Abstract
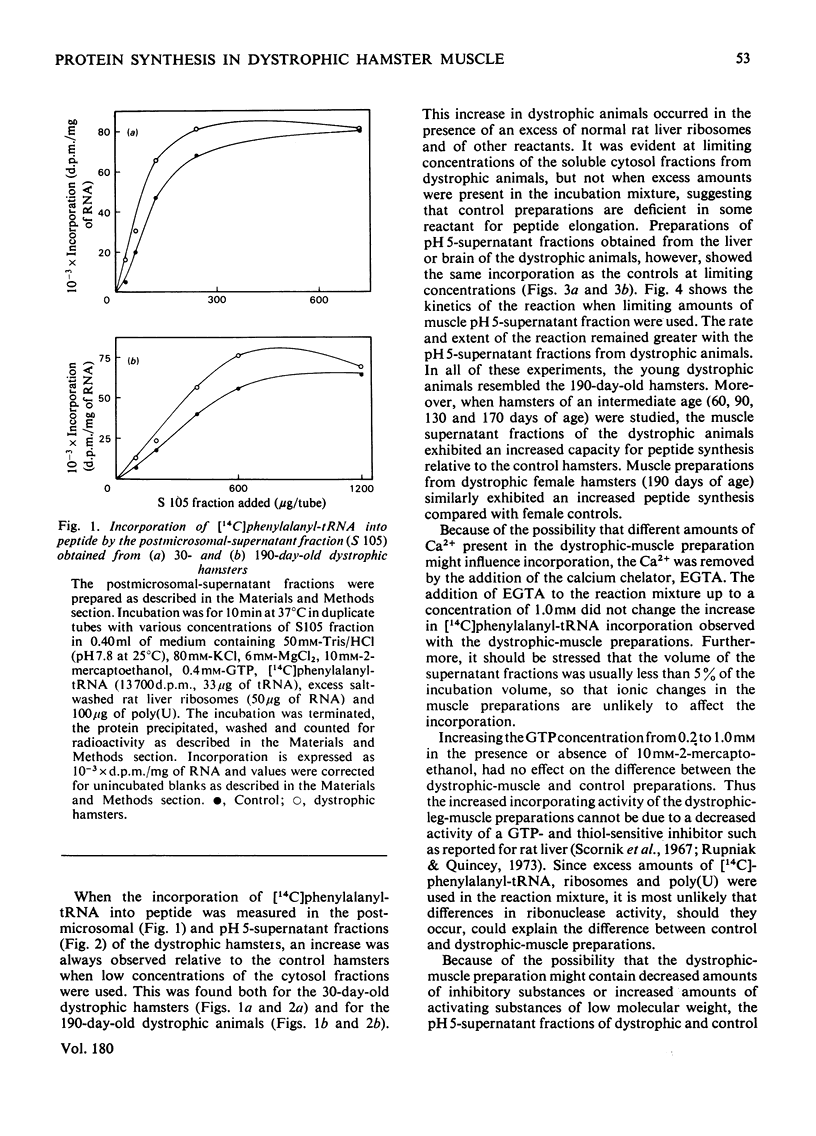
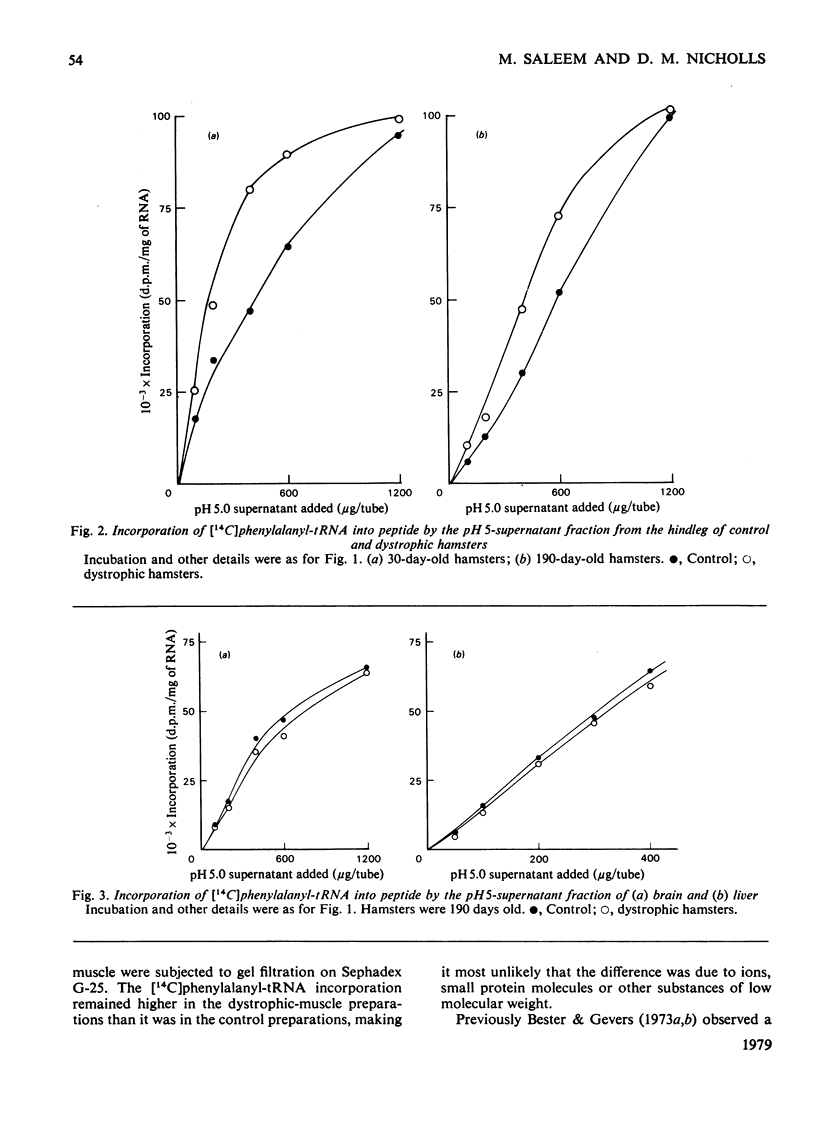
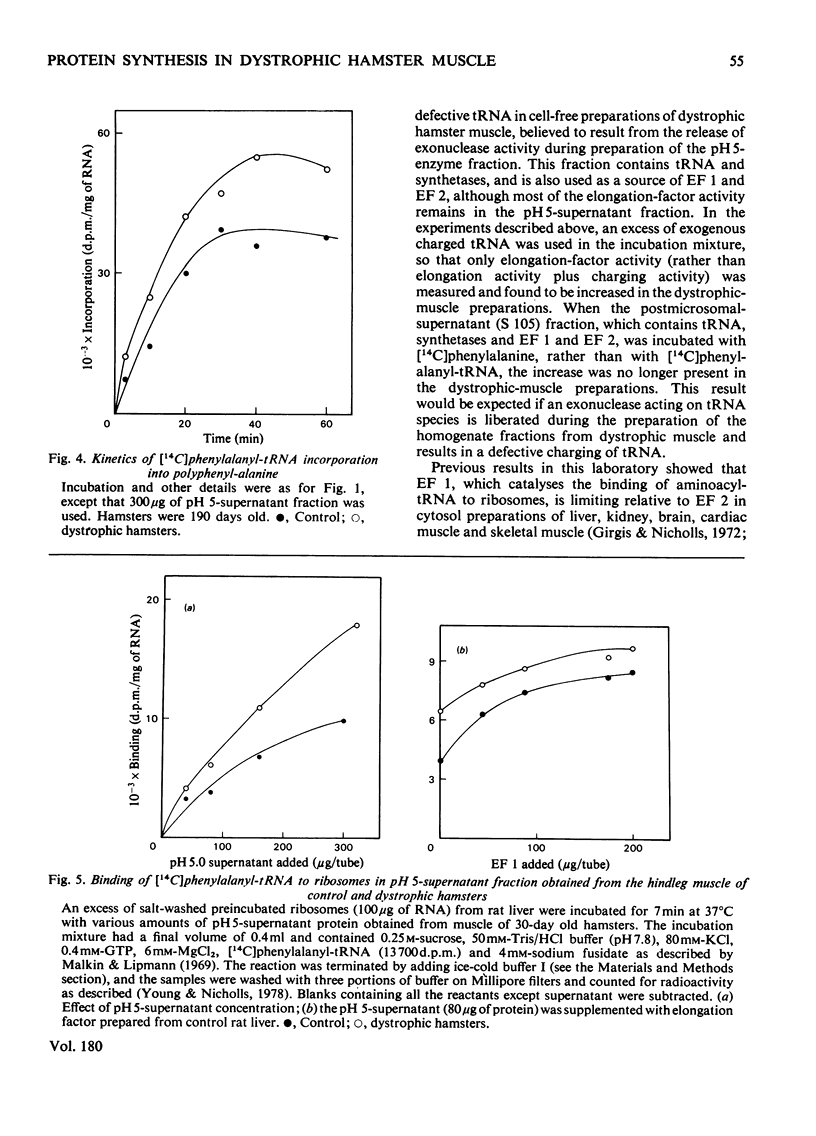
Homogenates of hindleg muscle were obtained from control and dystrophic male hamsters, 30 and 190 days of age, and were used to prepare the postmicrosomal pH5-supernatant fraction. The activity of this fraction in the incorporation of [14C]phenylalanyl-tRNA into peptides was increased in the dystrophic-muscle preparations. No such increase was found in brain or liver preparations from dystrophic hamsters. The increased capacity for aminoacyl-tRNA binding that was observed in preparations from dystrophic animals is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bester A. J., Gevers W. Cell-free protein synthesis in heart and skeletal muscles from polymyopathic hamsters. Biochem J. 1973 Feb;132(2):193–201. doi: 10.1042/bj1320193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bester A. J., Gevers W. Evidence for defective transfer ribonucleic acid in polymyopathic hamsters and its inhibitory effect on protein synthesis. Biochem J. 1973 Feb;132(2):203–214. doi: 10.1042/bj1320203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla R., Brot N. Age dependent changes in enzymes involved in macromolecular synthesis in Turbatrix aceti. Arch Biochem Biophys. 1975 Jul;169(1):227–236. doi: 10.1016/0003-9861(75)90336-7. [DOI] [PubMed] [Google Scholar]
- Bolla R., Weissbach H., Brot N. Multiple forms of elongation factor 1 in various rat tissues. Arch Biochem Biophys. 1975 Feb;166(2):683–684. doi: 10.1016/0003-9861(75)90433-6. [DOI] [PubMed] [Google Scholar]
- Bradley W. G., Jenkison M. Abnormalities of peripheral nerves in murine muscular dystrophy. J Neurol Sci. 1973 Feb;18(2):227–247. doi: 10.1016/0022-510x(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Cappon I. D., Nicholls D. M. DDT increases the aminoacyl-tRNA binding activity of liver pH 5 supernatant fraction. Chem Biol Interact. 1974 Sep;9(3):155–168. doi: 10.1016/s0009-2797(74)80001-3. [DOI] [PubMed] [Google Scholar]
- Girgis G. R., Nicholls D. M. Control of the increased protein synthesis in kidney of nephrotic rats by supernatant factors. Biochim Biophys Acta. 1971 Oct 14;247(2):335–347. doi: 10.1016/0005-2787(71)90681-2. [DOI] [PubMed] [Google Scholar]
- Girgis G. R., Nicholls D. M. Protein synthesis limited by transferase I. Biochim Biophys Acta. 1972 May 29;269(3):465–476. doi: 10.1016/0005-2787(72)90134-7. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F., Goldspink G. Age-related changes in protein turnover and ribonucleic acid of the diaphragm muscle of normal and dystrophic hamsters. Biochem J. 1977 Jan 15;162(1):191–194. doi: 10.1042/bj1620191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Wilson P. Mechanical properties of dystrophic mouse muscle. J Neurol Neurosurg Psychiatry. 1971 Oct;34(5):512–520. doi: 10.1136/jnnp.34.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. A., Engelhardt D. L. The regulation of protein synthesis in animal cells by serum factors. Biochemistry. 1976 Apr 6;15(7):1375–1381. doi: 10.1021/bi00652a004. [DOI] [PubMed] [Google Scholar]
- Homburger F., Nixon C. W., Eppenberger M., Baker J. R. Hereditary myopathy in the Syrian hamster: studies on pathogenesis. Ann N Y Acad Sci. 1966 Sep 9;138(1):14–27. doi: 10.1111/j.1749-6632.1966.tb41151.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki K., Mizumoto K., Tanaka M., Kaziro Y. A new protein factor required for polypeptide elongation in mammalian tissues. J Biochem. 1973 Oct;74(4):849–852. doi: 10.1093/oxfordjournals.jbchem.a130311. [DOI] [PubMed] [Google Scholar]
- Kitchin S. E., Watts D. C. Comparison of the turnover patterns of total and individual muscle proteins in normal mice and those with hereditary muscular dystrophy. Biochem J. 1973 Dec;136(4):1017–1028. doi: 10.1042/bj1361017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsiopoulos P. S., Mohr S. C. Protein synthesis elongation factor 1 from rat liver: a zinc metalloenzyme. Biochem Biophys Res Commun. 1975 Dec 1;67(3):979–987. doi: 10.1016/0006-291x(75)90771-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lochner A., Brink A., Brink A. J. Protein synthesis in the myocardiopathy and muscular dystrophy of the Syrian hamster. Clin Sci. 1971 Jan;40(1):89–94. doi: 10.1042/cs0400089. [DOI] [PubMed] [Google Scholar]
- Malkin M., Lipmann F. Fusidic acid: inhibition of factor T2 in reticulocyte protein synthesis. Science. 1969 Apr 4;164(3875):71–72. doi: 10.1126/science.164.3875.71. [DOI] [PubMed] [Google Scholar]
- McEwen Nicholls D., Carey J., Sendecki W. Growth of liver accompanied by an increased binding of aminoacyl-transfer ribonucleic acids. Biochem J. 1977 Sep 15;166(3):463–471. doi: 10.1042/bj1660463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeehan W. L., Hardesty B. Purification and partial characterization of the aminoacyl transfer ribonucleic acid binding enzyme from rabbit reticulocytes. J Biol Chem. 1969 Aug 25;244(16):4330–4339. [PubMed] [Google Scholar]
- Michelson A. M., Russell E. S., Harman P. J. Dystrophia Muscularis: A HEREDITARY PRIMARY MYOPATHY IN THE HOUSE MOUSE. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1079–1084. doi: 10.1073/pnas.41.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery A. Parabiotic reinnervation in normal and dystrophic mice. Part 1. Muscle weight and physiological studies. J Neurol Sci. 1975 Nov;26(3):401–423. doi: 10.1016/0022-510x(75)90211-7. [DOI] [PubMed] [Google Scholar]
- Montgomery A., Park D. C., Pennington R. J. Peptide hydrolases and AMP deaminase in extensor digitorum longus and soleus muscles from genetically dystrophic hamsters. Comp Biochem Physiol B. 1974 Nov 15;49(3):387–392. doi: 10.1016/0305-0491(74)90175-8. [DOI] [PubMed] [Google Scholar]
- Montgomery A. The isometric responses of fast and slow twitch muscles from normal and genetically dystrophic hamsters. Exp Neurol. 1975 Jan;46(1):87–102. doi: 10.1016/0014-4886(75)90034-5. [DOI] [PubMed] [Google Scholar]
- Nicholls D. M., Chan Y. P., Girgis G. R. Aminoacyl-tRNA binding activity in regenerating kidney following contralateral nephrectomy or administration of folic acid. Dev Biol. 1975 Nov;47(1):1–11. doi: 10.1016/0012-1606(75)90259-6. [DOI] [PubMed] [Google Scholar]
- Nicholls D. M., Petryshyn R., Warner L. Laser irradiation induces increased activity of liver elongation factor 1. Radiat Res. 1974 Oct;60(1):98–107. [PubMed] [Google Scholar]
- Nwagwu M. Activity of polyribosomes from the muscle of normal and dystrophic mice in cell-free amino-acid incorporation. Eur J Biochem. 1975 Aug 1;56(1):123–127. doi: 10.1111/j.1432-1033.1975.tb02214.x. [DOI] [PubMed] [Google Scholar]
- Pennington R. J. Biochemistry of dystrophic muscle. 2. Some enzyme changes in dystrophic mouse muscle. Biochem J. 1963 Jul;88(1):64–68. doi: 10.1042/bj0880064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryshyn R. A., Creasy R. C., Nicholls D. M. Protein synthesis in the heart of genetic dystrophic mice. Biochem Med. 1977 Oct;18(2):139–151. doi: 10.1016/0006-2944(77)90085-0. [DOI] [PubMed] [Google Scholar]
- Petryshyn R. A., Nicholls D. M. Studies of a factor from dystrophic mouse muscle inhibitory towards protein synthesis. Biochem J. 1978 Dec 15;176(3):907–917. doi: 10.1042/bj1760907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryshyn R., Nicholls D. M. Protein synthesis in dystrophic muscle. Activity of the pH 5 supernatant fraction of muscle in dystrophic mice. Biochim Biophys Acta. 1976 Jul 16;435(4):391–404. doi: 10.1016/0005-2787(76)90204-5. [DOI] [PubMed] [Google Scholar]
- Rowe R. W., Goldspink G. Muscle fibre growth in five different muscles in both sexes of mice. II. Dystrophic mice. J Anat. 1969 May;104(Pt 3):531–538. [PMC free article] [PubMed] [Google Scholar]
- Rupniak H. T., Quincey R. V. Mechanism of action of a microsomal inhibitor of protein synthesis potentiated by oxidized glutathione. Biochem J. 1973 Oct;136(2):335–342. doi: 10.1042/bj1360335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scornik O. A., Hoagland M. B., Pfefferkorn L. C., Bishop E. A. Inhibitors of protein synthesis in rat liver microsome fractions. J Biol Chem. 1967 Jan 10;242(1):131–139. [PubMed] [Google Scholar]
- Smith R. L., Baca O., Gordon J. Co-ordinate synthesis of ribosomes and elongation factors in the liver of immature chicks following a metabolic shift up. J Mol Biol. 1976 Jan 15;100(2):115–126. doi: 10.1016/s0022-2836(76)80143-x. [DOI] [PubMed] [Google Scholar]
- Srivastava U. Biochemical changes in progressive muscular dystrophy. IX. Synthesis of native myosin, actin, and tropomyosin in the skeletal muscle of mouse as a function of muscular dystrophy. Can J Biochem. 1972 Apr;50(4):409–415. doi: 10.1139/o72-055. [DOI] [PubMed] [Google Scholar]
- Weinstock I. M., Markiewicz L. Muscle protein synthesis during development of the normal and dystrophic chicken. Activity of supernatant elongation factors. Biochim Biophys Acta. 1974 Dec 6;374(2):197–206. doi: 10.1016/0005-2787(74)90363-3. [DOI] [PubMed] [Google Scholar]
- Wrogemann K., Quilliam N. M., Nylen E. G., Johnson M. D. Altered amounts of hemoglobin synthesis in livers of dystrophic hamsters. Can J Biochem. 1977 Aug;55(8):894–900. doi: 10.1139/o77-131. [DOI] [PubMed] [Google Scholar]
- Young E. T., Nicholls D. M. Liver enzyme induction by 1,1,1-trichloro-2,2-bis-(p-chlorophenyl)ethane (DDT) is accompanied by an increase in the specific activity of elongation factor 1. Biochem J. 1978 Jun 15;172(3):479–486. doi: 10.1042/bj1720479. [DOI] [PMC free article] [PubMed] [Google Scholar]


