference between CGM and SMBG/UC in pre-post TAR change (MD = −0.33% [95%CI: −0.75 to 0.09]) (Fig. 2c). This was invariant with outlier inclusion sensitivity analysis (eFigure 9a, eFigure 11f), CGM modality, study funding, and risk-of-bias but not insulin treatment (Fig. 2d, eFigure 9b-d). Studies without funding compared to commercially funded trials (B = 0.79, p = 0.015) and studies recruiting both insulin-treated and non-insulin-treated participants compared to studies recruiting only insulin-treated participants (B = −2.02, p = 0.0028) were associated with an increase and decrease in pre-post TBR respectively on meta-regression. Baseline BMI (B = −0.091, p = 0.027) and planned wear time of CGM devices (B = −0.054, p = 0.037) were also significantly associated with pre-post TBR decrease. (Supplementary Table 1d).
Funnel plots for TIR, TAR and TBR meta-analyses were symmetrical (eFigure 4d-f) and Egger’s regression test were insignificant for publication bias (Supplementary Table 2).
Patient reported outcome measures
Narrative synthesis
Eight SRs conducted a narrative synthesis on patient reported outcome measures (PROMs), of which five SRs [28, 29, 32, 34, 36] synthesized primary study findings relating to standardized PROM instruments, while the remaining three synthesized [33, 66, 72] qualitative descriptions of patient experience and sentiment post-CGM use. Amongst the five SRs that synthesized qualitative findings from primary studies, two SRs [32, 36] concluded no significant difference in quality-of-life between CGM and SMBG/UC patients, two SRs [28, 29] concluded a significant increase in quality-of-life amongst CGM patients over UC while one SR [34] described the evidence of CGM improving quality-of-life over UC to be mixed between significant and insignificant improvement. The three SRs [33, 66, 72] synthesizing qualitative findings concurred that CGM increased diabetes awareness and improved function in daily living amongst T2DM patients. However, many participants were still concerned with sensor injection pain, inconvenience of sensor wear, cutaneous complications and infections, and subsidies. Three SRs conducted a meta-analysis of standardized mean difference in pre-post change of PROMs, with one SR synthesizing satisfaction scores [38] while two SRs subgrouped outcomes into three domains [39, 42], namely diabetes-related distress, treatment satisfaction and quality-of-life. One SR demonstrated a significant increase in treatment satisfaction amongst CGM patients [38], one SR demonstrated no difference in pre-post changes across the three domains [42] while the remaining SR showed only a significant difference in treatment satisfaction favoring the SMBG arm [39]. There was high overlap in PROM meta-analysis (25.00%), thus a primary study-level meta-analysis was conducted.
Meta-analysis of primary studies
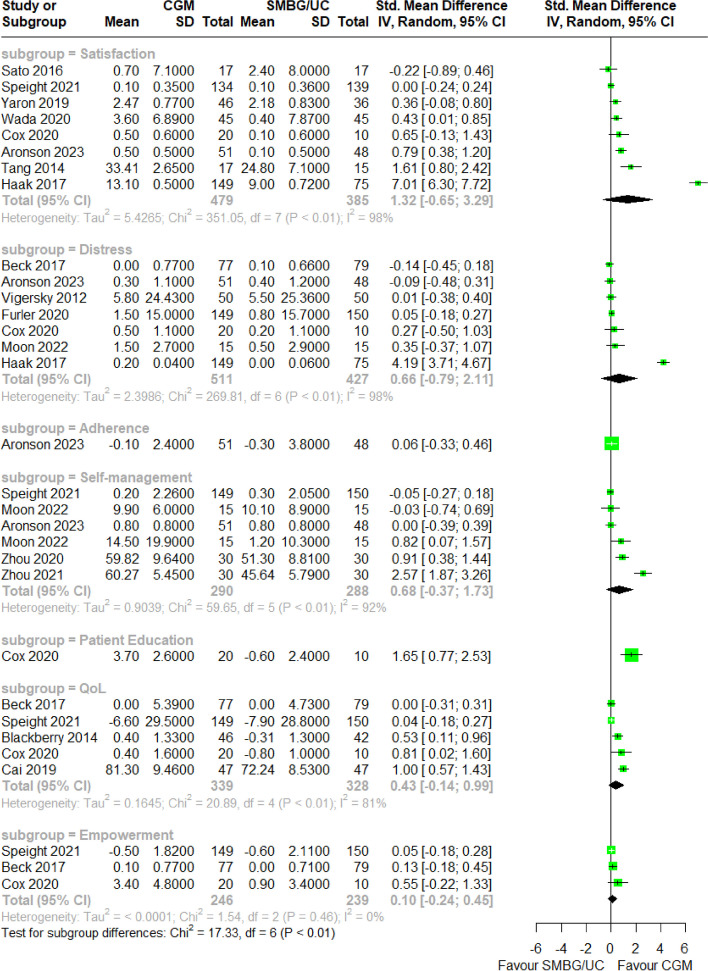
PROM scores were extracted from the aforementioned primary studies for meta-analysis. 21 unique instruments were utilized to collect PROMs across seven domains: treatment satisfaction, diabetes-related distress, treatment adherence, diabetes self-management, patient education, quality-of-life and patient empowerment. The most frequently utilized instruments for each domain were the Diabetes Distress Scale [119] for diabetes-related distress, World Health Organisation Quality Of Life [120] instrument for quality-of-life, Diabetes Treatment Satisfaction Questionnaire [121] for treatment satisfaction and Summary of Diabetes Self-Care Activities [122] measure for self-management. Distress scores were negated to align the scale direction with other domains due to the negative coding distress-related questionnaires. A meta-analysis of standardized mean difference in pre-post PROM scores between CGM and SMBG/UC groups was conducted for each aforementioned PROM domain. Sample size of meta-analyses with more than one study ranged from 485 to 938. Effect sizes ranged from SMD = 0.10 to SMD = 1.32, while heterogeneity ranged from I2 = 0% to I2 = 98%. pre-post change in all seven PROM domains was invariant between CGM and SMBG/UC arms (Fig. 3), and remained so after repeating the meta-analysis for studies utilising FGM, rCGM and rtCGM only respectively (eFigure 9a-c).
Fig. 3.
Forest plot for meta-analysis of primary studies comparing CGM with SMBG from 2014 to 2023 by pooling the standardized mean difference of pre-post change in patient reported outcome measures subgrouped by domains, specifically satisfaction, distress, adherence, self-management, patient education, quality-of-life and empowerment. None of the domains demonstrated a statistically significant effect size
Adverse events
Seven SRs [28, 32, 33, 35, 72, 74, 75] narratively synthesized adverse events with CGM use. Five of these SRs [28, 33, 35, 74, 75] summarized cutaneous complications presented in CGM patients, which mostly involved pain, itching and redness at the injection site that were easily managed with topicals and self-limiting. One SR [70] further calculated an overall incidence rate of cutaneous complications amongst CGM users, reporting 1090 events observed across 1158 participants, with 138 to 158 accumulated years of wear time, arriving at a rate of 1 cutaneous complication event for every 8 weeks of wear time. Three of these SRs summarized hypoglycemia incidence within CGM patients, of which two concluded [72, 74] that CGM users had no hypoglycemic events, while one reported [32] infrequent severe hypoglycemic events amongst CGM users. Two SRs conducted a meta-analysis of odds ratio of adverse events between CGM and SMBG/UC. One SR reported a higher risk of adverse events in the SMBG arm over CGM (RR: 1.22 [95% CI: 1.01 to 1.47]) [39]. The other SR reported no significant differences in the odds of severe hypoglycemia or macrovascular complications between CGM and SMBG/UC [77].
Cost effectiveness assessment
One SR [65] reported higher healthcare costs per annum for CGM patients (USD$23,021 p.a.) than patients on usual care (USD$21,502 p.a.).The same study concluded that healthcare resource utilization, emergency events and hospitalization were lower amongst CGM users.
One SR [69] used the results of their own meta-analysis of HbA1c outcomes in their included studies in a cost-effectiveness assessment. The SR utilized Markov modeling with end-stage complication events for a lifetime horizon based on annual cycles, simulating 2 cohorts of 1000 Spanish T2DM patients aged 57 years old. The SR concluded that the mean incremental quality-adjusted life years (QALY) of CGM patients over UC was 0.27, yielding an incremental cost-effectiveness ratio of €180,533 per QALY, which was significantly higher than an estimated willing-to-pay threshold of €25,000.
Other physiological measurements
Three SRs conducted a meta-analysis on pre-post change in body mass-related measurements, specifically weight and body mass index, demonstrating no significant difference in pre-post outcomes between CGM and SMBG/UC [34, 39, 42]. The same three SRs also conducted a meta-analysis on pre-post change in diastolic and systolic blood pressure, demonstrating no significant difference between CGM and SMBG/UC. Two SRs conducted a meta-analysis on cholesterol outcomes, demonstrating no significant difference in pre-post outcomes between CGM and SMBG/UC [39, 42].
GRADE
GRADE assessment was conducted for the meta-analysed outcomes, differences in pre-post change in HbA1c and follow-up PROM scores between CGM and SMBG/UCgroups. The evidence certainty was rated moderate for pre-post change in HbA1c, TIR and TAR outcomes, low for pre-post change in TBR and very low for pre-post change in PROM scores. Serious issues were raised regarding the methodological quality of the primary studies conducted, and the lack of precision and directness in outcome measurement for PROM. This is mainly due to the lack of consistency in the PROM instruments used, even within domains. The low number of studies and small within-study sample size also contributed to an imprecise estimate of PROM changes between CGM and SMBG/UC participants. (Table 2).
Table 2.
GRADE table reporting evidence certainty for HbA1c, TIR, TAR, TBR and PROM outcomes summarized from this umbrella review of systematic reviews evaluating the effectiveness of CGM from 2011 to 2024
| CONTINUOUS GLUCOSE MONITORING IN THE MANAGEMENT OF TYPE 2 DIABETES | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | Patients with Type 2 Diabetes in a Community Setting | |||||||||||
| Intervention | Continuous Glucose Monitoring | |||||||||||
| Comparator | Self-Monitoring of Blood Glucose | |||||||||||
| Certainty assessment | № of patients | Certainty | Key Message | |||||||||
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | CGM | SMBG |
Absolute (95% CI) |
|||
| pre-post HbA1c (%) change (follow-up: mean 5.08 months) | ||||||||||||
| 28 | randomized trials | serious [a] | not serious | not serious | not serious | none | 1434 | 1261 |
MD 0.42% lower (0.60 lower to 0.24 lower) |
⨁⨁⨁◯ Moderate |
HbA1c reduction was significantly greater amongst type 2 diabetes patients using CGM over SMBG/UC | |
| pre-post TIR (% day) change (follow-up: mean 5.26 months) | ||||||||||||
| 14 | randomized trials | serious [a] | not serious | not serious | not serious | none | 813 | 639 |
MD 6.00% higher (3.13 higher to 8.88 higher) |
⨁⨁⨁◯ Moderate |
TIR increase was significantly greater amongst type 2 diabetes patients using CGM over SMBG | |
| pre-post TAR (% day) change (follow-up: mean 4.62 months) | ||||||||||||
| 11 | randomized trials | serious [a] | not serious | not serious | serious | none | 653 | 460 |
MD 4.33% lower (8.37 lower to 0.28 lower) |
⨁⨁◯◯ Low |
TAR decrease was significantly greater amongst type 2 diabetes patients using CGM over SMBG | |
| pre-post TBR (% day) change (follow-up: mean 4.65 months) | ||||||||||||
| 14 | randomized trials | serious [a] | not serious | not serious | serious | none | 723 | 531 |
MD 0.33% lower (0.75 lower to 0.09 higher) |
⨁◯◯◯ Very low |
No difference in TBR changes betweem T2DM patients using CGM or SMBG/UC | |
| pre-post PROMs change [Adherence] (follow-up: mean 4 months) | ||||||||||||
| 1 | randomized trials | serious [a] | very serious [b] | serious [c] | serious [d] | none | 51 | 48 |
SMD 0.06 SD higher (0.33 lower to 0.46 higher) |
⨁◯◯◯ Very low |
No significant difference in treatment adherence change between CGM or SMBG/UC | |
| pre-post PROMs change [Distress] (follow-up: mean 6.43 months) | ||||||||||||
| 7 | randomized trials | serious [a] | very serious [b] | serious [c] | serious [d] | none | 511 | 427 |
SMD 0.66 SD higher (0.79 lower to 2.11 higher) |
⨁◯◯◯ Very low |
No significant difference in diabetes-related distress change between CGM or SMBG/UC | |
| pre-post PROMs change [Empowerment] (follow-up: mean 7.67 months) | ||||||||||||
| 3 | randomized trials | serious [a] | very serious [b] | serious [c] | serious [d] | none | 246 | 239 |
SMD 0.10 SD higher (0.24 lower to 0.45 higher) |
⨁◯◯◯ Very low |
No significant difference in patient empowerment change between CGM or SMBG/UC | |
| pre-post PROMs change [Patient Education] (follow-up: mean 5 months) | ||||||||||||
| 1 | randomized trials | serious [a] | very serious [b] | serious [c] | serious [d] | none | 20 | 10 |
SMD 0.26 SD higher (0.46 lower to 0.97 higher) |
⨁◯◯◯ Very low |
No significant difference in patient education change between CGM or SMBG/UC | |
| pre-post PROMs change [Quality-of-Life] (follow-up: mean 6.90 months) | ||||||||||||
| 5 | randomized trials | serious [a] | very serious [b] | serious [c] | serious [d] | none | 339 | 328 |
SMD 0.43 SD higher (0.14 lower to 0.99 higher) |
⨁◯◯◯ Very low |
No significant difference in quality-of-life change between CGM or SMBG/UC | |
| pre-post PROMs change [Satisfaction] (follow-up: mean 6.13 months) | ||||||||||||
| 8 | randomized trials | serious [a] | very serious [b] | serious [c] | serious [d] | none | 479 | 385 |
SMD 1.32 SD higher (0.65 lower to 3.29 higher) |
⨁◯◯◯ Very low |
No significant difference in treatment satisfaction change between CGM or SMBG/UC | |
| pre-post PROMs change [Self-Management] (follow-up: mean 6.17 months) | ||||||||||||
| 6 | randomized trials | serious [a] | very serious [b] | serious [c] | serious [d] | none | 290 | 288 |
SMD 0.68 SD higher (0.37 lower to 1.73 higher) |
⨁◯◯◯ Very low |
No significant difference in diabetes self-management change between CGM or SMBG/UC | |
CGM continuous glucose monitoring, CI confidence interval, MD Mean difference, PROMs Patient-reported outcome measure, SMBG/UC Self-monitoring of blood glucose or usual care, SD Standard deviation, SMD Standardized mean difference
Explanations
aPrimary studies poorly documented, or did not carry out, proper allocation concealment, and some studies had incomplete outcome reporting
bPrimary studies assessing quality-of-life used different quality-of-life questionnaires, which led to inconsistencies in data collection and outcome measurement, even within domains. This is especially so given the content and objectives of the questionnaires deployed were significantly different (e.g. comparing the PAID and DDS), which constitutes as heterogenous exposure to experience recall and outcome measurement amongst participants
cSome primary studies used questionnaires that studied not quality-of-life, but related constructs such as diabetes-related distress
dWithin-study variance was significant, and extensive subgroup analysis was unable to identify sources of heterogeneity across studies, leading to unexplained imprecision of results
Discussion
To our knowledge, this is the first UR to consolidate the evidence base for comparing CGM with SMBG/UC in T2DM outcomes. We found that currently published SRs strongly concur that the CGM was associated with greater HbA1c reduction and time-in-range (TIR) increase as compared to SMBG, yielding better outcomes in glycemic control. The SRs also concurred that the use of CGM was not associated with significant adverse events, except for cutaneous complications that resolved with topicals. However, the evidence for the impact of CGM use on patient reported outcome measures (PROMs) was mixed.
HbA1c
Our meta-analysis robustly concurred with the findings from previous SRs that CGM use led to significantly greater HbA1c reduction than SMBG. The mean difference in pre-post HbA1c change of 0.42% estimated by our meta-analysis of RCTs was greater than the minimal clinically important difference (MCID) of 0.30% for HbA1c change to be clinically and statistically significant in decreasing diabetic complications [123, 124]. Hence, it is reasonable to infer that CGM may be associated with improved diabetes outcomes compared to SMBG/UC. However, similar to the meta-analyses done by the included SRs, there was high heterogeneity amongst the primary studies. This was expected given the wide geographic distribution and temporal range of the primary studies, employment of different CGM modalities, and variability in insulin therapies initiated in recruited participants. The latter point reflects not only a therapeutic heterogeneity but possible heterogeneity in the T2DM severity of recruited populations across studies. Of note, insulin resistance was not robustly incorporated into sensitivity analyses across included SRs apart from meta-regression on baseline HbA1c values. This might be due to insufficient evaluation of insulin resistance across primary studies. Thus, future studies may consider quantitatively evaluating baseline insulin resistance through methods such as oral glucose tolerance tests or fasting insulin levels [125, 126].
CGM modality was a strong source of heterogeneity. Retrospective CGM (rCGM) and real-time CGM (rtCGM) subgroups had low heterogeneity, while the flash glucose monitoring (FGM) subgroup was highly heterogeneous. This might be so as FGM efficacy is significantly contingent on patient compliance, given that patients are required to actively scan their sensor to record a glycemic reading. A recent SR recommended the indication of FGM for patients with T2DM only if they (1) are highly motivated to scan their devices and (2) possess low hypoglycemia risk [127], highlighting the significant dependence of FGM on patient compliance for therapeutic benefit. Hence, variation in patient compliance to scanning across FGM trials might contribute to subgroup heterogeneity.
Many primary studies were funded by medical technology companies with products for diabetes management, especially CGM devices, in the market, which raises concerns over bias and conflicts of interest [128–132]. However, our meta-analysis demonstrated that summary effect size of changes in HbA1c, TIR, time-above-range (TAR) and time-below-range (TBR) were invariant with study funding. Hence, concerns of industrial and funding bias might not be significant.
Glycemic control and variability
TIR reporting is useful given the international consensus on its applicability as a clinical endpoint in diabetes management [133], with a call for standardization of CGM data reporting amongst clinicians and researchers alike. Previous studies have also established an inverse association between TIR and end-stage diabetes complications, such as retinopathy [23, 134], neuropathy [135], renal damage [23, 136], cardiovascular complications and all-cause mortality [137], reinforcing its clinical significance. Although included SRs largely concur that CGM use is associated with greater TIR, other glycemic variability (GV) measures were either unreported or vague.
Our meta-analysis findings on TIR robustly concurred with our narrative synthesis of SR findings, specifically that there was a significant increase in TIR amongst CGM participants over SMBG/UC participants. Furthermore, the mean difference in pre-post TIR change of 6.0% estimated by the meta-analysis was greater than the MCID of 5%, which approximates to an additional one hour of blood sugar levels within range and was associated with lower rates of diabetes-related complications [138, 139]. This finding concurred with the clinically meaningful difference in HbA1c change estimated in our meta-analysis with CGM use compared to SMBG/UC. However, a significant increase in TIR was only observed amongst rtCGM users in our meta-analysis, and not FGM or rCGM. This may be due to the unblinded nature of rtCGM which educates users on their day-to-day GV without the need for doctor’s consult or patient education from other healthcare professionals in rCGM or inconvenience of scanning the device in FGM [8, 140]. Meanwhile, our findings for TAR and TBR were not as conclusive, which concurred with our narrative synthesis of SR findings. This may be due to the smaller sample sizes and effect sizes observed. Of note, rtCGM subgroups were of lowest heterogeneity in both meta-analyses, which supported our findings on rtCGM in TIR.
Patient reported outcome measures
Our meta-analysis could not robustly conclude if CGM use was associated with increased PROM scores compared to SMBG/UC. This was in keeping with our narrative synthesis of PROM outcomes reported by included SRs. Furthermore, we noted a lack of standardization in testing of PROMs across CGM trials, both in the domains assessed and questionnaires used within a domain, which may decrease the comparability of findings in the field. This represented a gap in CGM research, where quality-of-life amongst patients with T2DM has not been adequately characterized. Beyond quantitative evaluations of quality-of-life, patient experience of CGM in diabetes management, extending to even caregivers, family and the workplace, has been thoroughly characterized for T1DM [141–145] by many qualitative studies, to the point where a meta-synthesis was done [146]. Yet, comparatively few qualitative studies have been done to understand the same for patients with T2DM exclusively. Of the few which did, a study found that amongst youths and adolescents, CGM was better received than SMBG as it eliminated the need to do multiple finger-pricks across the day, and the real-time blood glucose data empowered patients to make lifestyle adjustments [147]. Thus, more work is needed to better understand the experience of T2DM patients in using CGM for widespread clinical adoption and higher treatment compliance.
Methodological issues across primary studies and systematic reviews
The UR consolidated methodological gaps across the primary studies reviewed by the included SRs. It was concerning that all RCTs were either of moderate or high risk-of-bias, as it reduces confidence in the conclusion that CGM leads to better outcomes for patients with T2DM than SMBG. The primary study meta-analyses subgrouped by overall risk-of-bias were not particularly revealing as well, which we hypothesized to be due to consistent methodological gaps across these studies. However, a case may be made that blinding participants to outcomes measured using CGM devices in the respective studies would contradict the behavioral modification element of CGM-based therapy, especially for rtCGM and FGM modalities. Hence, hypothetical studies which blinded participants to CGM data would be irrelevant to clinical practice except in the case of rCGM. Furthermore, in the setting of CGM trials, consideration of adherence to CGM usage and wear-time must be made especially in pragmatic trials. With comprehensive reporting on such CGM usage metrics, investigators can better contextualize the efficacy of CGM in blood sugar control with respect to individual use patterns.
The UR incidentally found methodological gaps in the conduct of SRs consistent across included studies, as described in the Results. Many SRs failed to describe the rationale for including studies of a particular design or designs. Thus, inherent biases of study types, such as confounding in NRSIs, were not adequately explored and discussed, which lowered the certainty of the SRs’ conclusions. Many meta-analyses did not explore heterogeneity through subgroup analysis or meta-regression. This reduced the explanatory power and validity of the SRs’ meta-analyses. Lastly, insufficient reporting of funding sources of primary studies amongst SRs complicated the assessment of industry influence on the execution and outcomes of primary studies.
These methodological gaps are concerning as clinical practice guidelines (CPGs) worldwide are increasingly based on SRs published, as recommended by international consensus bodies [148, 149]. It follows that if SRs are conducted with low methodological rigor, then the resultant CPGs formulated based on those SRs may be founded on poor evidence, which may compromise patient care and safety. This observation is echoed in other fields as well using tools such as AMSTAR [150], AMSTAR-2 [151] and GRADE [152]. Thus, conducting an umbrella review would serve as an additional checkpoint on the evidence certainty and potential risks-of-bias in these SRs, thereby ensuring that subsequent CPGs are developed upon high quality evidence.
Research and practical recommendations
Medical boards worldwide can explore the incorporation of CGM into prevailing national CPGs for the management of T2DM. The United States has formally recommended CGM for the management of both T1DM and T2DM recently [153], which indicates a gradual acceptance of CGM as a standard of care for T2DM. Beyond incorporation into CPGs, institutions can explore training healthcare professionals to be competent in (1) the indication of the appropriate CGM therapy for the appropriate T2DM patient and (2) interpreting the CGM results holistically apart from the HbA1c and TIR values solely.
A related development in patient-centric T2DM therapeutics is that of closed-loop automatic insulin delivery (AID) systems, where insulin is automatically infused to a patient according to their glycemic status as monitored by a CGM [154]. Recent trials demonstrated the efficacy of AIDs in improving glycemic control whilst minimizing hypoglycemic events [155, 156]. Given the difficulty of glycemic management in T2DM patients due to insulin resistance and higher body mass, AIDs are a potentially safer and less distressing alternative to insulin injections for T2DM management.
Another development in T2DM therapeutics is that of novel pharmacologics, especially sodium-glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP1-RA). Although these drugs have additional benefits such as cardioprotective effects and weight loss [157], the risk of hypoglycemia is not well-understood when these drugs are taken with conventional OHAs or insulin. As such, recent studies have utilized CGM to understand the blood sugar dynamics of patients on these novel drug combinations [158–160], as well as that of high-risk populations, such as people who are fasting [161], using SGLT2is and GLP1-RAs. Thus, CGM remains a powerful tool to evaluate glycemic changes following novel interventions so as to better understand their safety profile.
Of note, patient education is an increasingly important factor in the management of chronic diseases, including that of T2DM. CGM has been studied as a patient education tool where practitioners used CGM to help patients with T2DM understand how certain lifestyle and dietary choices would change their blood sugar [162–164]. This was associated with not only better glycemic control, but also greater satisfaction and lower diabetes-related distress [11, 164]. Yet, these benefits are contingent on sufficient counseling on the healthcare professional’s part to help patients learn how to interpret the results on their CGM interfaces as well as diet, exercise and glucose excursion education [162, 163]. Thus, more work might be needed to better understand the role of CGM in patient education and activation towards optimal glycemic control amongst adults with T2DM.
There are multiple gaps in CGM research. Firstly, multi-center trials recruiting more participants should be conducted to further validate the efficacy of CGM in all aspects across other demographics. Secondly, more cost-effectiveness studies should be done to guide healthcare resource allocation and public copayment models for potential CGM subsidies. Thirdly, more quantitative and qualitative studies on PROMs with CGM use should be done to further improve CGM devices and therapies for patient-centric use.
Strengths and limitations
The strengths of our UR include the comprehensive search of literature conducted and the breadth of sensitivity analysis done in the meta-analysis. To ensure that the results produced were not spurious, we were conservative in our imputation to avoid overestimating effect estimates. Sensitivity analyses with different correlation coefficients confirmed that imputation did not cause the data to vary significantly and were reported in the Supplement to address methodological issues in data imputation. A protocol was also clearly defined for handling and reporting overlaps in meta-analyses included in our SRs. Hence, our meta-analysis is the most contemporaneous at the point of writing and devoid of overlaps. Lastly, our meta-analysis also comprehensively explored sources of heterogeneity amongst the included primary studies, eventually drawing conclusions on how CGM modality, commercial funding and insulin therapy might affect the effectiveness of CGM.
There were limitations in the included studies and meta-analysis. Firstly, many SRs included studies investigating both T1DM and T2DM patients. This may have reduced the extent of outcome reporting and synthesis for T2DM patients on CGM by these SRs. We mitigated this by ensuring that extracted results discussed in this UR were specific for T2DM patients. Secondly, a significant number of outcomes were imputed in the HbA1c reduction meta-analysis, as some studies did not report a pre-post within-group HbA1c change score. We mitigated this through the aforementioned sensitivity analyses by varying the correlation coefficient for imputation. Furthermore, comparing our final meta-analysis with that of the included meta-analyses, we noticed no significant differences in effect size and only moderate differences in the standard deviation for the same studies, respectively. Lastly, although the most recent SRs included in our study was published in 2024, yet the most recent primary study included across the SRs and analyzed in this UR was published in 2022. This represented a gap in CGM primary data, which may have incorporated recent developments in therapeutics and patient education discussed previously.
Conclusion
CGM was demonstrated to be associated with greater HbA1c and TAR reductions and TIR increase than SMBG/UC in patients with T2DM, and this finding was of moderate evidence certainty. Our findings were mostly invariant with funding status, pre-existing insulin treatment, CGM modality and risk-of-bias of primary studies.. Various PROMs were found to not differ significantly between groups using CGM and SMBG/UC, and the overall evidence was of very low certainty due to the significant inconsistency in assessing PROMs and the low sample size. Countries can explore the incorporation of CGM into standard care and clinical practice guidelines for the management of T2DM. Qualitative studies exploring the experience and acceptability of CGM in T2DM patients are also strongly recommended to facilitate wider clinical adoption and greater patient acceptability of CGM.
Supplementary Information
Acknowledgements
Not applicable.
Authors’ contributions
YYT conceptualized the methodology, did the literature review, designed and executed the systematic review, performed the meta-analysis and wrote the manuscript. EHS participated in the title-abstract selection, data extraction and quality appraisal. GCKH conceptualized the study and edited the manuscript. SBS participated in the title-abstract selection, data extraction and quality appraisal. ST conceptualized the study, provided oversight for the systematic review and edited the manuscript. All authors have read and approved the submitted manuscript.
Authors’ information
Not applicable.
Funding
The authors report no funding for this study.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes – Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE. Relation of Glycemic Control to Diabetic Complications and Health Outcomes. Diabetes Care. 1998;21:C39–43. [DOI] [PubMed] [Google Scholar]
- 3.Stolar M. Glycemic Control and Complications in Type 2 Diabetes Mellitus. Am J Med. 2010;123:S3–11. [DOI] [PubMed] [Google Scholar]
- 4.Andersson DK, Svärdsudd K. Long-Term Glycemic Control Relates to Mortality in Type II Diabetes. Diabetes Care. 1995;18:1534–43. [DOI] [PubMed] [Google Scholar]
- 5.Klonoff DC (2005) Continuous Glucose Monitoring. DIABETES CARE 28: [DOI] [PubMed]
- 6.Mazze RS, Strock E, Wesley D, Borgman S, Morgan B, Bergenstal R, Cuddihy R. Characterizing Glucose Exposure for Individuals with Normal Glucose Tolerance Using Continuous Glucose Monitoring and Ambulatory Glucose Profile Analysis. Diabetes Technol Ther. 2008;10:149–59. [DOI] [PubMed] [Google Scholar]
- 7.Ong WM, Chua SS, Ng CJ. Barriers and facilitators to self-monitoring of blood glucose in people with type 2 diabetes using insulin: a qualitative study. Patient Prefer Adherence. 2014;8:237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sergel-Stringer OT, Wheeler BJ, Styles SE, Boucsein A, Lever CS, Paul RG, Sampson R, Watson A, de Bock MI. Acceptability and experiences of real-time continuous glucose monitoring in adults with type 2 diabetes using insulin: a qualitative study. J Diabetes Metab Disord. 2024;23:1163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vettoretti M, Facchinetti A. Combining continuous glucose monitoring and insulin pumps to automatically tune the basal insulin infusion in diabetes therapy: a review. Biomed Eng OnLine. 2019;18:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubelj K, Stipančić G, La Grasta SL, Požgaj Šepec M. Continuous glucose monitoring and type 1 diabetes mellitus control in child, adolescent and young adult population – arguments for its use and effects. Acta Clin Croat. 2021;60:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor PJ, Thompson CH, Brinkworth GD. Effectiveness and acceptability of continuous glucose monitoring for type 2 diabetes management: A narrative review. J Diabetes Investig. 2018;9:713–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ten Brinke R, Dekker N, de Groot M, Ikkersheim D. Lowering HbA1c in type 2 diabetics results in reduced risk of coronary heart disease and all-cause mortality. Prim Care Diabetes. 2008;2:45–9. [DOI] [PubMed] [Google Scholar]
- 13.Luk AOY, Ma RCW, Lau ESH, Yang X, Lau WWY, Yu LWL, Chow FCC, Chan JCN, So W-Y. Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: prospective analysis of the Hong Kong Diabetes Registry. Diabetes Metab Res Rev. 2013;29:384–90. [DOI] [PubMed] [Google Scholar]
- 14.Ishibashi F, Taniguchi M, Kosaka A, Uetake H, Tavakoli M. Improvement in Neuropathy Outcomes With Normalizing HbA1c in Patients With Type 2 Diabetes. Diabetes Care. 2018;42:110–8. [DOI] [PubMed] [Google Scholar]
- 15.Genis-Mendoza AD, González-Castro TB, Tovilla-Vidal G, Juárez-Rojop IE, Castillo-Avila RG, López-Narváez ML, Tovilla-Zárate CA, Sánchez-de la Cruz JP, Fresán A, Nicolini H. Increased Levels of HbA1c in Individuals with Type 2 Diabetes and Depression: A Meta-Analysis of 34 Studies with 68,398 Participants. Biomedicines. 2022;10:1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold LW, Wang Z. The HbA1c and All-Cause Mortality Relationship in Patients with Type 2 Diabetes is J-Shaped: A Meta-Analysis of Observational Studies. Rev Diabet Stud RDS. 2014;11:138–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright LA-C, Hirsch IB (2017) Metrics Beyond Hemoglobin A1C in Diabetes Management: Time in Range, Hypoglycemia, and Other Parameters. Diabetes Technol Ther 19:S-16 [DOI] [PMC free article] [PubMed]
- 18.Qu Y, Jacober SJ, Zhang Q, Wolka LL, DeVries JH. Rate of hypoglycemia in insulin-treated patients with type 2 diabetes can be predicted from glycemic variability data. Diabetes Technol Ther. 2012;14:1008–12. [DOI] [PubMed] [Google Scholar]
- 19.Monnier L, Wojtusciszyn A, Colette C, Owens D. The contribution of glucose variability to asymptomatic hypoglycemia in persons with type 2 diabetes. Diabetes Technol Ther. 2011;13:813–8. [DOI] [PubMed] [Google Scholar]
- 20.Beyond A1C Writing Group. Need for Regulatory Change to Incorporate Beyond A1C Glycemic Metrics. Diabetes Care. 2018;41:e92–4. [DOI] [PubMed] [Google Scholar]
- 21.Danne T, Nimri R, Battelino T, et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care. 2017;40:1631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raj R, Mishra R, Jha N, Joshi V, Correa R, Kern PA. Time in range, as measured by continuous glucose monitor, as a predictor of microvascular complications in type 2 diabetes: a systematic review. BMJ Open Diabetes Res Care. 2022;10:e002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li Z, Brown AS, Close KL. Validation of Time in Range as an Outcome Measure for Diabetes Clinical Trials. Diabetes Care. 2018;42:400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee I, Probst D, Klonoff D, Sode K. Continuous glucose monitoring systems - Current status and future perspectives of the flagship technologies in biosensor research -. Biosens Bioelectron. 2021;181:113054. [DOI] [PubMed] [Google Scholar]
- 25.Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: A review of the technology and clinical use. Diabetes Res Clin Pract. 2017;133:178–92. [DOI] [PubMed] [Google Scholar]
- 26.Di Molfetta S, Caruso I, Cignarelli A, Natalicchio A, Perrini S, Laviola L, Giorgino F. Professional continuous glucose monitoring in patients with diabetes mellitus: A systematic review and meta-analysis. Diabetes Obes Metab. 2023;25:1301–10. [DOI] [PubMed] [Google Scholar]
- 27.Maiorino MI, Signoriello S, Maio A, Chiodini P, Bellastella G, Scappaticcio L, Longo M, Giugliano D, Esposito K. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care. 2020;43:1146–56. [DOI] [PubMed] [Google Scholar]
- 28.Ang E, Lee ZX, Moore S, Nana M. Flash glucose monitoring (FGM): A clinical review on glycaemic outcomes and impact on quality of life. J Diabetes Complications. 2020;34: 107559. [DOI] [PubMed] [Google Scholar]
- 29.Cowart K, Updike W, Bullers K. Systematic Review of Randomized Controlled Trials Evaluating Glycemic Efficacy and Patient Satisfaction of Intermittent-Scanned Continuous Glucose Monitoring in Patients with Diabetes. Diabetes Technol Ther. 2020;22:337–45. [DOI] [PubMed] [Google Scholar]
- 30.Evans M, Welsh Z, Ells S, Seibold A. The Impact of Flash Glucose Monitoring on Glycaemic Control as Measured by HbA1c: A Meta-analysis of Clinical Trials and Real-World Observational Studies. Diabetes Ther. 2020;11:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Y, Zhou M, Xu X, Chen WY. Effects of flash glucose monitoring on glycemic control in participants with diabetes mellitus: A meta-analysis of randomized controlled trials. J Diabetes Complications. 2022;36:108314. [DOI] [PubMed] [Google Scholar]
- 32.(2019) Flash Glucose Monitoring System for People with Type 1 or Type 2 Diabetes: A Health Technology Assessment. Ont Health Technol Assess Ser 19:1–108 [PMC free article] [PubMed]
- 33.Asarani NAM, Reynolds AN, Boucher SE, de Bock M, Wheeler BJ. Cutaneous Complications With Continuous or Flash Glucose Monitoring Use: Systematic Review of Trials and Observational Studies. J Diabetes Sci Technol. 2020;14:328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ida S, Kaneko R, Murata K. Utility of Real-Time and Retrospective Continuous Glucose Monitoring in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. J Diabetes Res. 2019;2019:e4684815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoeks LBEA, Greven WL, de Valk HW. Real-time continuous glucose monitoring system for treatment of diabetes: a systematic review. Diabet Med. 2011;28:386–94. [DOI] [PubMed] [Google Scholar]
- 36.Dicembrini I, Mannucci E, Monami M, Pala L. Impact of technology on glycaemic control in type 2 diabetes: A meta-analysis of randomized trials on continuous glucose monitoring and continuous subcutaneous insulin infusion. Diabetes Obes Metab. 2019;21:2619–25. [DOI] [PubMed] [Google Scholar]
- 37.Lu J, Ying Z, Wang P, Fu M, Han C, Zhang M. Effects of continuous glucose monitoring on glycaemic control in type 2 diabetes: A systematic review and network meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2024;26:362–72. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira ROM, Trevisan T, Pasqualotto E, Chavez MP, Marques BF, Lamounier RN, van de Sande-Lee S. Continuous Glucose Monitoring Systems in Noninsulin-Treated People with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Technol Ther. 2024;26:252–62. [DOI] [PubMed] [Google Scholar]
- 39.Seidu S, Kunutsor SK, Ajjan RA, Choudhary P. Efficacy and Safety of Continuous Glucose Monitoring and Intermittently Scanned Continuous Glucose Monitoring in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis of Interventional Evidence. Diabetes Care. 2023;47:169–79. [DOI] [PubMed] [Google Scholar]
- 40.Uhl S, Choure A, Rouse B, Loblack A, Reaven P. Effectiveness of Continuous Glucose Monitoring on Metrics of Glycemic Control in Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J Clin Endocrinol Metab. 2024;109:1119–31. [DOI] [PubMed] [Google Scholar]
- 41.Gandhi GY, Kovalaske M, Kudva Y, et al. Efficacy of Continuous Glucose Monitoring in Improving Glycemic Control and Reducing Hypoglycemia: A Systematic Review and Meta-Analysis of Randomized Trials. J Diabetes Sci Technol. 2011;5:952–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong S-Y, Cho M-K. Effects of Continuous Glucose Monitoring on Glycemic Control in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Healthcare. 2024;12:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gates M, Gates A, Pieper D, et al. Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement. BMJ. 2022;378:e070849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollock M, Fernandes R, Becker L, Pieper D, Hartling L Chapter V: Overviews of Reviews In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
- 45.da Costa Santos CM, de Mattos Pimenta CA, Nobre MRC. The PICO strategy for the research question construction and evidence search. Rev Lat Am Enfermagem. 2007;15:508–11. [DOI] [PubMed] [Google Scholar]
- 46.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. [DOI] [PubMed] [Google Scholar]
- 48.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 49.Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG, Group ISTC, Group ECSTC (2003) Evaluating non-randomised intervention studies. Health Technol Assess Winch Engl 7:iii–173 [DOI] [PubMed]
- 50.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016. 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pieper D, Antoine S-L, Mathes T, Neugebauer EAM, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67:368–75. [DOI] [PubMed] [Google Scholar]
- 52.Pérez-Bracchiglione J, Meza N, Bangdiwala SI, Niño de Guzmán E, Urrútia G, Bonfill X, Madrid E. Graphical Representation of Overlap for OVErviews: GROOVE tool. Res Synth Methods. 2022;13:381–8. [DOI] [PubMed] [Google Scholar]
- 53.Tan BL, Tong HJ, Narashimhan S, Banihani A, Nazzal H, Duggal MS. Tooth autotransplantation: An umbrella review. Dent Traumatol. 2023;39:2–29. [DOI] [PubMed] [Google Scholar]
- 54.R Core Team R, others (2013) R: A language and environment for statistical computing.
- 55.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 56.Balduzzi S, Rücker G, Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 153–160 [DOI] [PMC free article] [PubMed]
- 57.Deeks JJ, Higgins J, Altman DG, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1. 0 (updated March 2011). Cochrane Collab 2:5
- 58.Langan D, Higgins JPT, Jackson D, Bowden J, Veroniki AA, Kontopantelis E, Viechtbauer W, Simmonds M. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10:83–98. [DOI] [PubMed] [Google Scholar]
- 59.Sterne JA, Egger M (2005) Regression methods to detect publication and other bias in meta-analysis. Publ Bias Meta-Anal Prev Assess Adjust 99–110
- 60.Hariton E, Locascio JJ. Randomised controlled trials—the gold standard for effectiveness research. BJOG Int J Obstet Gynaecol. 2018;125:1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baujat B, Mahé C, Pignon J-P, Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med. 2002;21:2641–52. [DOI] [PubMed] [Google Scholar]
- 62.Jenkins DG, Quintana-Ascencio PF. A solution to minimum sample size for regressions. PLoS ONE. 2020;15:e0229345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. [DOI] [PubMed] [Google Scholar]
- 64.Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr. 2013;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aggarwal A, Pathak S, Goyal R. Clinical and economic outcomes of continuous glucose monitoring system (CGMS) in patients with diabetes mellitus: A systematic literature review. Diabetes Res Clin Pract. 2022;186:109825. [DOI] [PubMed] [Google Scholar]
- 66.Azhar A, Gillani SW, Mohiuddin G, Majeed RA. A systematic review on clinical implication of continuous glucose monitoring in diabetes management. J Pharm Bioallied Sci. 2020;12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bidonde J, Fagerlund BC, Frønsdal KB, Lund UH, Robberstad B (2017) FreeStyle Libre Flash Glucose Self-Monitoring System: A Single-Technology Assessment. Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH), Oslo, Norway [PubMed]
- 68.Castellana M, Parisi C, Molfetta SD, Gioia LD, Natalicchio A, Perrini S, Cignarelli A, Laviola L, Giorgino F. Efficacy and safety of flash glucose monitoring in patients with type 1 and type 2 diabetes: a systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2020;8:e001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.García-Lorenzo B, Rivero-Santana A, Vallejo-Torres L, Castilla-Rodríguez I, García-Pérez S, García-Pérez L, Perestelo-Pérez L. Cost-effectiveness analysis of real-time continuous monitoring glucose compared to self-monitoring of blood glucose for diabetes mellitus in Spain. J Eval Clin Pract. 2018;24:772–81. [DOI] [PubMed] [Google Scholar]
- 70.Janapala RN, Jayaraj JS, Fathima N, Kashif T, Usman N, Dasari A, Jahan N, Sachmechi I Continuous Glucose Monitoring Versus Self-monitoring of Blood Glucose in Type 2 Diabetes Mellitus: A Systematic Review with Meta-analysis. Cureus 11:e5634 [DOI] [PMC free article] [PubMed]
- 71.Kieu A, King J, Govender RD, Östlundh L. The Benefits of Utilizing Continuous Glucose Monitoring of Diabetes Mellitus in Primary Care: A Systematic Review. J Diabetes Sci Technol. 2023;17:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mattishent K, Loke Y. Detection of asymptomatic drug-induced hypoglycemia using continuous glucose monitoring in older people–systematic review. J Diabetes Complications. 2018;32:805–12. [DOI] [PubMed] [Google Scholar]
- 73.Meade LT. The use of continuous glucose monitoring in patients with type 2 diabetes. Diabetes Technol Ther. 2012;14:190–5. [DOI] [PubMed] [Google Scholar]
- 74.Park C, Le QA. The Effectiveness of Continuous Glucose Monitoring in Patients with Type 2 Diabetes: A Systematic Review of Literature and Meta-analysis. Diabetes Technol Ther. 2018;20:613–21. [DOI] [PubMed] [Google Scholar]
- 75.Pleus S, Ulbrich S, Zschornack E, Kamann S, Haug C, Freckmann G. Documentation of Skin-Related Issues Associated with Continuous Glucose Monitoring Use in the Scientific Literature. Diabetes Technol Ther. 2019;21:538–45. [DOI] [PubMed] [Google Scholar]
- 76.Torres Roldan VD, Urtecho M, Nayfeh T, et al. A Systematic Review Supporting the Endocrine Society Guidelines: Management of Diabetes and High Risk of Hypoglycemia. J Clin Endocrinol Metab. 2023;108:592–603. [DOI] [PubMed] [Google Scholar]
- 77.Jancev M, Vissers TACM, Visseren FLJ, van Bon AC, Serné EH, DeVries JH, de Valk HW, van Sloten TT. Continuous glucose monitoring in adults with type 2 diabetes: a systematic review and meta-analysis. Diabetologia. 2024;67:798–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blackberry ID, Furler JS, Ginnivan LE, et al. An exploratory trial of basal and prandial insulin initiation and titration for type 2 diabetes in primary care with adjunct retrospective continuous glucose monitoring: INITIATION study. Diabetes Res Clin Pract. 2014;106:247–55. [DOI] [PubMed] [Google Scholar]
- 79.Cai ZQ, Wu S. Observation on the effect of scanning glucose monitoring system in diabetic participants. Contemp Med. 2019;25:173–4. [Google Scholar]
- 80.Ehrhardt NM, Chellappa M, Walker MS, Fonda SJ, Vigersky RA. The Effect of Real-Time Continuous Glucose Monitoring on Glycemic Control in Patients with Type 2 Diabetes Mellitus. J Diabetes Sci Technol. 2011;5:668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Furler J, O’Neal D, Speight J, et al. Use of professional-mode flash glucose monitoring, at 3-month intervals, in adults with type 2 diabetes in general practice (GP-OSMOTIC): a pragmatic, open-label, 12-month, randomised controlled trial. Lancet Diabetes Endocrinol. 2020;8:17–26. [DOI] [PubMed] [Google Scholar]
- 82.Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline J-P, Rayman G. Use of Flash Glucose-Sensing Technology for 12 months as a Replacement for Blood Glucose Monitoring in Insulin-treated Type 2 Diabetes. Diabetes Ther. 2017;8:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Q, Xuan W, Zhang Z. Analysis of the effect of different blood glucose monitoring methods on blood glucose control in participants with type 2 diabetes mellitus. Med Innov China. 2020;17:136–9. [Google Scholar]
- 84.Tildesley HD, Wright AM, Chan JHM, Mazanderani AB, Ross SA, Tildesley HG, Lee AM, Tang TS, White AS. A Comparison of Internet Monitoring with Continuous Glucose Monitoring in Insulin-Requiring Type 2 Diabetes Mellitus. Can J Diabetes. 2013;37:305–8. [DOI] [PubMed] [Google Scholar]
- 85.Wada E, Onoue T, Kobayashi T, et al. Flash glucose monitoring helps achieve better glycemic control than conventional self-monitoring of blood glucose in non-insulin-treated type 2 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care. 2020;8:e001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang HL. The application of ambulatory glucose monitoring in participants with type 2 diabetes mellitus. Med Forum. 2021;25:1031–3. [Google Scholar]
- 87.Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178–84. [DOI] [PubMed] [Google Scholar]
- 88.Yeoh E, Lim BK, Fun S, Tong J, Yeoh LY, Sum CF, Subramaniam T, Lim SC. Efficacy of self-monitoring of blood glucose versus retrospective continuous glucose monitoring in improving glycaemic control in diabetic kidney disease patients. Nephrology. 2018;23:264–8. [DOI] [PubMed] [Google Scholar]
- 89.Zhang W, Liu P, Ma SR, et al. The value of rapid glucose monitoring in participants with type 2 diabetes mellitus. Chin J Cardiovasc Rehabil. 2021;30:416–9. [Google Scholar]
- 90.Zhang X, Yan Y, Peng L, et al. The application of transient scanning glucose monitoring system in elderly diabetic participants. Med Inf. 2021;34:180–2. [Google Scholar]
- 91.Zhou M, Li X, Tu N, et al. The application of ambulatory glucose monitoring system in the management of type 2 diabetes mellitus. Chin J Prev Control Chronic Dis. 2021;29:700–2. [Google Scholar]
- 92.Zhou M, Tu N, Xu X, et al. Effectiveness of FGMS-based combined health coaching techniques in the management of participants with type 2 diabetes mellitus. Chin J Gerontol. 2020;40:3839–43. [Google Scholar]
- 93.Cox DJ, Banton T, Moncrief M, Conaway M, Diamond A, McCall AL (2020) Minimizing Glucose Excursions (GEM) With Continuous Glucose Monitoring in Type 2 Diabetes: A Randomized Clinical Trial. J Endocr Soc 4:bvaa118 [DOI] [PMC free article] [PubMed]
- 94.Price DA, Deng Q, Kipnes M, Beck SE. Episodic Real-Time CGM Use in Adults with Type 2 Diabetes: Results of a Pilot Randomized Controlled Trial. Diabetes Ther. 2021;12:2089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ajjan RA, Jackson N, Thomson SA. Reduction in HbA1c using professional flash glucose monitoring in insulin-treated type 2 diabetes patients managed in primary and secondary care settings: a pilot, multicentre, randomised controlled trial. Diab Vasc Dis Res. 2019;16:385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Allen NA, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract. 2008;80:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cosson E, Hamo-Tchatchouang E, Dufaitre-Patouraux L, Attali J-R, Pariès J, Schaepelynck-Bélicar P. Multicentre, randomised, controlled study of the impact of continuous sub-cutaneous glucose monitoring (GlucoDay®) on glycaemic control in type 1 and type 2 diabetes patients. Diabetes Metab. 2009;35:312–8. [DOI] [PubMed] [Google Scholar]
- 98.Ilany J, Bhandari H, Nabriski D, Toledano Y, Konvalina N, Cohen O. Effect of prandial treatment timing adjustment, based on continuous glucose monitoring, in patients with type 2 diabetes uncontrolled with once-daily basal insulin: A randomized, phase IV study. Diabetes Obes Metab. 2018;20:1186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tang TS, Digby EM, Wright AM, Chan JHM, Mazanderani AB, Ross SA, Tildesley HG, Lee AM, White AS, Tildesley HD. Real-time continuous glucose monitoring versus internet-based blood glucose monitoring in adults with type 2 diabetes: A study of treatment satisfaction. Diabetes Res Clin Pract. 2014;106:481–6. [DOI] [PubMed] [Google Scholar]
- 100.Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and Long-Term Effects of Real-Time Continuous Glucose Monitoring in Patients With Type 2 Diabetes. Diabetes Care. 2011;35:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ajjan RA, Abougila K, Bellary S, Collier A, Franke B, Jude EB, Rayman G, Robinson A, Singh BM. Sensor and software use for the glycaemic management of insulin-treated type 1 and type 2 diabetes patients. Diab Vasc Dis Res. 2016;13:211–9. [DOI] [PubMed] [Google Scholar]
- 102.Anjana RM, Kesavadev J, Neeta D, et al. A Multicenter Real-Life Study on the Effect of Flash Glucose Monitoring on Glycemic Control in Patients with Type 1 and Type 2 Diabetes. Diabetes Technol Ther. 2017;19:533–40. [PubMed] [Google Scholar]
- 103.Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous Glucose Monitoring Versus Usual Care in Patients With Type 2 Diabetes Receiving Multiple Daily Insulin Injections: A Randomized Trial. Ann Intern Med. 2017;167:365–74. [DOI] [PubMed] [Google Scholar]
- 104.Fantasia KL, Stockman M-C, Ju Z, Ortega P, Crable EL, Drainoni M-L, Walkey AJ, Bergstrom M, O’Brien K, Steenkamp D. Professional continuous glucose monitoring and endocrinology eConsult for adults with type 2 diabetes in primary care: Results of a clinical pilot program. J Clin Transl Endocrinol. 2021;24:100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kesavadev J, Vigersky R, Shin J, Pillai PBS, Shankar A, Sanal G, Krishnan G, Jothydev S. Assessing the Therapeutic Utility of Professional Continuous Glucose Monitoring in Type 2 Diabetes Across Various Therapies: A Retrospective Evaluation. Adv Ther. 2017;34:1918–27. [DOI] [PubMed] [Google Scholar]
- 106.Kim SK, Kim HJ, Kim T, Hur KY, Kim SW, Lee M-K, Min Y-K, Kim K-W, Chung JH, Kim JH. Effectiveness of 3-Day Continuous Glucose Monitoring for Improving Glucose Control in Type 2 Diabetic Patients in Clinical Practice. Diabetes Metab J. 2014;38:449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rivera-Ávila DA, Esquivel-Lu AI, Salazar-Lozano CR, Jones K, Doubova SV. The effects of professional continuous glucose monitoring as an adjuvant educational tool for improving glycemic control in patients with type 2 diabetes. BMC Endocr Disord. 2021;21:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sato J, Kanazawa A, Ikeda F, et al. Effect of treatment guidance using a retrospective continuous glucose monitoring system on glycaemic control in outpatients with type 2 diabetes mellitus: A randomized controlled trial. J Int Med Res. 2016;44:109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sierra JA, Shah M, Gill MS, Flores Z, Chawla H, Kaufman FR, Vigersky R. Clinical and economic benefits of professional CGM among people with type 2 diabetes in the United States: analysis of claims and lab data. J Med Econ. 2018;21:225–30. [DOI] [PubMed] [Google Scholar]
- 110.Thielen V, Scheen A, Bringer J, Renard E. Attempt to improve glucose control in type 2 diabetic patients by education about real-time glucose monitoring. Diabetes Metab. 2010;36:240–3. [DOI] [PubMed] [Google Scholar]
- 111.Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73–9. [DOI] [PubMed] [Google Scholar]
- 112.Simonson GD, Bergenstal RM, Johnson ML, Davidson JL, Martens TW. Effect of Professional CGM (pCGM) on Glucose Management in Type 2 Diabetes Patients in Primary Care. J Diabetes Sci Technol. 2021;15:539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Speight J, Holmes-Truscott E, Thuraisingam S, et al. Impact of quarterly professional-mode flash glucose monitoring in adults with type 2 diabetes in general practice (GP-OSMOTIC): Secondary psychological and self-care outcomes of a pragmatic, open-label, 12-month, randomised controlled trial. Diabetes Res Clin Pract. 2021;179:108994. [DOI] [PubMed] [Google Scholar]
- 114.Moon SJ, Kim K-S, Lee WJ, Lee MY, Vigersky R, Park C-Y. Efficacy of intermittent short-term use of a real-time continuous glucose monitoring system in non-insulin–treated patients with type 2 diabetes: A randomized controlled trial. Diabetes Obes Metab. 2023;25:110–20. [DOI] [PubMed] [Google Scholar]
- 115.Bergenstal RM, Mullen DM, Strock E, Johnson ML, Xi MX. Randomized comparison of self-monitored blood glucose (BGM) versus continuous glucose monitoring (CGM) data to optimize glucose control in type 2 diabetes. J Diabetes Complications. 2022;36:108106. [DOI] [PubMed] [Google Scholar]
- 116.Aronson R, Brown RE, Chu L, Bajaj HS, Khandwala H, Abitbol A, Malakieh N, Goldenberg R. IMpact of flash glucose Monitoring in pEople with type 2 Diabetes Inadequately controlled with non-insulin Antihyperglycaemic ThErapy (IMMEDIATE): A randomized controlled trial. Diabetes Obes Metab. 2023;25:1024–31. [DOI] [PubMed] [Google Scholar]
- 117.Taylor PJ, Thompson CH, Luscombe-Marsh ND, Wycherley TP, Wittert G, Brinkworth GD. Efficacy of Real-Time Continuous Glucose Monitoring to Improve Effects of a Prescriptive Lifestyle Intervention in Type 2 Diabetes: A Pilot Study. Diabetes Ther. 2019;10:509–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ajjan RA, Heller SR, Everett CC, et al. Multicenter Randomized Trial of Intermittently Scanned Continuous Glucose Monitoring Versus Self-Monitoring of Blood Glucose in Individuals With Type 2 Diabetes and Recent-Onset Acute Myocardial Infarction: Results of the LIBERATES Trial. Diabetes Care. 2022;46:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, Jackson RA. Assessing Psychosocial Distress in Diabetes: Development of the Diabetes Distress Scale. Diabetes Care. 2005;28:626–31. [DOI] [PubMed] [Google Scholar]
- 120.Group TW. Development of the World Health Organization WHOQOL-BREF Quality of Life Assessment. Psychol Med. 1998;28:551–8. [DOI] [PubMed] [Google Scholar]
- 121.Bradley C. Diabetes treatment satisfaction questionnaire. Change version for use alongside status version provides appropriate solution where ceiling effects occur. Diabetes Care. 1999;22:530–2. [DOI] [PubMed] [Google Scholar]
- 122.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23:943–50. [DOI] [PubMed] [Google Scholar]
- 123.Lind M, Odén A, Fahlén M, Eliasson B. The shape of the metabolic memory of HbA1c: re-analysing the DCCT with respect to time-dependent effects. Diabetologia. 2010;53:1093–8. [DOI] [PubMed] [Google Scholar]
- 124.Lind M, Polonsky W, Hirsch IB, et al. Continuous Glucose Monitoring vs Conventional Therapy for Glycemic Control in Adults With Type 1 Diabetes Treated With Multiple Daily Insulin Injections: The GOLD Randomized Clinical Trial. JAMA. 2017;317:379–87. [DOI] [PubMed] [Google Scholar]
- 125.Antuna-Puente B, Disse E, Rabasa-Lhoret R, Laville M, Capeau J, Bastard J-P. How can we measure insulin sensitivity/resistance? Diabetes Metab. 2011;37:179–88. [DOI] [PubMed] [Google Scholar]
- 126.McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, Duncan AW. Diagnosing insulin resistance in the general population. Diabetes Care. 2001;24:460–4. [DOI] [PubMed] [Google Scholar]
- 127.Edelman SV, Argento NB, Pettus J, Hirsch IB. Clinical Implications of Real-time and Intermittently Scanned Continuous Glucose Monitoring. Diabetes Care. 2018;41:2265–74. [DOI] [PubMed] [Google Scholar]
- 128.Krimsky S (2006) Publication Bias, Data Ownership, and the Funding Effect in Sciece: Threats to the Integrity of Biomedical Research. In: eweb:291343. https://repository.library.georgetown.edu/handle/10822/976495. Accessed 23 Aug 2023
- 129.Yaphe J, Edman R, Knishkowy B, Herman J. The association between funding by commercial interests and study outcome in randomized controlled drug trials. Fam Pract. 2001;18:565–8. [DOI] [PubMed] [Google Scholar]
- 130.Probst P, Knebel P, Grummich K, Tenckhoff S, Ulrich A, Büchler MW, Diener MK. Industry Bias in Randomized Controlled Trials in General and Abdominal Surgery. Ann Surg. 2016;264:87–92. [DOI] [PubMed] [Google Scholar]
- 131.Lexchin J. Sponsorship bias in clinical research. Int J Risk Saf Med. 2012;24:233–42. [DOI] [PubMed] [Google Scholar]
- 132.Landefeld CS. Commercial support and bias in pharmaceutical research. Am J Med. 2004;117:876–8. [DOI] [PubMed] [Google Scholar]
- 133.Battelino T, Danne T, Bergenstal RM, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019;42:1593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu J, Ma X, Zhou J, et al. Association of Time in Range, as Assessed by Continuous Glucose Monitoring, With Diabetic Retinopathy in Type 2 Diabetes. Diabetes Care. 2018;41:2370–6. [DOI] [PubMed] [Google Scholar]
- 135.Mayeda L, Katz R, Ahmad I, Bansal N, Batacchi Z, Hirsch IB, Robinson N, Trence DL, Zelnick L, de Boer IH. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care. 2020;8:e000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jin X, Yang X, Xu Y, et al. Differential correlation between time in range and eGFR or albuminuria in type 2 diabetes. Diabetol Metab Syndr. 2023;15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lu J, Wang C, Shen Y, et al. Time in Range in Relation to All-Cause and Cardiovascular Mortality in Patients With Type 2 Diabetes: A Prospective Cohort Study. Diabetes Care. 2020;44:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gabbay MAL, Rodacki M, Calliari LE, et al. Time in range: a new parameter to evaluate blood glucose control in patients with diabetes. Diabetol Metab Syndr. 2020;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dovc K, Battelino T. Time in range centered diabetes care. Clin Pediatr Endocrinol. 2021;30:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Soni A, Wright N, Agwu J, et al. A practical approach to continuous glucose monitoring (rtCGM) and FreeStyle Libre systems (isCGM) in children and young people with Type 1 diabetes. Diabetes Res Clin Pract. 2022;184:109196. [DOI] [PubMed] [Google Scholar]
- 141.Crocket H, Elbashy MM, Kavanagh T, et al. Parental experiences of short term supported use of a do-it-yourself continuous glucose monitor (DIYrtCGM): A qualitative study. Diabet Med. 2022;39:e14731. [DOI] [PubMed] [Google Scholar]
- 142.Lawton J, Blackburn M, Allen J, Campbell F, Elleri D, Leelarathna L, Rankin D, Tauschmann M, Thabit H, Hovorka R. Patients’ and caregivers’ experiences of using continuous glucose monitoring to support diabetes self-management: qualitative study. BMC Endocr Disord. 2018;18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vloemans AF, van Beers C, a. J, de Wit M, Cleijne W, Rondags SM, Geelhoed-Duijvestijn PH, Kramer MHH, Serné EH, Snoek FJ,. Keeping safe. Continuous glucose monitoring (CGM) in persons with Type 1 diabetes and impaired awareness of hypoglycaemia: a qualitative study. Diabet Med. 2017;34:1470–6. [DOI] [PubMed] [Google Scholar]
- 144.Polonsky WH, Hessler D. What Are the Quality of Life-Related Benefits and Losses Associated with Real-Time Continuous Glucose Monitoring? A Survey of Current Users. Diabetes Technol Ther. 2013;15:295–301. [DOI] [PubMed] [Google Scholar]
- 145.Litchman ML, Allen NA. Real-Time Continuous Glucose Monitoring Facilitates Feelings of Safety in Older Adults With Type 1 Diabetes: A Qualitative Study. J Diabetes Sci Technol. 2017;11:988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Messer LH, Johnson R, Driscoll KA, Jones J. Best friend or spy: a qualitative meta-synthesis on the impact of continuous glucose monitoring on life with Type 1 diabetes. Diabet Med. 2018;35:409–18. [DOI] [PubMed] [Google Scholar]
- 147.Chesser H, Srinivasan S, Puckett C, Gitelman SE, Wong JC (2022) Real-Time Continuous Glucose Monitoring in Adolescents and Young Adults With Type 2 Diabetes Can Improve Quality of Life. J Diabetes Sci Technol 19322968221139873 [DOI] [PMC free article] [PubMed]
- 148.Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P. Guidelines International Network: Toward International Standards for Clinical Practice Guidelines. Ann Intern Med. 2012;156:525–31. [DOI] [PubMed] [Google Scholar]
- 149.Steinberg E, Greenfield S, Wolman DM, Mancher M, Graham R, others (2011) Clinical Practice Guidelines We Can Trust. In: Institute Of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. National Academies Press [PubMed]
- 150.Kataoka Y, Oide S, Ariie T, Tsujimoto Y, Furukawa TA. The Methodological Quality Score of COVID-19 Systematic Reviews is Low, Except for Cochrane Reviews: A Meta-epidemiological Study. Ann Clin Epidemiol. 2021;3:46–55. [Google Scholar]
- 151.Siemens W, Schwarzer G, Rohe MS, Buroh S, Meerpohl JJ, Becker G. Methodological quality was critically low in 9/10 systematic reviews in advanced cancer patients—A methodological study. J Clin Epidemiol. 2021;136:84–95. [DOI] [PubMed] [Google Scholar]
- 152.Pandis N, Fleming PS, Worthington H, Salanti G. The Quality of the Evidence According to GRADE Is Predominantly Low or Very Low in Oral Health Systematic Reviews. PLoS ONE. 2015;10:e0131644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2020 Executive Summary. Endocr Pract. 2020;26:107–39. [DOI] [PubMed] [Google Scholar]
- 154.Beck RW. Closing in on closed-loop systems for type 2 diabetes. Nat Med. 2023;29:33–4. [DOI] [PubMed] [Google Scholar]
- 155.Thabit H, Hartnell S, Allen JM, Lake A, Wilinska ME, Ruan Y, Evans ML, Coll AP, Hovorka R. Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Lancet Diabetes Endocrinol. 2017;5:117–24. [DOI] [PubMed] [Google Scholar]
- 156.Boughton CK, Tripyla A, Hartnell S, Daly A, Herzig D, Wilinska ME, Czerlau C, Fry A, Bally L, Hovorka R. Fully automated closed-loop glucose control compared with standard insulin therapy in adults with type 2 diabetes requiring dialysis: an open-label, randomized crossover trial. Nat Med. 2021;27:1471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sharma P, Singh S, Thakur V, Sharma N, Grewal AS. Novel and emerging therapeutic drug targets for management of type 2 Diabetes Mellitus. Obes Med. 2021;23:100329. [Google Scholar]
- 158.Yamada K, Nakayama H, Yoshinobu S, et al. Effects of a sodium glucose co-transporter 2 selective inhibitor, ipragliflozin, on the diurnal profile of plasma glucose in patients with type 2 diabetes: A study using continuous glucose monitoring. J Diabetes Investig. 2015;6:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Torimoto K, Okada Y, Koikawa K, Tanaka Y. Early effects of sodium-glucose co-transporter 2 inhibitors in type 2 diabetes: study based on continuous glucose monitoring. Diabetol Metab Syndr. 2017;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hirsch IB, Parkin CG, Cavaiola TS, Bergenstal RM Use of continuous glucose monitoring when initiating glucagon-like peptide-1 receptor agonist therapy in insulin-treated diabetes. Diabetes Obes Metab. 10.1111/dom.15883 [DOI] [PubMed]
- 161.Abdelgadir E, Rashid F, Bashier A, Al Saeed M, Khalifa A, Alawadi F, Hassanein M. Use of flash glucose monitoring system in assessing safety of the SGLT2 inhibitors during Ramadan fasting in high risk insulin treated patients with type 2 diabetes. Diabetes Metab Syndr Clin Res Rev. 2019;13:2927–32. [DOI] [PubMed] [Google Scholar]
- 162.Cox DJ, Taylor AG, Moncrief M, Diamond A, Yancy WS, Hegde S, McCall AL. Continuous Glucose Monitoring in the Self-management of Type 2 Diabetes: A Paradigm Shift. Diabetes Care. 2016;39:e71–73. [DOI] [PubMed] [Google Scholar]
- 163.Allen N, Whittemore R, Melkus G. A continuous glucose monitoring and problem-solving intervention to change physical activity behavior in women with type 2 diabetes: a pilot study. Diabetes Technol Ther. 2011;13:1091–9. [DOI] [PubMed] [Google Scholar]
- 164.Hermanns N, Ehrmann D, Schipfer M, Kröger J, Haak T, Kulzer B. The impact of a structured education and treatment programme (FLASH) for people with diabetes using a flash sensor-based glucose monitoring system: Results of a randomized controlled trial. Diabetes Res Clin Pract. 2019;150:111–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.