Abstract
Though studies have investigated the association between air pollution and respiratory health outcomes in children, few have focused on night cough. The study objective was to simultaneously evaluate family factors (i.e., race, gender, maternal and paternal asthma, and breastfeeding), health (allergen sensitization and wheezing symptoms), home factors (dog, cat, mold, endotoxin, and dust mite), and other environmental exposures (traffic exhaust and second-hand tobacco smoke) for associations with recurrent dry night cough (RNC) during early childhood. A structural equation model with repeat measures was developed assessing RNC at ages one, two, and three. The prevalence of RNC was relatively large and similar at ages, one, two, and three at 21.6%, 17.3%, and 21.1%, respectively. Children exposed to the highest tertile of traffic exhaust had an estimated 45% increase in risk of RNC compared with children less exposed (adjusted OR 1.45, 95% CI: 1.09, 1.94). Also, wheezing was associated with a 76% higher risk of RNC (adjusted OR 1.76, 95% CI: 1.36, 2.26). A protective trend for breastfeeding was found with a 27% reduction in risk associated with breastfeeding (adjusted OR 0.73, 95% CI: 0.53, 1.01). No other factors were significant. These results suggest that traffic exhaust exposure may be a risk factor for night cough in young children.
Keywords: trafic exhaust, cough, wheez, asthma, children, structural equation model
The prevalence of night cough in children is estimated to be 14.5% at age one, 16% at age two, and increasing to 22% by age four (1–3). Frequent episodes of child cough, especially at night, can cause loss of sleep to both parent and child, leading to poor school and work performance. The occurrence of a dry night cough is frequent in asthmatic children and asthma is the most commonly identified cause of chronic cough in this age group (4). Among asthmatic children, the prevalence of night cough has been estimated to be 14.5% at age seven decreasing to 9.5% by 12 yrs of age (5). Night cough, in particular, is highly indicative of asthma with a sensitivity for predicting the asthma phenotype of 91% (6, 7).
Exposure to air pollution has been consistently associated with poor respiratory outcomes in children including wheezing (8–10), hospital admissions for asthma symptoms (11), wheezing bronchitis (12), and incidence of asthma (13). Few studies, however, have evaluated the etiology for dry night cough despite cough being among the most common symptoms of pediatric asthma presented to family physicians (14). In a German cohort study, Morgenstern et al. (15) found a positive association between dry night cough and nitrogen dioxide (NO2) exposure in the first year of life, but this finding was not confirmed in the second year of life. In this same study, particulate matter <2.5 μm (PM2.5) exposure was not found to be significantly associated with dry night cough at either age (15). In a previous study, the authors had found significant associations between PM2.5 and NO2 and dry cough at night at age one and attenuated effects at age two (16). In a UK cohort study, Pierse et al. (17) found night cough to be associated with particulate matter <10 μm (PM10) exposure, but did not report on PM2.5 exposure. In a cohort study conducted in the Netherlands, Brauer et al. (2) estimated home PM2.5 exposures and found a positive but not significant association with dry night cough in children two yrs of age. Using self-reported traffic exposure, a German survey study observed a positive association between the prevalence of night cough and truck traffic among 13–14 yrs old children, but no association was found among younger children age six to seven yrs (18). Therefore, the association between night cough and air pollution exposure remains unclear.
Air pollution in urban areas is a complex mixture of particles and gas-phase pollutants arising from a myriad of sources. The association between traffic-related air pollution and respiratory health effects in children is of recent interest due to the toxicological effects of the air pollution mixture arising from mobile sources, i.e., gasoline and diesel combustion engines (19). In particular, fine and ultrafine particulate matter (PM2.5 and PM1.0, respectively), is derived primarily from vehicular exhaust and, in contrast to PM10, has a larger fraction of elemental and organic carbon (20). Diesel exhaust particles (DEP) are a major component of PM2.5, particularly in urban areas where diesel exhaust is the largest single source of airborne PM from vehicles (21, 22). As such, DEP have been widely studied with respect to adverse respiratory health effects (21) where it has been demonstrated that they are associated with increased inflammatory cells, increased cytokine levels, decreased macrophage function, and increased airway resistance (21). Though the mechanisms by which DEP exert their toxicological effects remain unknown, the heterogeneous mixture of diesel exhaust is likely associated with the generation of reactive oxygen species (ROS) and inflammation.
The Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) is a prospective birth cohort study that is currently underway with the goal of determining if infants who are exposed to traffic-related particles, in particular DEP, are at an increased risk for the development of allergic disease and asthma. This analysis was undertaken to investigate the hypothesis that increased traffic-related particle exposure will be associated with increased risk of night cough in children after adjusting for wheezing and other covariates. In this study, we describe the association between family, health, home, and environmental exposures with night cough during early childhood.
Methods
Cohort description
The CCAAPS study methods have been described in detail (23). Briefly, infants were identified through birth records, between 2001 and 2003 in the greater Cincinnati Ohio area. To be eligible for this study, at least one parent had to be atopic defined as having at least one allergy symptom and also having allergic sensitization to at least one of 15 aeroallergens. Since the goal of the study was to investigate exposure to traffic exhaust, eligibility was restricted to families that resided within 400 m or further than 1500 m from the nearest major highway or interstate (9, 10). Families were enrolled when the child was approximately eight months of age.
Yearly clinical exams were conducted to obtain extensive medical history and to perform a complete physical examination. In addition, yearly questionnaires were personally administered to obtain exposure information and general health symptoms and conditions. The health outcome of interest in this study is recurrent dry night cough, hereafter abbreviated as RNC. RNC was defined as the parental report of their child coughing at night two or more times in the past year, apart from a cold or chest infection and was obtained at child’s age one, two, and three.
Covariates
Family covariates included child’s race (African–American or not), child’s gender, whether or not the mother or father had been diagnosed with asthma (yes or no), and whether or not the child was breastfed during the first year (ever or never). The health covariates included a positive or negative skin prick test (SPT) to a panel of 15 aeroallergens and report of recurrent wheeze defined as the parental report of the child wheezing two or more times in the past 12 months apart from a cold. The SPT panel included two types of grass pollen: timothy and fescue; four types of tree pollen: white oak, maple mix, American elm, and red cedar; short ragweed; four types of molds: Alternaria alternata, Aspergillus fumigatus, Penicillium mix, and Cladosporium herbarum; house dust mite mix; German cockroach; cat; and dog (23). A positive test was defined by a wheal size greater than or equal to three millimeters over that for the saline control after 15 min. These health covariates were collected at age one, two, and three.
Clinical evaluation and on-site home assessments were conducted by trained study team members when the child reached age one. The home covariates were obtained from the home inspections. During this visit, signs of mold and water damage were noted and their area measured. The homes were categorized in three groups: no mold, low mold, and high mold, as described by Cho et al. (24). House dust samples were collected and used to measure endotoxin and dust mite concentration. Dust samples were vacuumed from the infant’s primary activity room floor as described by Cho et al. (24). Samples were collected from an area of two square meters (m2) at a vacuuming rate of two min/m2 for carpeted floors and one sample of the entire room was collected at a rate of one min/m2 for non-carpeted floors. The home dust samples were sieved (355 μm sieve) and fine dust was stored at −20°C before analysis. The presence of a dog and/or cat in the home was obtained from the questionnaire at the time of family enrollment.
The environmental covariates included second-hand tobacco smoke exposure and estimated levels of exposure to traffic exhaust. Child’s second-hand tobacco smoke exposure was obtained from the year one questionnaire responses to whether or not the mother smokes.
Particulate matter2.5 was measured from air samples collected at 27 monitoring stations using a source signature and land use regression model and is explained in detail by Ryan et al. (10). Air samples at each monitoring station were collected over different seasons providing average annual exposure estimates (25). Air filters captured PM2.5 and were analyzed for elemental carbon (EC) concentrations as elemental carbon is commonly used for assessing traffic-related emissions (20). In addition, elemental carbon is commonly used as a marker of the diesel contribution to PM2.5 as approximately 75% of the chemical composition of DEP is elemental carbon in contrast to 4–15% of the overall chemical composition of PM2.5 (26, 27). The fraction of elemental carbon attributable to traffic (ECAT) sources was determined (28). A regression model was then used to determine the significant characteristics that predict average daily ECAT levels at each monitoring location. The final model accounted for 75% of the variability at the monitoring stations in the sampled levels of ECAT sources (R2 = 0.75) (10). Individual child traffic exposure estimates were derived using the parameter estimates obtained from this model and by calculating the elevation, average daily truck count on major roads within 400 m, and the length of bus routes within 100 m for each child’s residence at birth. Exposure assessment was calculated at one time point based on the child’s address at birth.
Statistical analyses
A structural equation model (s.e.m.) was developed to simultaneously examine the occurrence of RNC at all ages, while assessing multiple independent covariates of family, health, potential allergens in the home, and environmental exposures on their association with RNC. With a dichotomous outcome, the logistic link function was used, and the method of analysis was maximum likelihood estimation. Covariate effects (odds ratios (OR) and 95% confidence intervals (CI)) on RNC during early childhood were estimated by this model. This model provided an average effect over the first three yrs of life by using a linear combination of the outcome RNC at age one, two, and three. Age one, two, and three responses for wheeze and the clinical evaluation of SPT positive/negative were included in the model as independent covariates. Due to skewed distributions, traffic exhaust, endotoxin, and house dust mite were dichotomized with levels above the highest tertile of exposure defined as the highest exposure group (10). Covariate interactions were explored and a backward elimination procedure determined the final model. A second s.e.m. was fit in order to investigate child wheeze as an intervening variable in the pathway from each covariate to recurrent RNC. The ability to include an intervening pathway in the model is one of the benefits of using a s.e.m. over a traditional Generalized Estimating Equations (GEE) type model. In addition, s.e.m. allows us to consider the temporal ordering of covariates and the mechanistic relationships among the covariates (29). The model can be specified such that, for example, traffic exhaust exposure predicts child’s wheezing symptom which then predicts RNC, and at the same time traffic exhaust exposure independently predicts RNC. Statistical analyses were performed using sas (SAS Institute Inc., Cary, NC, USA) and Mplus (version 4.2, Muthen & Muthen).
Results
A total of 762 children fulfilled the eligibility requirements and enrolled into the CCAAPS cohort. Of these children, 550 (72%) had information available for all three clinical exams and were included in this analysis. Demographic and exposure variables for infants with and without RNC during the three yrs of follow-up are presented in Table 1. Fewer children were breastfed in the RNC group compared to the no RNC group, 66% vs. 75% (p = 0.02). About 45% of parents reported their child was wheezing in the RNC group compared to only 24% in the no RNC group (p < 0.01). There were more children with high traffic exhaust exposure in the RNC group compared to the no RNC group, 42% vs. 29%, respectively (p < 0.01). There were no significant differences with any other variables.
Table 1.
Summary of demographic and exposure variables by recurrent dry night cough (RNC) ever vs. never at age one, two, and/or three
| No RNC (n = 335)* | RNC at Age 1, 2, and/or 3 (n = 215)* | χ2 p value | |
|---|---|---|---|
| Female (n = 251) | 153 (45.7%) | 98 (45.6%) | 0.98 |
| African–American (n = 99) | 53 (15.8%) | 46 (21.4%) | 0.10 |
| Breastfed (n = 391) | 250 (74.6%) | 141 (65.6%) | 0.02 |
| Wheeze (n = 175) | 79 (23.6%) | 96 (44.7%) | <0.01 |
| Mother smokes (n = 68) | 39 (11.6%) | 29 (13.5%) | 0.52 |
| Maternal asthma (n = 134) | 76 (22.7%) | 58 (27.0%) | 0.25 |
| Paternal asthma (n = 76) | 45 (13.5%) | 31 (14.4%) | 0.75 |
| Dog in home (n = 198) | 120 (35.8%) | 78 (36.3%) | 0.91 |
| Cat in home (n = 127) | 78 (23.3%) | 49 (22.8%) | 0.89 |
| Visible mold (n = 276) | 170 (54.1%) | 106 (54.6%) | 0.91 |
| Highest traffic exhaust† (n = 198) | 104 (31.1%) | 94 (43.7%) | <0.01 |
| Highest endotoxin† (n = 162) | 103 (33.6%) | 59 (30.7%) | 0.51 |
| Highest dust mite† (n = 188) | 118 (37.7%) | 70 (35.5%) | 0.62 |
Denominators may vary slightly.
Highest = above the top tertile of exposure: traffic exhaust = 0.375 μg/m3, endotoxin = 133 EU/mg, dust mite = 22 EU/mg.
The prevalence and incidence of RNC at age one, two, and three are shown in Table 2. The prevalence of RNC was similar at all three yrs of age ranging from 17.3% to 21.6%. The incidence of RNC steadily decreased from 21.6% to 13.5% and 10.2% for ages one, two, and three, respectively.
Table 2.
Prevalence and incidence of recurrent dry night cough (RNC) at age one, two, and three
| Age 1 | Age 2 | Age 3 | |
|---|---|---|---|
| Prevalence | 21.6%(119/550) | 17.3%(95/550) | 21.1%(116/550) |
| Incidence | 21.6%(119/550) | 13.5%(58/431) | 10.2%(38/373) |
The full structural equation model including all variables is depicted in Fig. 1. From the final model after backward elimination, wheezing was the strongest significant predictor of RNC (adjusted OR = 1.76; 95% CI: 1.36, 2.26) (Fig. 2). Even after adjustment for wheezing, there remained a 45% significant increase in the risk of RNC with high traffic exhaust exposure (adjusted OR = 1.45; 95% CI: 1.09, 1.94) (Fig. 2). This model provides an average effect of traffic exhaust exposure on RNC over the first three yrs of life. However, strong associations between high traffic exhaust exposure and RNC was observed at all three yrs separately (year one unadjusted OR = 1.58; 95% CI: 1.04, 2.39; year two unadjusted OR = 1.77; 95% CI: 1.13, 2.77; year three unadjusted OR = 1.67; 95% CI: 1.10, 2.53). A 27% reduction in risk of RNC was found for those children who were breastfed compared to not breastfed (adjusted OR = 0.73; 95% CI: 0.53, 1.01; p = 0.06) (Fig. 2). All other predictor variables were not statistically associated with RNC.
Fig. 1.
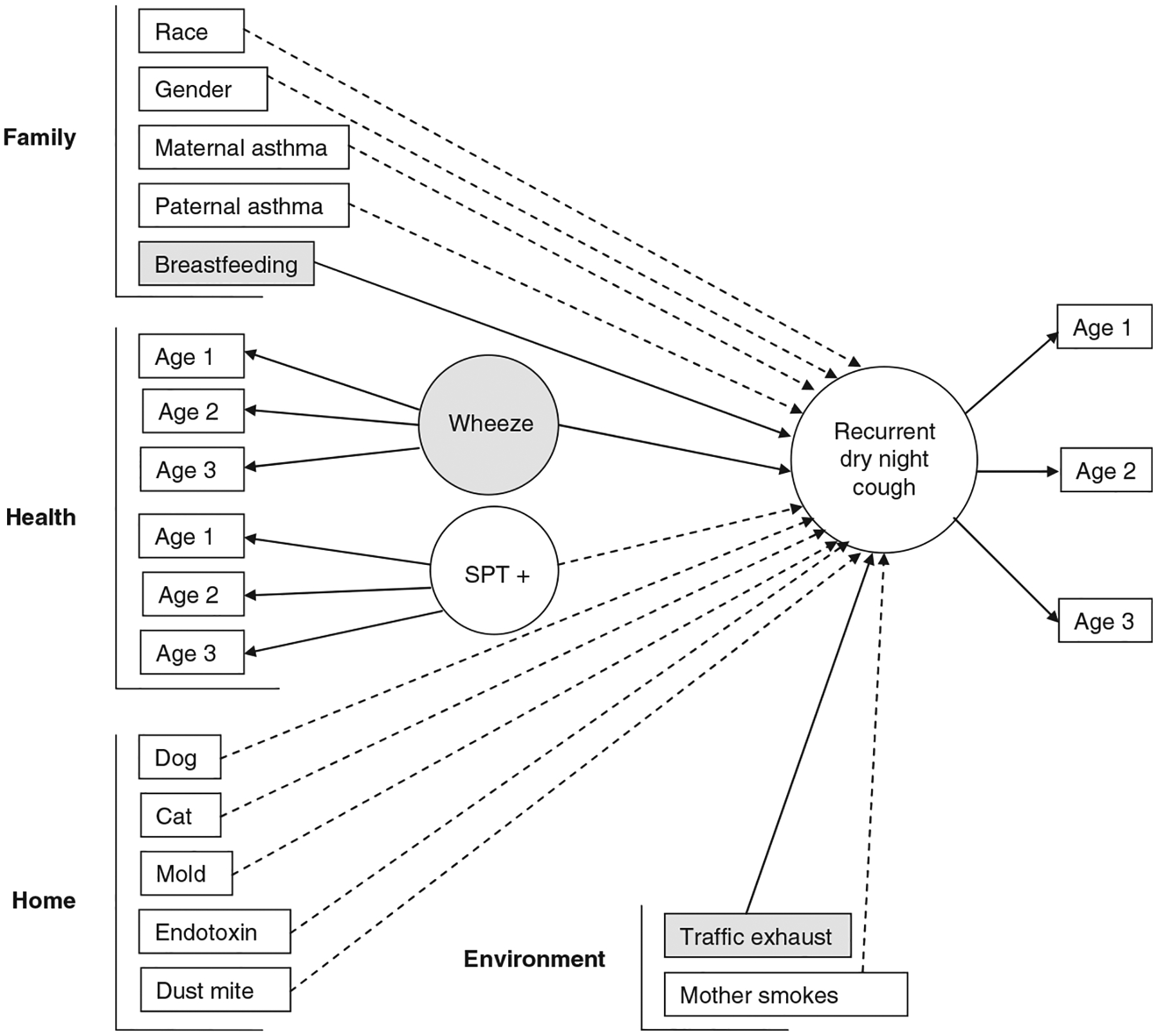
Structural equation model with pathways from each covariate to recurrent dry night cough (RNC). By convention, latent variables are depicted as ovals and rectangles represent indicator and other measured variables. For example, the latent variable wheeze is measured by the indicator variable wheeze at age one, wheeze at age two, and wheeze at age three. Solid lined arrows indicate significant associations and dashed lined arrows show associations that were not significant. Significant covariates are also shaded. The directions of the arrows distinguish between predictors and outcomes and do not necessarily imply causality.
Fig. 2.
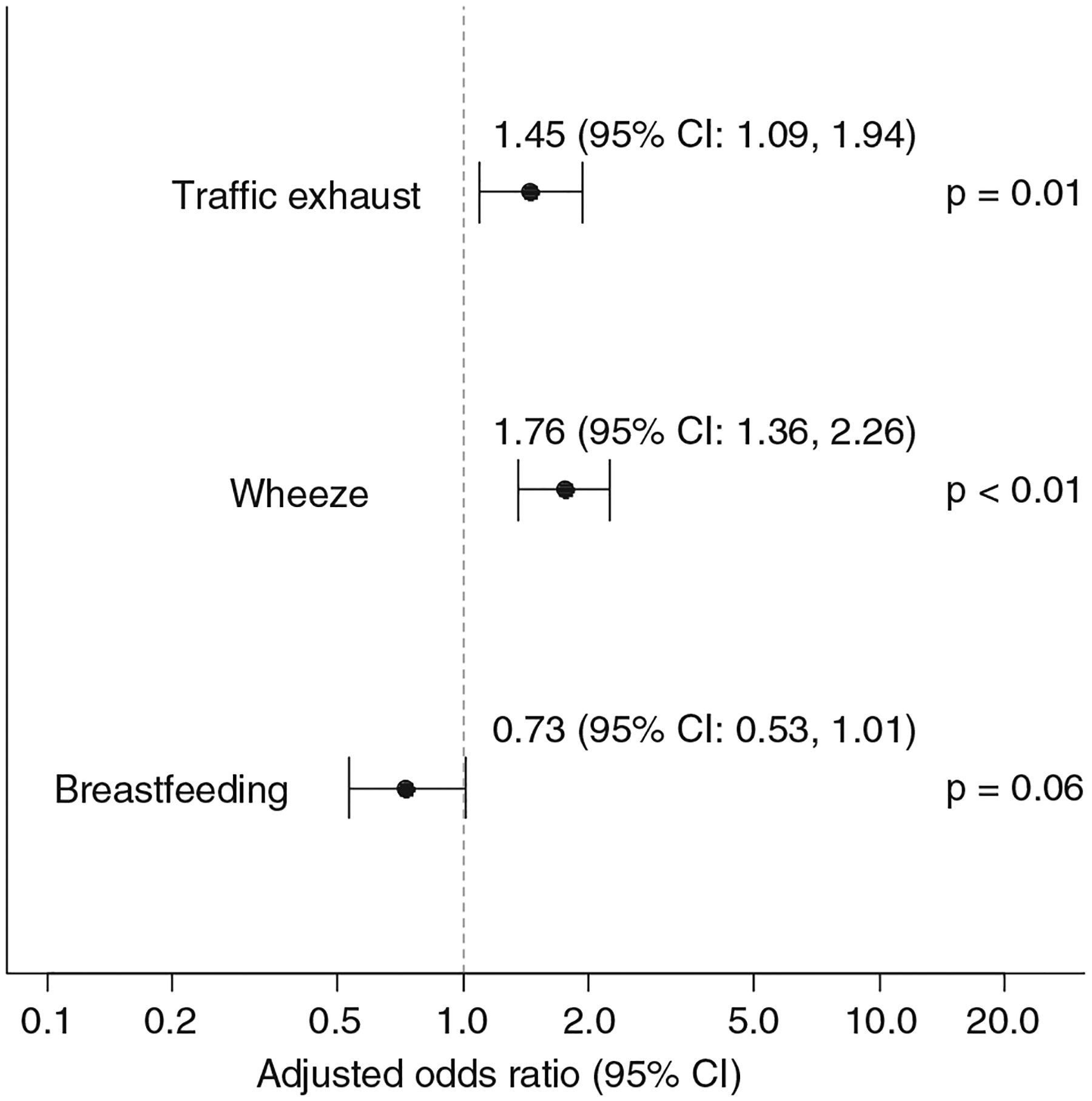
Adjusted odds ratios and 95% confidence intervals from the final structural equation model after backward elimination of insignificant covariates.
To determine if a child’s wheezing symptom acted as an intervening condition in the causal pathway for RNC a second model was constructed allowing pathways through wheeze to RNC (Fig. 3). After backward elimination, there remained an almost identical risk for having RNC for those who have wheezing symptoms as well as high traffic exhaust exposure. Additionally, by allowing pathways from each variable to also predict wheeze, a significant increase in wheeze was also seen with high traffic exhaust exposure (adjusted OR = 1.29; 95% CI: 1.01, 1.64; p = 0.04), but this effect was not as strong as it was for RNC (Fig. 4). Children whose fathers were diagnosed with asthma also had a significant increased risk for wheeze (adjusted OR = 1.68; 95% CI: 1.19, 2.36) as did children with mothers who smoked (adjusted OR = 1.60; 95% CI: 1.17, 2.21) (Fig. 4). In this model, however, there was no longer a trend of lower risk of RNC for those children who were breastfed. All other predictor variables were not statistically associated with RNC or wheeze.
Fig. 3.
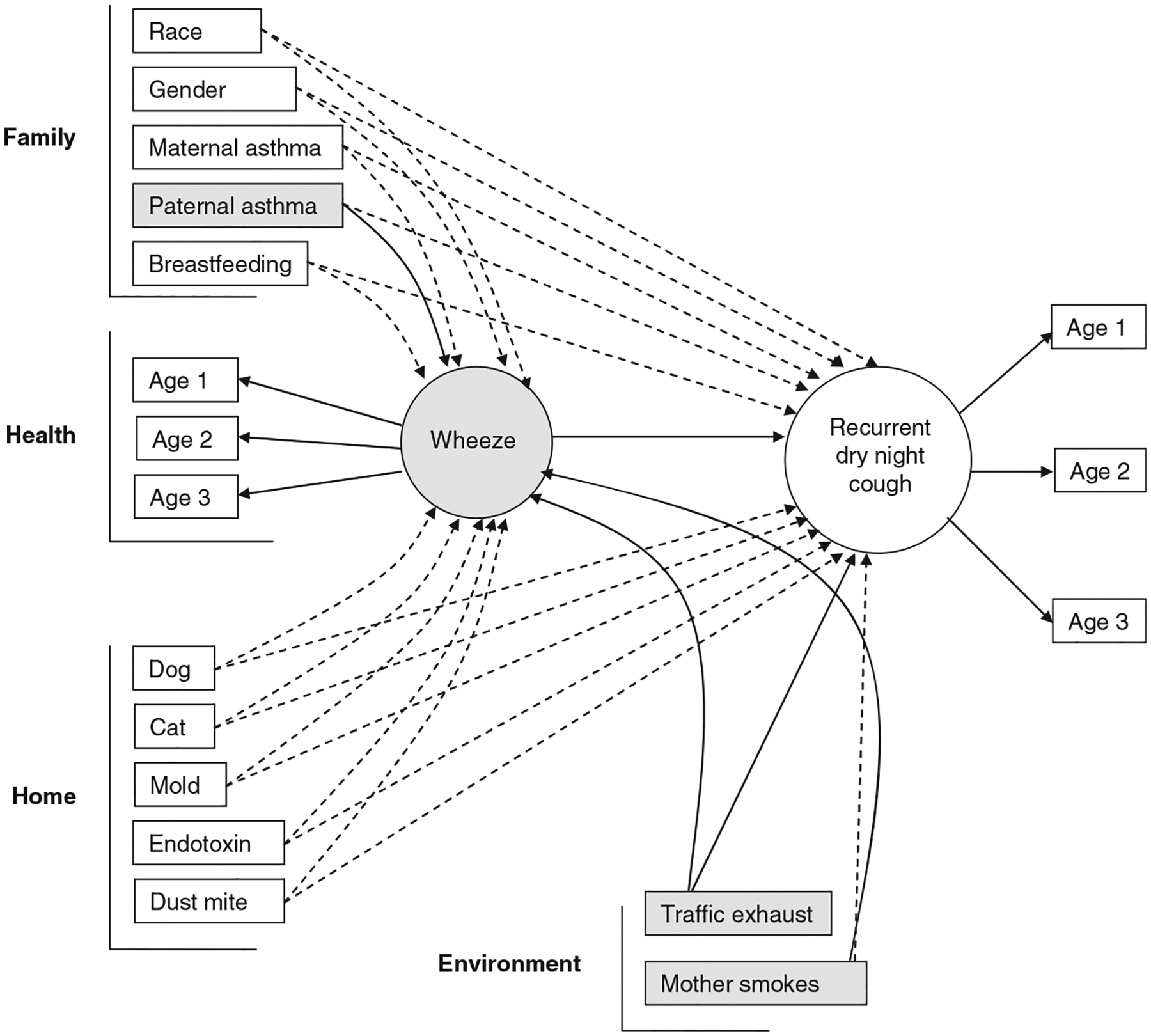
Intervening structural equation model with temporal pathways from each covariate to wheeze then to recurrent dry night cough (RNC).
Fig. 4.
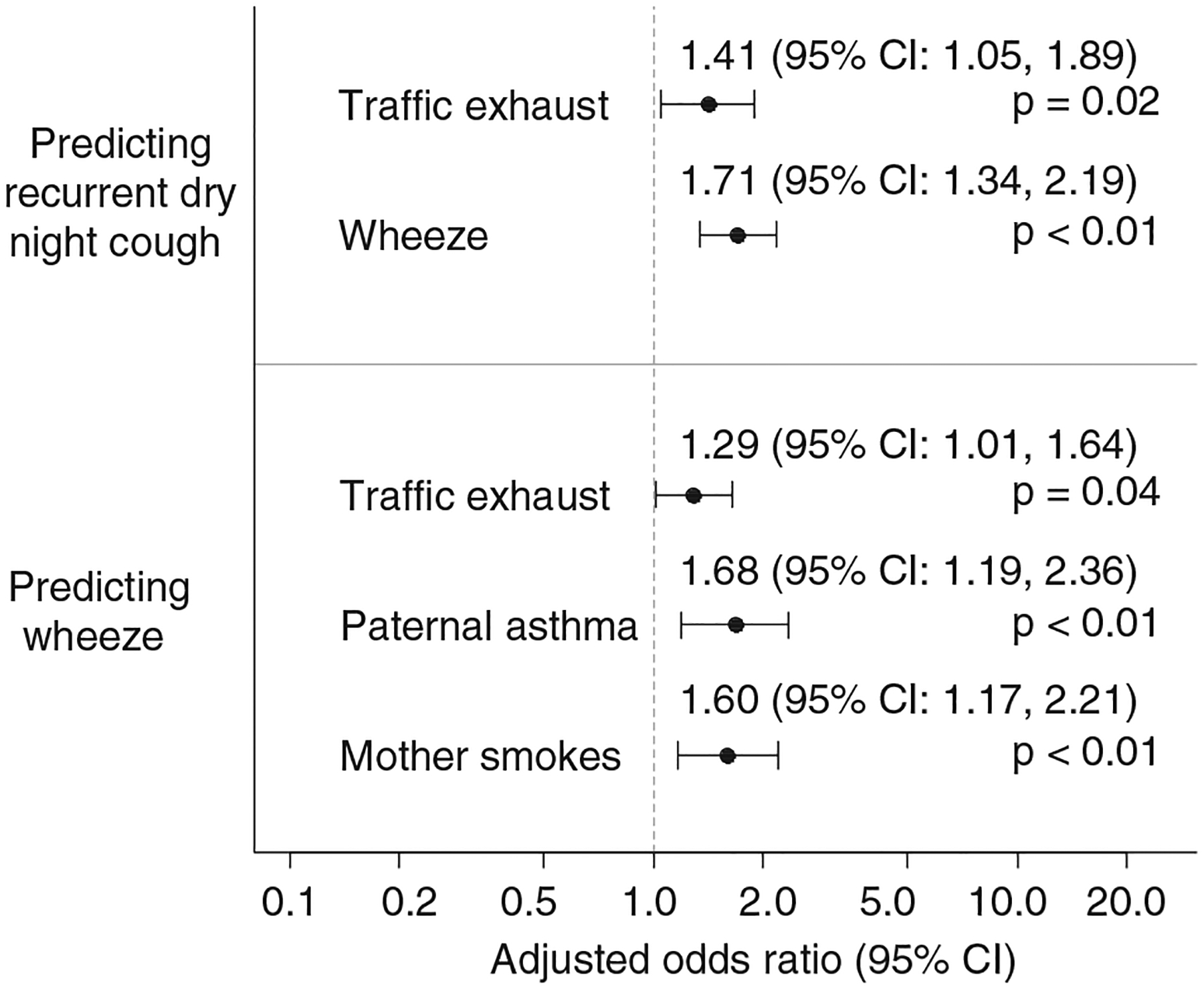
Adjusted odds ratios and 95% confidence intervals from the final intervening structural equation model with temporal pathways through wheeze after backward elimination of insignificant covariates.
Discussion
This study adds to the evidence of an association between air pollution and poor respiratory health outcomes in children, although RNC has infrequently been examined. An estimated 45% increase in the risk of RNC was found to be associated with increased traffic exhaust exposure. This finding is consistent with the Hirsch et al. (30) study, who reported an increase in the prevalence of cough in children associated with an increase in exposure to traffic-related air pollution, based on a cross-sectional study in Germany. Also, a cross-sectional study among children residing in Switzerland with lower PM10 levels showed a decline in dry night cough (31).
In the intervening model, a 41% increase in the risk of RNC and a 29% increase in the risk of wheeze were both significantly associated with high traffic exhaust exposure. Traffic exhaust exposure is a strong predictor of RNC and not just for children who wheeze, as we have previously shown (9, 10). In addition, infants with wheezing symptoms were found to have an estimated 71% higher risk of RNC compared to non-wheezers. This result is consistent with the findings by Powell and Primhak (32) who reported that children with RNC were more likely to develop wheezing if they had a family history of atopy. The children in the CCAAPS cohort also have a family history of atopy, since at least one parent had to have one or more symptoms of allergy and a SPT positive result.
Infants who were breastfed were 27% less likely to have RNC compared to those infants that were not breastfed (p = 0.06). The health benefits associated with breastfeeding have long been advocated. In a cohort study, Cushing et al. (33) found that full breastfeeding was associated with a lower risk of lower respiratory illness and a reduced duration of all respiratory illnesses in the first 6 months of life. The protective effect of breastfeeding on the development of asthma, however, is still controversial. Our findings suggest that breastfeeding may have limited protective effect on RNC.
In the intervening model, we found that children of fathers diagnosed with asthma were at increased risk of wheeze, but paternal asthma was not directly associated with the risk of RNC. Similarly, children of mothers who smoked were at increased risk of wheeze, but mother smoking was not directly associated with the risk of RNC. This model implies that these factors may be more important in predicting wheezing symptoms which, in turn, are strongly associated with RNC.
These findings have some limitations. The limitations include the potential for misclassification of exposure estimates, although the exposure regression modeling techniques may reduce this possible bias. Findings, also, may not be generalizable to all infants, since these were children at higher risk for allergic symptoms. Further, all infants resided in the greater Cincinnati area, a region with the convergence of five interstates (I-75, I-74, I-275, I-475, and I-71) which contributes to high truck traffic with over 60,000 trucks passing through it daily (34).
One of the benefits of this study is the use of longitudinal data that allowed for the estimation of exposure effects of RNC over three yrs of follow up during early childhood. To our knowledge, this is the first study to use a s.e.m. to assess multiple independent variables on the outcome of RNC during early childhood. With this model, the intervening pathways, i.e., through childhood wheezing symptoms, can be specified in addition to direct pathways to the outcome of interest, RNC. Hence, this model helps to dissect the complex relationships among variables and symptoms. This study is unique as it also utilized objective measures of traffic exhaust as well as other exposures, whereas other studies have relied solely on distance from a major road as a surrogate of air pollution exposure.
Acknowledgments
We thank all the members of the CCAAPS study team and the participating families.
Funding
This study was supported by the National Institute of Environmental Health Sciences (NIEHS) grants ES10957 and ES11170.
References
- 1.Clarisse B, Demattei C, Nikasinovic L, et al. Bronchial obstructive phenotypes in the first year of life among Paris birth cohort infants. Pediatr Allergy Immunol 2009: 20: 126–33. [DOI] [PubMed] [Google Scholar]
- 2.Brauer M, Hoek G, Van Vliet P, et al. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Crit Care Med 2002: 166: 1092–8. [DOI] [PubMed] [Google Scholar]
- 3.Brauer M, Hoek G, Smit HA, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J 2007: 29: 879–88. [DOI] [PubMed] [Google Scholar]
- 4.de Benedictis FM, Selvaggio D, de Benedictis D. Cough, wheezing and asthma in children: lesson from the past. Pediatr Allergy Immunol 2004: 15: 386–93. [DOI] [PubMed] [Google Scholar]
- 5.Robertson CF, Heycock E, Bishop J, Nolan T, Olinsky A, Phelan PD. Prevalence of asthma in Melbourne school children: changes over 26 yr. BMJ 1991: 302: 1116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall CB, Wakefield D, Rowe TM, Carlisle PS, Cloutier MM. Diagnosing pediatric asthma: validating the easy breathing survey. J Pediatr 2001: 139: 267–72. [DOI] [PubMed] [Google Scholar]
- 7.Leung AK, Robson WL, Tay-Uyboco J. Chronic cough in children. Can Fam Physician 1994: 40: 531–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Venn AJ, Lewis SA, Cooper M, Hubbard R, Britton J. Living near a main road and the risk of wheezing illness in children. Am J Respir Crit Care Med 2001: 164: 2177–80. [DOI] [PubMed] [Google Scholar]
- 9.Ryan PH, LeMasters G, Biagini J, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic J Allergy Clin Immunol 2005: 116: 279–84. [DOI] [PubMed] [Google Scholar]
- 10.Ryan PH, LeMasters GK, Biswas P, et al. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environ Health Perspect 2007: 115: 278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards J, Walters S, Griffiths RK. Hospital admissions for asthma in pre-school children: relationship to major roads in Birmingham, United Kingdom. Arch Environ Health 1994: 49: 223–7. [DOI] [PubMed] [Google Scholar]
- 12.Pershagen G, Rylander E, Norberg S, Eriksson M, Nordvall SL. Air pollution involving nitrogen dioxide exposure and wheezing bronchitis in children. Int J Epidemiol 1995: 24: 1147–53. [DOI] [PubMed] [Google Scholar]
- 13.Zmirou D, Gauvin S, Pin I, et al. Traffic related air pollution and incidence of childhood asthma: results of the Vesta case-control study. J Epidemiol Community Health 2004: 58: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbi E, Longo G. Chronic and recurrent cough, sinusitis and asthma. Much ado about nothing. Pediatr Allergy Immunol 2007: 18: 22–4. [DOI] [PubMed] [Google Scholar]
- 15.Morgenstern V, Zutavern A, Cyrys J, et al. Respiratory health and individual estimated exposure to traffic-related air pollutants in a cohort of young children. Occup Environ Med 2007: 64: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gehring U, Cyrys J, Sedlmeir G, et al. Traffic-related air pollution and respiratory health during the first 2 yrs of life. Eur Respir J 2002: 19: 690–8. [DOI] [PubMed] [Google Scholar]
- 17.Pierse N, Rushton L, Harris RS, Kuehni CE, Silverman M, Grigg J. Locally generated particulate pollution and respiratory symptoms in young children. Thorax 2006: 61: 216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behrens T, Taeger D, Maziak W, et al. Self-reported traffic density and atopic disease in children. Results of the ISAAC Phase III survey in Muenster, Germany. Pediatr Allergy Immunol 2004: 15: 331–9. [DOI] [PubMed] [Google Scholar]
- 19.Nel A Air pollution-related illness: effects of particles. Science 2005: 308: 804–6. [DOI] [PubMed] [Google Scholar]
- 20.Holguin F. Traffic, outdoor air pollution, and asthma. Immunol and Allergy Clin N Am 2008: 28: 577–88. [DOI] [PubMed] [Google Scholar]
- 21.Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. J Allergy Clin Immunol 2005: 115: 221–8. [DOI] [PubMed] [Google Scholar]
- 22.Wan JX, Diaz-Sanchez D. Antioxidant enzyme induction: a new protective approach against the adverse effects of diesel exhaust particles. Inhalation Toxicology 2007: 19: 177–82. [DOI] [PubMed] [Google Scholar]
- 23.LeMasters GK, Wilson K, Levin L, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr 2006: 149: 505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho SH, Reponen T, Bernstein D, et al. The effect of home characteristics on dust antigen concentrations and loads in homes. Sci Total Environ 2006: 371: 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martuzevicius D, Grinshpun SA, Reponen T, et al. Spatial and temporal variations of PM2.5 concentration and composition throughout an urban area with high freeway density – the Greater Cincinnati Study. Atmos Environ 2004: 38: 1091–105. [Google Scholar]
- 26.Wichmann HE. Diesel exhaust particles. Inhalation Toxicology 2007: 19: 241–4. [DOI] [PubMed] [Google Scholar]
- 27.U.S. EPA (Environmental Protection Agency). Health assessment document for diesel engine exhaust. Prepared by the National Center for Environmental Assessment, Washington DC, for the Office of Transportation and Air Quality; 2002. EPA/600/8–90/057F. Available: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=29060 [accessed 12 October 2006]. [Google Scholar]
- 28.Hu S, McDonald R, Martuzevicius D, et al. UNMIX modeling of ambient PM2.5 near an interstate highway in Cincinnati, OH, USA. Atmos Environ 2006: 40: 378–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buncher C, Succop P, Dietrich K. Structural equation modeling in environmental risk assessment. Environ Health Perspect 1991: 90: 209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsch T, Weiland SK, von Mutius E, et al. Inner city air pollution and respiratory health and atopy in children. Eur Respir J 1999: 14: 669–77. [DOI] [PubMed] [Google Scholar]
- 31.Bayer-Oglesby L, Grize L, Gassner M, et al. Decline of ambient air pollution levels and improved respiratory health in Swiss children. Environ Health Perspect 2005: 113: 1632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell CVE, Primhak RA. Stability of respiratory symptoms in unlabelled wheezy illness and nocturnal cough. Arch Dis Child 1996: 75: 385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cushing AH, Samet JM, Lambert WE, et al. Breastfeeding reduces risk of respiratory illness in infants. Am J Epidemiol 1998: 147: 863–70. [DOI] [PubMed] [Google Scholar]
- 34.Ohio Department of Transportation. Traffic Survey Report 2005. Available: http://www2.dot.state.oh.us/techservsite/offceorg/traffmonit/countinformation/ [accessed 14 January 2009].


