Abstract
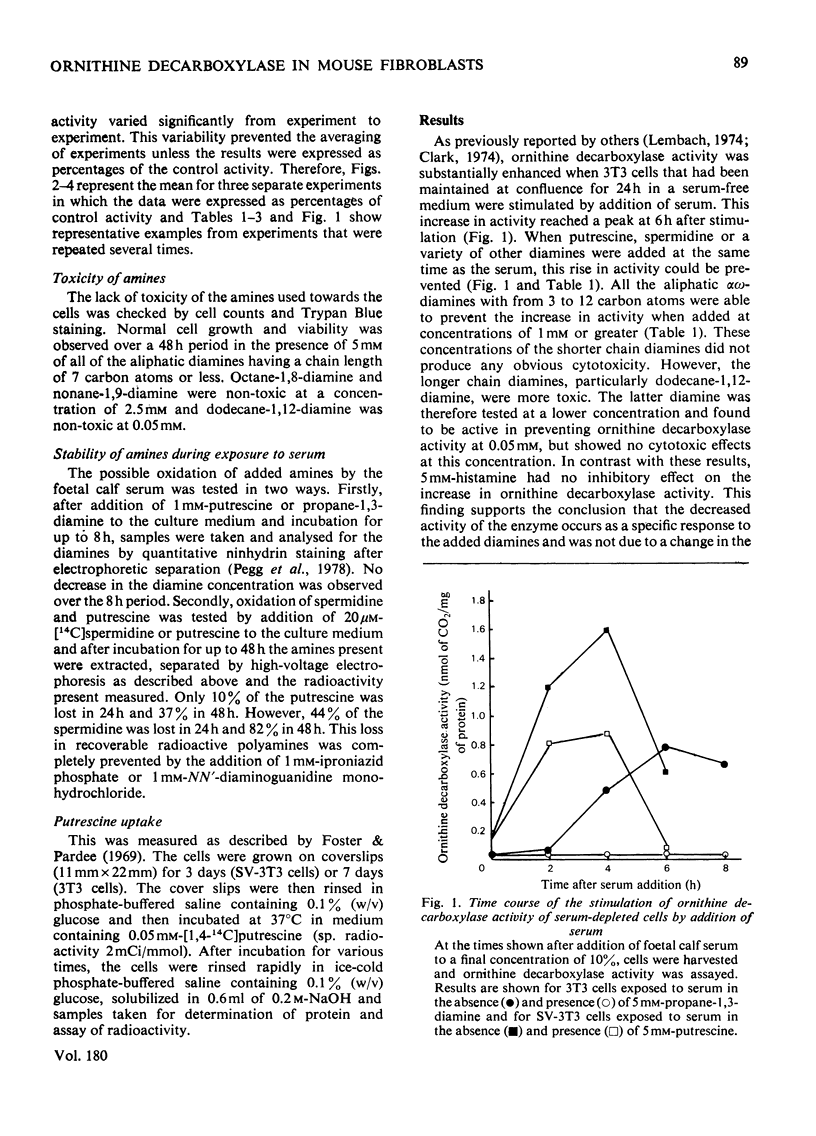
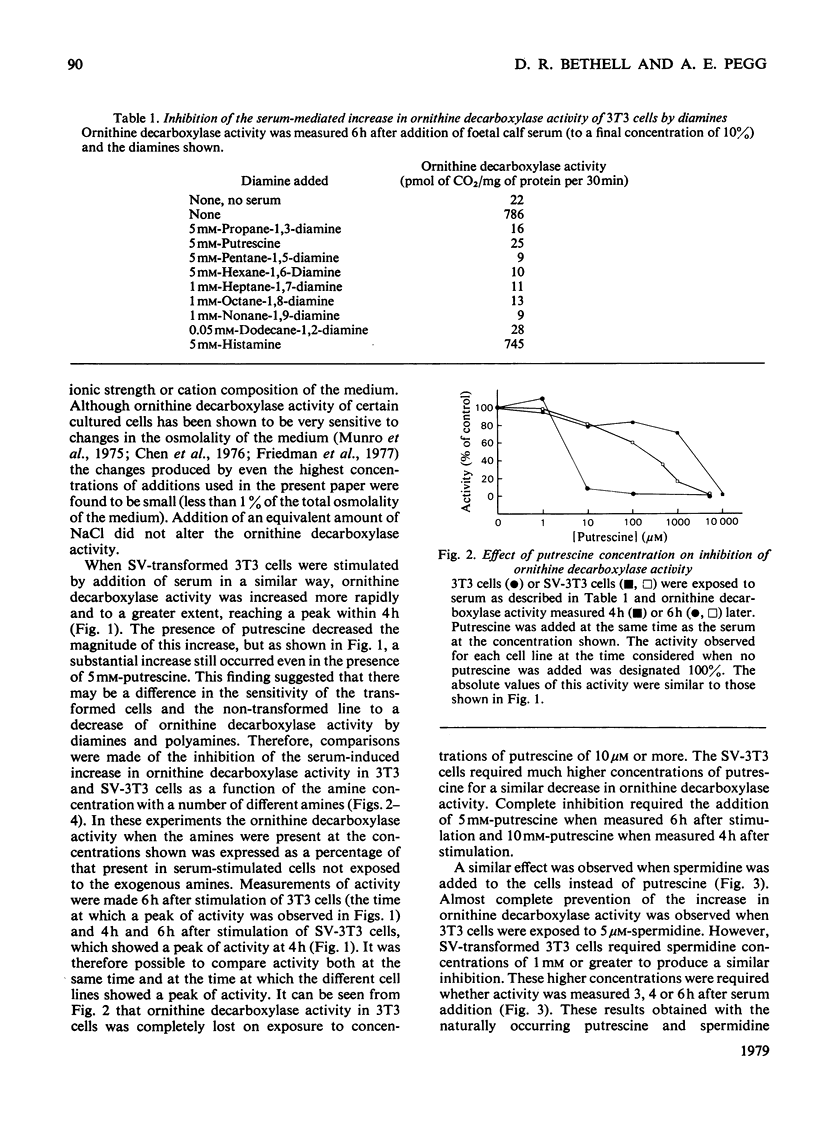
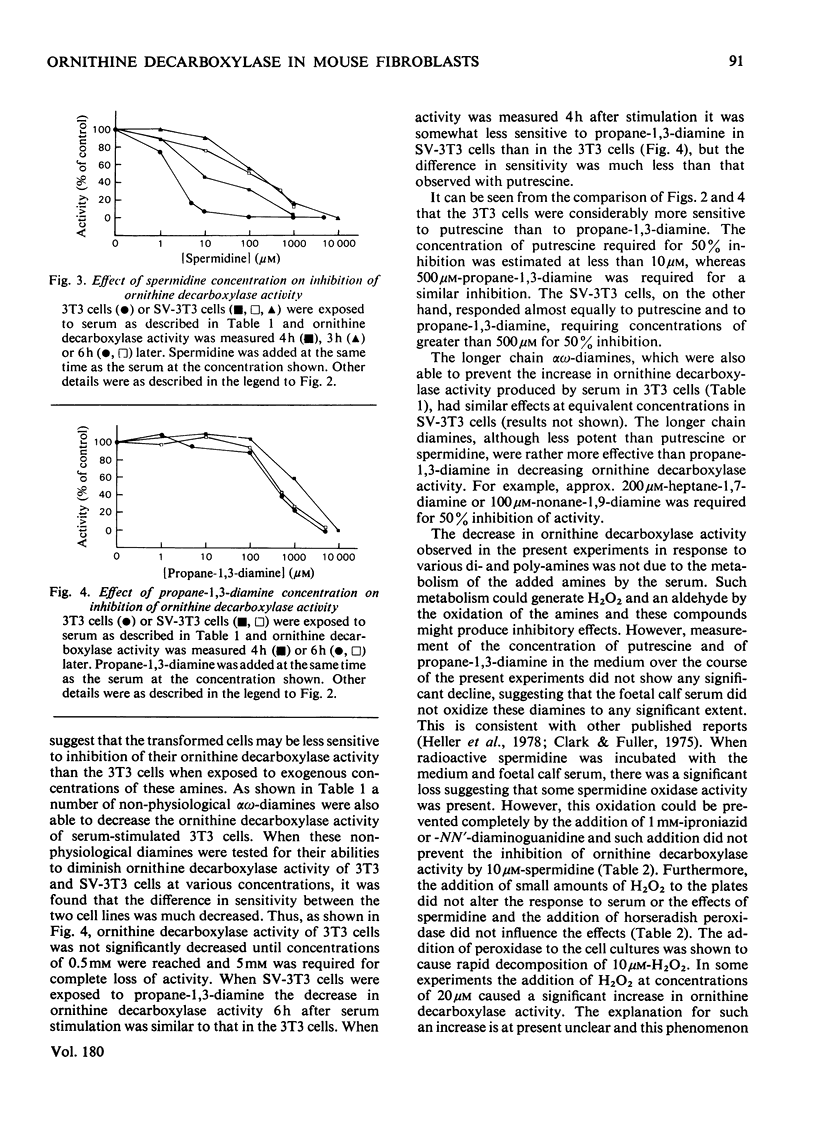
1. The induction of ornithine decarboxylase activity in mouse 3T3 fibroblasts or an SV-40 transformed 3T3 cell line by serum was prevented by addition of the naturally occurring polyamines putrescine (butane-1,4-diamine) and spermidine. Much higher concentrations of these amines were required to fully suppress ornithine decarboxylase activity in the transformed SV-3T3 cells than in the 3T3 fibroblasts. 2. Synthetic alpha omega-diamines with 3--12 carbon atoms also prevented the increase in ornithine decarboxylase activity induced by serum in these cells. The longer chain diamines were somewhat more potent than propane-1,3-diamine in this effect, but the synthetic diamines were less active than putrescine in the 3T3 cells. There was little difference between the responses of 3T3 and SV-3T3 cells to the synthetic diamines propane-1,3-diamine and heptane-1,7-diamine. 3. These results are discussed in relation to the control of polyamine synthesis in mammalian cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachrach U. Polyamines as chemical markers of malignancy. Ital J Biochem. 1976 Jan-Feb;25(1):77–93. [PubMed] [Google Scholar]
- Bartos F., Bartos D., Dolney A. M., Grettie D. P., Campbell R. A. Radioimmunoassay of spermidine in human serum. Res Commun Chem Pathol Pharmacol. 1978 Feb;19(2):295–309. [PubMed] [Google Scholar]
- Chen K., Heller J. S., Canellakis E. S. The inhibition of the induction of ornithine decarboxylase by cations. Biochem Biophys Res Commun. 1976 May 3;70(1):212–220. doi: 10.1016/0006-291x(76)91130-x. [DOI] [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Regulation of ornithine decarboxylase in 3T3 cells by putrescine and spermidine: indirect evidence for translational control. Biochemistry. 1975 Oct 7;14(20):4403–4409. doi: 10.1021/bi00691a010. [DOI] [PubMed] [Google Scholar]
- Clark J. L. Specific induction of ornithine decarboxylase in 3T3 mouse fibroblasts by pituitary growth factors: cell density-dependent biphasic response and alteration of half-life. Biochemistry. 1974 Oct 22;13(22):4668–4674. doi: 10.1021/bi00719a031. [DOI] [PubMed] [Google Scholar]
- Dubrow R., Pardee A. B., Pollack R. 2-amino-isobutyric acid and 3-O-methyl-D-glucose transport in 3T3, SV 40-transformed 3T3 and revertant cell lines. J Cell Physiol. 1978 May;95(2):203–211. doi: 10.1002/jcp.1040950210. [DOI] [PubMed] [Google Scholar]
- Durie B. G., Salmon S. E., Russell D. H. Polyamines as markers of response and disease activity in cancer chemotherapy. Cancer Res. 1977 Jan;37(1):214–221. [PubMed] [Google Scholar]
- Fong W. F., Heller J. S., Canellakis E. S. The appearance of an ornithine decarboxylase inhibitory protein upon the addition of putrescine to cell cultures. Biochim Biophys Acta. 1976 Apr 23;428(2):456–465. doi: 10.1016/0304-4165(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Pardee A. B. Transport of amino acids by confluent and nonconfluent 3T3 and polyoma virus-transformed 3T3 cells growing on glass cover slips. J Biol Chem. 1969 May 25;244(10):2675–2681. [PubMed] [Google Scholar]
- Friedman Y., Park S., Levasseur S., Burke G. Activation of thyroid ornithine decarboxylase (ODC) in vitro by hypotonicity; a possible mechanism for ODC induction. Biochem Biophys Res Commun. 1977 Jul 11;77(1):57–64. doi: 10.1016/s0006-291x(77)80164-2. [DOI] [PubMed] [Google Scholar]
- Guha S. K., Jänne J. Inhibition of ornithine decarboxylase in vivo in rat ovary. Biochem Biophys Res Commun. 1977 Mar 7;75(1):136–142. doi: 10.1016/0006-291x(77)91300-6. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Chen K. Y., Kyriakidis D. A., Fong W. F., Canellakis E. S. The modulation of the induction of ornithine decarboxylase by spermine, spermidine and diamines. J Cell Physiol. 1978 Aug;96(2):225–234. doi: 10.1002/jcp.1040960211. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isselbacher K. J. Increased uptake of amino acids and 2-deoxy-D-glucose by virus-transformed cells in culture. Proc Natl Acad Sci U S A. 1972 Mar;69(3):585–589. doi: 10.1073/pnas.69.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Pegg A. E. Studies on ornithine decarboxylase activity in the isolated perfused rat liver. Biochim Biophys Acta. 1977 Sep 15;484(1):177–187. doi: 10.1016/0005-2744(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Jänne J., Hölttä E. Regulation of ornithine decarboxylase activity by putrescine and spermidine in rat liver. Biochem Biophys Res Commun. 1974 Nov 27;61(2):449–456. doi: 10.1016/0006-291x(74)90977-2. [DOI] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- Kallio A., Löfman M., Pösö H., Jänne J. Inhibition of ornithine decarboxylase by diamines in regenerating rat liver. Evidence for direct action on the accumulation of the enzyme protein. FEBS Lett. 1977 Jul 1;79(1):195–199. [PubMed] [Google Scholar]
- Kallio A., Pösö H., Guha S. K., Jänne J. Polyamines and their biosynthetic enzymes in Ehrlich ascites-carcinoma cells. Modification of tumour polyamine pattern by diamines. Biochem J. 1977 Jul 15;166(1):89–94. doi: 10.1042/bj1660089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., Pösö H., Scalabrino G., Jänne J. Regulation of ornithine decarboxylase by diamines in regenerating rat liver. FEBS Lett. 1977 Feb 1;73(2):229–234. doi: 10.1016/0014-5793(77)80987-3. [DOI] [PubMed] [Google Scholar]
- Kano K., Oka T. Polyamine transport and metabolism in mouse mammary gland. General properties and hormonal regulation. J Biol Chem. 1976 May 10;251(9):2795–2800. [PubMed] [Google Scholar]
- Kay J. E., Lindsay V. J. Control of ornithine decarboxylase activity in stimulated human lymphocytes by putrescine and spermidine. Biochem J. 1973 Apr;132(4):791–796. doi: 10.1042/bj1320791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lembach K. J. Regulation of growth in vitro. I. Control of ornithine decarboxylase levels in untransformed and transformed mouse fibroblasts by serum. Biochim Biophys Acta. 1974 Jun 20;354(1):88–100. doi: 10.1016/0304-4165(74)90056-7. [DOI] [PubMed] [Google Scholar]
- Munro G. F., Miller R. A., Bell C. A., Verderber E. L. Effects of external osmolality on polyamine metabolism in HeLa cells. Biochim Biophys Acta. 1975 Dec 5;411(2):263–281. doi: 10.1016/0304-4165(75)90306-2. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Conover C., Wrona A. Effects of aliphatic diamines on rat liver ornithine decarboxylase activity. Biochem J. 1978 Mar 15;170(3):651–660. doi: 10.1042/bj1700651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Biosynthesis of putrescine in the prostate gland of the rat. Biochem J. 1968 Jul;108(4):533–539. doi: 10.1042/bj1080533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piik K., Pösö H., Jänne J. Reversible inhibition of rat liver regeneration by 1,3-diamino-2-propanol, an inhibitor of ornithine decarboxylase. FEBS Lett. 1978 May 15;89(2):307–312. doi: 10.1016/0014-5793(78)80243-9. [DOI] [PubMed] [Google Scholar]
- Pohjanpelto P. Putrescine transport is greatly increased in human fibroblasts initiated to proliferate. J Cell Biol. 1976 Mar;68(3):512–520. doi: 10.1083/jcb.68.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pösö H., Guha S. K., Jänne J. Stabilization of ornithine decarboxylase in rat liver. Biochim Biophys Acta. 1978 Jun 9;524(2):466–473. doi: 10.1016/0005-2744(78)90185-7. [DOI] [PubMed] [Google Scholar]
- Pösö H., Jänne J. Inhibition of polyamine accumulation and deoxyribonucleic acid synthesis in regenerating rat liver. Biochem J. 1976 Aug 15;158(2):485–488. doi: 10.1042/bj1580485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pösö H., Kallio A., Scalabrino G., Jänne J. Specific inhibition of the synthesis of putrescine and spermidine by 1,3-diaminopropane in rat liver in vivo. Biochim Biophys Acta. 1977 Mar 29;497(1):288–297. doi: 10.1016/0304-4165(77)90162-3. [DOI] [PubMed] [Google Scholar]
- Raina A., Jänne J. Physiology of the natural polyamines putrescine, spermidine and spermine. Med Biol. 1975 Jun;53(3):121–147. [PubMed] [Google Scholar]
- Rennert O. M., Lawson D. L., Shukla J. B., Miale T. D. Cerebrospinal fluid polyamine monitoring in central nervous system leukemia. Clin Chim Acta. 1977 Mar 15;75(3):365–369. doi: 10.1016/0009-8981(77)90354-0. [DOI] [PubMed] [Google Scholar]
- Schuler M. F., Jefferson L. S., Gorodecki J., Glaser R., Dietz J., Lipton A. Selective growth of transformed cell lines by rat liver perfusate. Cancer Res. 1977 Jun;37(6):1662–1665. [PubMed] [Google Scholar]
- Sunkara P. S., Rao P. N., Nishioka K. Putrescine biosynthesis in mammalial cells: essential for DNA synthesis but not for mitosis. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1125–1153. doi: 10.1016/0006-291x(77)91635-7. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Transport systems for 1,4-diaminobutane, spermidine, and spermine in Escherichia coli. J Biol Chem. 1966 Aug 25;241(16):3714–3723. [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Biosynthesis and metabolism of 1,4-diaminobutane, spermidine, spermine, and related amines. Adv Enzymol Relat Areas Mol Biol. 1972;36:203–268. doi: 10.1002/9780470122815.ch7. [DOI] [PubMed] [Google Scholar]
- Van Nest G. A., Grimes W. J. A comparison of membrane components of normal and transformed BALB/c cells. Biochemistry. 1977 Jun 28;16(13):2902–2908. doi: 10.1021/bi00632a016. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Hale A. H., Yau T. M., Buckman T., Johnson M., Brady T. M., LaRossa D. D. Transport changes associated with growth control and malignant transformation. J Cell Physiol. 1976 Dec;89(4):711–721. doi: 10.1002/jcp.1040890431. [DOI] [PubMed] [Google Scholar]
- Wiegand L., Pegg A. E. Effects of inhibitors of S-adenosylmethionine decarboxylase and ornithine decarboxylase on DNA synthesis in rat liver after partial hepatectomy. Biochim Biophys Acta. 1978 Jan 26;517(1):169–180. doi: 10.1016/0005-2787(78)90044-8. [DOI] [PubMed] [Google Scholar]


