Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is a common chronic liver disease in human history and it is expected to surpass other causes of liver disease mortality by 2030. Therefore, finding an alternative way to diagnose steatosis in the early stage when imaging modalities are not available is crucial. This study decided to validate the optimal cut-off points and the sensitivity and specificity of the Fatty Liver Index (FLI) based on the Iranian population compared to ultrasonography.
Methods
The data of 367 individuals, 108 males and 259 females over 35, were analyzed. Hepatic steatosis was identified by ultrasound. FLI was determined from waist circumference, gamma-glutamyl transferase, triglyceride, and body mass index data. The receiver operating characteristic curve (ROC) was used to determine the best FLI index cut point for diagnosing nonalcoholic fatty liver. The sensitivity and specificity indices were calculated for the determined cut point.
Results
The AUC of the FLI index in diagnosing NAFLD in the total population was 0.733 (95% CI: 0.68–0.77, specificity = 0.6705, sensitivity = 0.7320) with the optimal COP of 40.6. There was a statistically significant association between non-alcoholic liver disease and FLI-based ultrasound (p < 0.0001). Furthermore, the sex-specific optimal COPs of FLI was 33.4, specificity = 0.6071, sensitivity = 0.8462 in men vs. 27.8, sensitivity = 0.8233, specificity = 0.7655 in women.
Conclusion
FLI is a reliable tool for identifying individuals with NAFLD. It has the potential to aid in detecting and managing this condition in large-scale populations while other methods are not available. We also determine an optimal COP of 40.6 with sensitivity and specificity of 73.20% and 67.05% in the general population, respectively.
Keywords: Fatty liver index, Ultrasound, Non-alcoholic fatty liver, Sensitivity, Specificity
Background
Non-alcoholic fatty liver disease (NAFLD) is a common chronic liver disease in human history, characterized by the excessive accumulation of lipid granules in liver hepatocytes (steatosis) in the absence of other etiologies, such as consumption Excessive alcohol intake or using hepatotoxic drugs [1–5]. (More than a daily intake of 30 g for men and 20 g for women) Although NAFLD is often asymptomatic, it includes a spectrum ranging from non-alcoholic steatohepatitis (NASH), steatohepatitis, liver tissue scarring (fibrosis), and hepatocellular carcinoma [6–9].
With the significantly increasing prevalence of metabolic syndrome-related disorders, like type ΙΙ diabetes, which are NAFLD’s leading causes, it is expected to surpass other causes of liver disease mortality by 2030 [10–12]. About 33% of people worldwide have NAFLD, with rates increasing from 25 to 38% in the last thirty years [13]. Yet, it is poorly understood by global health communities and the general population [14].
While liver biopsy is considered the gold standard technique for diagnosing hepatic steatosis, it is invasive and may result in clinical complications [15, 16]. Some non-invasive imaging techniques have been proposed as alternative methods to liver biopsy, which include transient elastography, magnetic resonance imaging (MRI), and computed tomography. However, these can be expensive and not readily available [6]. Conversely, ultrasound is a convenient and safe imaging method that can be performed easily, even on conscious patients.; it is broadly available and relatively cheap. Therefore, ultrasound is the primary diagnostic method for detecting NAFLD in most cases [17–19].
Early diagnosis of NAFLD is necessary to prevent its progression, and finding an alternative way to diagnose steatosis when imaging modalities are unavailable is crucial [20]. by using simple clinical and laboratory parameters such as waist circumference, triglycerides, gamma-glutamyl transferase, and body mass index to calculate Fatty Liver Index (FLI), it is a convenient index for screening and identifying high-risk patients for NAFLD [21, 22]. It has become a popular screening tool for NAFLD due to its simplicity and cost-effectiveness [20, 23]. However, optimal cut-off points for FLI may vary across different populations, including the Iranian population [24]. Therefore, due to the variation among population lifestyles and the differing cut-off points (COPs) for FLI parameters, this study aimed to identify the optimal cut-off points, as well as the specificity and sensitivity of the Fatty Liver Index based on the Iranian population compared to ultrasonography.
Methods
Participants
This cross-sectional study gathered data from the Fasa Cohort Study as a branch of the PERSIAN cohort, focusing on non-communicable diseases [25]. Informed consent was obtained from all subjects. Legal guardians were involved to ensure that consent was appropriately managed for any individuals who may have cognitive impairments or other conditions that could affect their ability to provide informed consent independently. Age over 35, no alcohol consumption, no history of congenital hepatic diseases, B, C, or autoimmune hepatitis were considered as inclusion criteria. The individuals who underwent abdominal surgery within the past six months and those with a history of drug use that could result in liver steatosis, like consumption of corticosteroids and valproate sodium, were excluded.
Anthropometric and biochemical assessment
Demographic data and the participants’ status of alcohol consumption, physical activity, smoking, and medical history, including blood hypertension, cardiovascular disease, diabetes, gastrointestinal disease, and stroke, were recorded in the questionnaire. Weight and height were measured and recorded by a trained healthcare worker (Behvarz). Blood pressure was taken two times, with a 15-minute gap between each measurement. After twelve hours of fasting, venous blood samples were drawn to analyze serum lipid profiles, aspartate aminotransferase, alanine transaminase, Gamma-glutamyl transpeptidase, and fasting blood sugar. Samples were analyzed at the Noncommunicable Diseases Research Center (NCDRC) laboratory. Physical activity was evaluated by the answers given in questionnaires. More information is explained in the cohort protocol [25, 26].
NAFLD diagnosis
Ultrasonography
Ultrasonography (US) was performed with a Samsung WS80A ultrasound machine by a trained radiologist at the ultrasound center of Valiasr Hospital in Fasa. To ensure accuracy and reliability, the US films of the patients were recorded and subsequently double-checked by another experienced radiologist. In cases where discrepancies between the radiologists’ interpretations of ultrasound results arise, the opinion of a senior radiologist would typically be accepted to resolve any differences and establish a conclusion. Based on the echogenicity of the liver parenchyma and the comparison with the echogenicity of the renal cortex (for patients with parenchymal renal disease, the liver parenchyma was compared with the spleen), participants were separated into two groups: without NAFLD and with NAFLD [27].
Fatty liver index
FLI was determined based on the following calculation:
FLI = [ 0.139 × BMI + e0.953 × ln (TG) + 0.718 × ln (GGT) + 0.053 × WC − 15.745 / (1 + e0.953 × ln (TG) + 0.718 × ln (GGT) + 0.139 × BMI + 0.053 × WC − 15.745)] × 100.
The cut-off points (COPs) of the FLI used in this study were based on previous literature, specifically at values of 30 and 60, as established in earlier research [20, 22].
Statistical analysis
All statistical analyses were performed using MedCalc20.0.26. Results were described as mean ± standard deviations for quantitative data and number and percentage for qualitative data. T-tests were used to compare means between groups, while chi-square tests were applied to evaluate associations between categorical variables. Receiver operating characteristic curve (ROC) analysis was employed to determine the optimal FLI cut-off point for diagnosing nonalcoholic fatty liver disease. The sensitivity and specificity indices were calculated for the determined cut point. Discrimination was assessed using the C-statistic (Area Under the Curve, AUC). Calibration was also evaluated through calibration plots, although specific metrics were not reported because the sample size was insufficient to provide reliable estimates. Confidence intervals (CIs) for sensitivity and specificity were calculated and reported. A p-value < 0.05 was considered significant in the analysis.
Result
This cross-sectional study evaluated 367 individuals, 108 males, and 259 females, with a mean age of 49.3 ± 8.94 years. The mean and standard deviation of BMI and physical activity index were 26.95 ± 4.60 kg/m2 and 40.66 ± 9.30 weekly MET (Metabolic Equivalent of Task)-minutes, respectively.
Demographic and clinical variables of nonalcoholic fatty liver disease and non-NAFLD diagnosed in the US are reported in Table 1. A total of 194 people (52.9%) had NAFLD. T-test analysis revealed that age (P = 0.007) and physical activity index (P = 0.034) are associated with NAFLD. Moreover, our study showed a significant statistical association between diabetes mellitus (DM) and nonalcoholic fatty liver disease based on chi-square (p = 0.018) (Table 1).
Table 1.
General Characteristics of Ultrasound Diagnosed NAFLD and Non-NAFLD
| Variables | NAFLD, N (%) | Non-NAFLD, N (%) | P Value | |
|---|---|---|---|---|
| Sex | 0.253 | |||
| Male | 52 (26.8) | 56 (32.4) | ||
| Female | 142 (73.2) | 117 (67.6) | ||
| DM | 0.018 | |||
| Yes | 42 (21.6) | 21 (12.1) | ||
| No | 152 (78.4) | 152 (87.9) | ||
| HTN | 0.251 | |||
| Yes | 46 (23.7) | 32 (18.5) | ||
| No | 148 (76.3) | 141 (81.5) | ||
| Smoking | 0.377 | |||
| Yes | 25 (12.9) | 28 (16.2) | ||
| No | 169 (87.1) | 145 (83.8) | ||
| Mean (SD) | Mean (SD) | |||
| Age | 48.68 (8.16) | 50 (9.72) | 0.007 | |
| Physical activity index | 39.58 (10.04) | 41.88 (8.48) | 0.034 | |
| FLI | 56.13 (25.62) | 33.71(25.37) | < 0.001 | |
| BMI | 28.75 (4.37) | 24.94 (3.99) | < 0.001 | |
| TG | 152.47(74.39) | 129.01(89.61) | < 0.001 | |
| GGT | 28.76(28.14) | 21.42(19.83) | < 0.001 | |
| WC | 100.79(9.86) | 91.84(10.68) | < 0.001 | |
Abbreviations: BMI, body mass index; DM, diabetes mellitus; FLI: Fatty Liver Index ⁏ GGT: gamma-glutamyl transferase⁏ HTN, hypertension; N, number; NAFLD, non-alcoholic fatty liver disease; SD, standard deviation; TG: triglycerides⁏ US, ultrasonography⁏ WC: waist circumference. Statistical tests: Chi-square test, independent t-test
Table 2 shows the frequency of NAFLD based on FLI’s different cut-off points and its relations between the study’s variables. Physical activity index and HTN are related to NAFLD in cut-off points 30 and 60 (FLI ≥ 30: p < 0.01, p = 0.017. FLI ≥ 60: p = 0.003, p = 0.014). DM is related to the NAFLD in the FLI cut-off point ≥ 60 (p = 0.014) (Table 2).
Table 2.
Association offattyyliverrindexx and variables with and without NAFLD
| Variable | FLI ≥ 30 | FLI ≥ 60 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NAFLD (Yes / No) | Mean (SD) | t | P Value | NAFLD (Yes / No) | Mean (SD) | t | P Value | |||
| Age (years) | -1.14 | 0.255 | -1.566 | 0.434 | ||||||
| Yes | 49.70(8.55) | Yes | 50.30 (8.87) | |||||||
| No | 48.59(9.58) | No | 48.77 (8.95) | |||||||
| BMI (kg/m2) | -17.547 | 0.000 | -16.347 | 0.078 | ||||||
| Yes | 29.30(3.83) | Yes | 31.04 (4.03) | |||||||
| No | 22.82(2.44) | No | 24.76 (3.18) | |||||||
| Physical Activity Index (weekly MET-minutes) | 3.595 | 0.000 | 2.876 | 0.003 | ||||||
| Yes | 39.37(8.15) | Yes | 38.77 (7.27) | |||||||
| No | 42.94(10.71) | No | 41.68 (10.10) | |||||||
| NAFLD, N (%) | Non-NAFLD, N (%) | P Value | NAFLD, N (%) | Non-NAFLD, N (%) | P Value | |||||
| Sex | 0.813 | 1.000 | ||||||||
| Male | 70 (29.9) | 38 (28.6) | 38 (29.7) | 70 (29.3) | ||||||
| Female | 164 (70.1) | 95 (71.4) | 90 (70.3) | 169 (70.7) | ||||||
| DM | 0.113 | 0.014 | ||||||||
| Yes | 46 (19.7) | 17 (12.8) | 24 (18.8) | 200 (83.7) | ||||||
| No | 188. (80.3) | 116 (87.2) | 39 (16.3) | 104 (81.3) | ||||||
| HTN | 0.017 | 0.014 | ||||||||
| Yes | 59 (25.2) | 114 (85.7) | 36 (28.1) | 42 (17.6) | ||||||
| No | 19 (14.3) | 175 (74.8) | 92 (71.9) | 197 (82.4) | ||||||
| Smoking | 1.000 | 0.372 | ||||||||
| Yes | 200 (85.5) | 114 (85.7) | 108 (84.4) | 206 (86.2) | ||||||
| No | 34 (14.5) | 19 (14.3) | 20 (15.6) | 33 (13.8) | ||||||
Abbreviations: BMI, body mass index; DM, diabetes mellitus; FLI, Fatty Liver Index; HTN, hypertension; MET: Metabolic Equivalent of Task; N, number; NAFLD, non-alcoholic fatty liver disease; SD, standard deviation
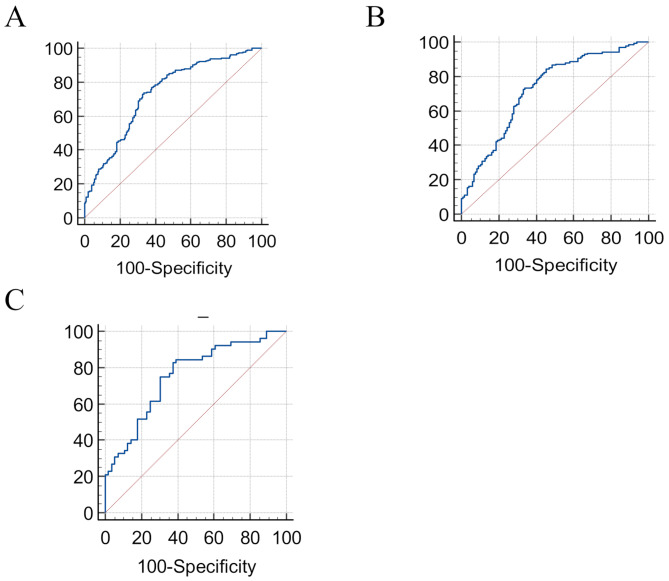
The AUC of the FLI index in the diagnosis of nonalcoholic fatty liver disease in the total population was 0.733 (95% CI: 0.68–0.77, specificity = 0.6705, sensitivity = 0.7320, 95% CI for sensitivity = [0.67–0.76], 95% CI for specificity = [0.61–0.71]) and the optimal cut-off is 40.6. Analysis showed that there was a statistically significant association between the non-alcoholic fatty liver disease and FLI based on ultrasound (p < 0.0001) (Table 3) (Fig. 1). Furthermore, the sex-specific optimal COP of FLI was 33.4 in men vs. 27.8 in women. The AUC of FLI in males was 0.75 (95% CI: 0.66–0.83, specificity = 0.6071, sensitivity = 0.8462, 95% CI for sensitivity = [0.75–0.89], 95% CI for specificity = [0.50–0.69]) (p < 0.0001) while in female was 0.72 (95% CI: 0.66–0.78, sensitivity = 0.8233, specificity = 0.7655, 95% CI for sensitivity = [0.79–0.88], 95% CI for specificity = [0.47–0.60]) (p < 0.0001) (Table 3) (Fig. 1).
Table 3.
The cut-off points of fatty liver index based on the results of liver US
| Variable | NAFLD, N (%) | AUC | Sensitivity (95% CI) | Specificity (95% CI) | PV | |
|---|---|---|---|---|---|---|
| Total n = (367) | 0.733 | 0.73 (0.67–0.76) | 0.67(0.61–0.71) | < 0.0001 | ||
| Yes | 194 (52.86) | |||||
| No | 173 (47.14) | |||||
| Male n = (108) | 0.755 | 0.84(0.75–0.89) | 0.60(0.50–0.69) | < 0.0001 | ||
| Yes | 52 (48.15) | |||||
| No | 56 (51.58) | |||||
| Female n = (259) | 0.727 | 0.84(0.79–0.88) | 0.54(0.47–0.60) | < 0.0001 | ||
| Yes | 142 (54.83) | |||||
| No | 117 (45.17) |
Abbreviation: AUC: area under the curve, CI: confidence interval, NAFLD: non-alcoholic fatty liver disease
Fig. 1.
Receiver Operating Characteristic Curves (ROC) of FLI with a cut-off point of A: 40.6 in total population. B: 27.8 in females. C: 33.4 in males
The area under the curve was calculated for the predictive model. Sensitivity and specificity were higher in males than in females. The AUC values indicated consistent performance across sexes, illustrating the model’s robustness in various populations.
Discussion
Nonalcoholic fatty liver disease (NAFLD) serves as an important indicator of metabolic syndrome and is associated with various metabolic-related conditions, including cardiovascular disease (CVD), diabetes mellitus, renal disease, thyroid dysfunction, polycystic ovarian syndrome (PCOS), and colorectal cancer [15, 28]. Given the increasing prevalence of NAFLD among individuals with these conditions, establishing an accessible, non-invasive, and cost-effective screening method is essential [29, 30].
Liver biopsy is an invasive procedure that may result in clinical complications and MRI, while accurate for detecting steatosis, is costly and not widely available [31]. Transient elastography is a straightforward, quick, and non-invasive method that can predict NAFLD in lean patients, but its accuracy in diagnosing NAFLD in obese patients may be limited [32]. Ultrasonography is widely available and relatively cheap, and it has become a standard method used for diagnosing hepatic steatosis based on increasing echogenicity of the liver by fat accumulation in hepatocytes. However, it is operator-dependent [33, 34]. A study by Irene Cantero et al. found that in the absence of MRI and biopsy, ultrasound (with ROC-AUC: 0.746) demonstrated the highest correlation with these methods [35]. The Fatty Liver Index is also a helpful method that can be easily calculated in a medical setting. Research has shown that FLI is strongly correlated with NAFLD diagnosed by ultrasonography [24]. FLI can be utilized for screening individuals with fatty liver disease and for identifying those at high risk for metabolic and cardiovascular disorders [36]. However, it is crucial to consider potential variations in waist and BMI cut-offs due to factors such as ethnicity, diet, and the environment [37]. Therefore, validation of FLI is necessary when implementing it in diverse populations.
Previous studies based on Western populations showed acceptable accuracy of cut-off points for FLI, with an AUC of 0.81–0.84. These studies suggested that an FLI below 30 effectively rules out hepatic steatosis with a sensitivity of 87%, while an FLI above 60 predicts the condition with a specificity of 86% [20, 22]. Juan Wu also in the American population compared both transient elastography and US with FLI and proposed a value of 45.60 and 59.54 for the optimal COP of FLI, AUC of 0.833 and 0.681, specificity of 70.50%, and 75.15%, sensitivity of 80.85% and 55.53%, respectively [38]. In an Asian population, similar values yielded an acceptable AUC of 0.87 [39]. In the Iranian population, Dehnavi et al. validated 26.2 as the optimal cut-off point based on a controlled attenuation parameter technique with an AUC of 0.85, sensitivity of 0.83, and specificity of 0.7 [40]. In the present study, we compared the use of abdominal ultrasonography, finding the optimal COP of FLI to be 40.6, with sensitivity and specificity of 73.20% and 67.05%. The variations in optimal cut-off points, sensitivity, and specificity of FLI across different studies may stem from differences in diagnostic methods, study populations, sample sizes, and ethnic backgrounds. Therefore, further research is needed to establish standardized guidelines for FLI interpretation, taking into account the diverse characteristics of populations and settings. The findings regarding Body Mass Index and diabetes mellitus indicate that a lower cut-off point of 30 is more sensitive for detecting early stages of liver fat accumulation, as it shows a significant difference in BMI between NAFLD and non-NAFLD. In contrast, no such difference is observed at a cut-off of 60. Additionally, it is important to consider the effective sample size of individuals with liver steatosis at FLI values of 30 and 60, as this can significantly impact the robustness and reliability of the results. A larger sample size at lower thresholds may provide more reliable insights into the association with NAFLD. This suggests that BMI may be a more effective marker for identifying at-risk individuals when using lower thresholds. Similarly, the relationship between DM and NAFLD becomes less pronounced at higher FLI cut-off points, indicating that metabolic implications are clearer at lower thresholds. Notably, using a cut-off point of 30 yielded a prevalence of NAFLD of 63.8% (234 individuals), compared to 34.9% (128 individuals) at a cut-off of 60, emphasizing the impact of cut-off selection on diagnostic results and highlighting the potential for overdiagnosis at lower thresholds, which could affect clinical practice and patient management. The studies of Bi-Ling Yang et al. [41] and Dehnavi et al. [40] showed that the optimal COPs of the FLI are higher in males than females. In line with these findings, in the current study, we calculated the optimal COPs of Fatty Liver Index 33.4 and 27.8 for males and females, respectively. The sensitivity and specificity of FLI were higher in males than in females, with sensitivity exceeding 82% and specificity exceeding 60% for both genders. Inconsistent with our findings, in a study conducted in 2016 on 5052 subjects, the cut-off value in females was suggested higher than in males [23]. Resolving these conflicting results is challenging; however, the differences in optimal cut-off points may partly be explained by the observation that males generally have a higher body mass index and more severe metabolic disturbances, contributing to the development of NAFLD. As males age, they experience an increase in visceral fat, leading to fatty liver disease and insulin resistance due to the release of adipocytokines and free fatty acids [41]. In women Estrogen plays a role in suppressing the accumulation of visceral fat and triglycerides though they have an increased risk of being obese and metabolic syndrome after menopause, suggesting a potential protective effect of estrogen in preventing the onset of fatty liver disease [42, 43].
Based on our findings, if FLI is considered more than 30, there was no significant relationship between NAFLD and the independent variables of gender, diabetes, and smoking. Body mass index, physical activity index, and hypertension (HTN) were significantly related to NAFLD. Moreover, with a cut-off point of more than 60 for FLI, no statistically significant relationship was observed between NAFLD and gender, DM, and smoking; however, a significant correlation was found between physical activity index and increased blood pressure with NAFLD. A study by Bi-Ling Yang involving 23,797 participants indicated positive relations between age, gender, BMI, fasting blood sugar, blood pressure, and FLI [44]. Variations in sample size, population characteristics, and different cut-off points for FLI may account for discrepancies in results. Acknowledging that relying on abdominal ultrasonography as a comparison technique for diagnosing NAFLD may introduce potential limitations is essential. Ultrasound results can be influenced by the experience of the radiologist and may be subject to interpretation variability. Future studies should consider incorporating additional diagnostic techniques to enhance the accuracy and reliability of NAFLD diagnosis. In the present study, we focused on the area under the curve for evaluating the effectiveness of the FLI. This approach provides a more comprehensive understanding of diagnostic performance across different populations. Differences in AUC values across studies can be attributed to various factors, including sample size, population characteristics, and diagnostic methods. Despite limitations related to sample variation and diagnostic methods, findings from comparative studies support the efficiency of FLI in predicting NAFLD. Therefore, we recommend the Fatty Liver Index as a reliable method for detecting NAFLD and suggest determining optimal cut-off points based on population characteristics.
Conclusion
The Fatty Liver Index is a reliable tool for identifying individuals with NAFLD. It has the potential to aid in the detection and management of this condition in large-scale populations where other methods may not be available. We determined an optimal cut-off point of 40.6, with sensitivity and specificity of 73.20% and 67.05% in the general population, respectively.
Acknowledgements
The authors would like to thank Shiraz and Fasa University of Medical Sciences for supporting this project and the NCD research center at FUMS for their cooperation.
Abbreviations
- AUC
Area under the curve
- BMI
Body mass index
- CVD
Cardiovascular disease
- COPs
Cut-off points
- DM
Diabetes mellitus
- FLI
Fatty Liver Index
- FUMS
Fasa University of Medical Sciences
- GGT
Gamma-glutamyl transferase
- HTN
Hypertension
- MET
Metabolic Equivalent of Task
- MRI
Magnetic resonance imaging
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- NCDRC
Noncommunicable Diseases Research Center
- PCOs
Polycystic ovarian syndrome
- ROC
Receiver operating characteristic curve
- SD
Standard deviation
- TG
Triglyceride
- US
Ultrasonography
- WC
Waist circumference
Author contributions
A.S., S.K., F.Z., M.F. gathered the data; analysis and interpretation of data: A.D., A.S., S.N.; drafting of the manuscript: S.N., A.S., M.F.; critical revision of the manuscript for important intellectual content: M.F., A.S., S.N., S.K.; statistical analysis: A.D., R.H., M.F.; administrative, technical, and material support: M.F., R.H., A.D.; study supervision: M.F., S.K., F.Z.; All authors discussed the results and contributed to the final manuscript.
Funding
No fund was received for this article.
Data availability
Data supporting this study’s findings are available from the Noncommunicable Disease Research Center of Fasa University of Medical Sciences. Still, restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. The data are, however, available from the corresponding author upon reasonable request and with the permission of the Noncommunicable Disease Research Center of Fasa University of Medical Sciences.
Declarations
Declarations
The results/data/figures in this manuscript have not been published elsewhere, nor are they under consideration by another publisher.
While preparing this work, the author used ChatGPT to paraphrase some parts of the article. After using this tool, the author reviewed and edited the content as needed and took full responsibility for the publication’s content.
Ethics approval and consent to participate
The study protocol was registered and approved by the Ethics Committee of Fasa University of Medical Sciences (FUMS) by No: IR.FUMS.REC.1400.095 Furthermore, the study was performed following the Declaration of Helsinki. Informed consent was obtained from all subjects and/or their legal guardian(s).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sepideh Niknejad, Email: Sepideh.niknejad@yahoo.com.
Mojtaba Farjam, Email: farjam.phd@gmail.com.
References
- 1.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40:S17–29. [DOI] [PubMed] [Google Scholar]
- 2.Serfaty L, Lemoine M. Definition and natural history of metabolic steatosis: clinical aspects of NAFLD, NASH and cirrhosis. Diabetes Metab. 2008;34(6):634–7. [DOI] [PubMed] [Google Scholar]
- 3.Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158(7):1851–64. [DOI] [PubMed] [Google Scholar]
- 4.Ruhl C, Everhart J. Fatty liver indices in the multiethnic United States national health and nutrition examination survey. Aliment Pharmacol Ther. 2015;41(1):65–76. [DOI] [PubMed] [Google Scholar]
- 5.Ekstedt M, Franzén LE, Holmqvist M, Bendtsen P, Mathiesen UL, Bodemar G, et al. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2009;44(3):366–74. [DOI] [PubMed] [Google Scholar]
- 6.Byra M, Styczynski G, Szmigielski C, Kalinowski P, Michałowski Ł, Paluszkiewicz R, et al. Transfer learning with deep convolutional neural network for liver steatosis assessment in ultrasound images. Int J Comput Assist Radiol Surg. 2018;13:1895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szanto KB, Li J, Cordero, Oben JA. Ethnic differences and heterogeneity in genetic and metabolic makeup contributing to nonalcoholic fatty liver disease. Diabetes Metabolic Syndrome Obesity: Targets Therapy. 2019;357:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamali R, Jamali A. Non-alcoholic fatty liver disease. KAUMS J (Feyz). 2010;14(2):169–79. [Google Scholar]
- 9.Ahmed A, Wong RJ, Harrison SA. Nonalcoholic fatty liver disease review: diagnosis, treatment, and outcomes. Clin Gastroenterol Hepatol. 2015;13(12):2062–70. [DOI] [PubMed] [Google Scholar]
- 10.Vernon G, Baranova A, Younossi Z. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–85. [DOI] [PubMed] [Google Scholar]
- 11.Fleischman MW, Budoff M, Zeb I, Li D, Foster T. NAFLD prevalence differs among hispanic subgroups: the multi-ethnic study of atherosclerosis. World J Gastroenterology: WJG. 2014;20(17):4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machado MV, Diehl AM. Pathogenesis of nonalcoholic steatohepatitis. Gastroenterology. 2016;150(8):1769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling nafld disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. [DOI] [PubMed] [Google Scholar]
- 15.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–609. [DOI] [PubMed] [Google Scholar]
- 16.Cao W, Zhao C, Shen C, Wang Y. Cytokeratin 18, alanine aminotransferase, platelets and triglycerides predict the presence of nonalcoholic steatohepatitis. PLoS ONE. 2013;8(12):e82092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265–9. [DOI] [PubMed] [Google Scholar]
- 18.Abd El-Kader SM, El-Den Ashmawy EMS. Non-alcoholic fatty liver disease: the diagnosis and management. World J Hepatol. 2015;7(6):846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karthik L, Kumar G, Keswani T, Bhattacharyya A, Chandar SS, Bhaskara Rao K. Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. PLoS ONE. 2014;9(3):e90972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganji A, Safavi M, Nouraie S, Nasseri-Moghadam S, Merat S, Vahedi H, et al. Digestive and liver diseases statistics in several referral centers in Tehran, 2000–2004. Govaresh. 2006;11(1):33–8. [Google Scholar]
- 22.Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. 2013;11(9):1201–4. [DOI] [PubMed] [Google Scholar]
- 23.Motamed N, Sohrabi M, Ajdarkosh H, Hemmasi G, Maadi M, Sayeedian FS, et al. Fatty liver index vs waist circumference for predicting non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22(10):3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calès P, Boursier J, Chaigneau J, Lainé F, Sandrini J, Michalak S, et al. Diagnosis of different liver fibrosis characteristics by blood tests in non-alcoholic fatty liver disease. Liver Int. 2010;30(9):1346–54. [DOI] [PubMed] [Google Scholar]
- 25.Farjam M, Bahrami H, Bahramali E, Jamshidi J, Askari A, Zakeri H, et al. A cohort study protocol to analyze the predisposing factors to common chronic non-communicable diseases in rural areas: Fasa Cohort Study. BMC Public Health. 2016;16(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Homayounfar R, Farjam M, Bahramali E, Sharafi M, Poustchi H, Malekzadeh R, et al. Cohort Profile: the Fasa adults Cohort Study (FACS): a prospective study of non-communicable diseases risks. Int J Epidemiol. 2023;52(3):e172–8. [DOI] [PubMed] [Google Scholar]
- 27.Goel AJJ, Di Muzio B. Diffuse hepatic steatosis (grading). Reference article, Radiopaedia.orgAccessed on 13 Oct (2023) 10.53347/rID-33279
- 28.Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174–97. [DOI] [PubMed] [Google Scholar]
- 29.Saponaro C, Gaggini M, Gastaldelli A. Nonalcoholic fatty liver disease and type 2 diabetes: common pathophysiologic mechanisms. Curr Diab Rep. 2015;15:1–13. [DOI] [PubMed] [Google Scholar]
- 30.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13(4):511–31. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen D, Talwalkar JA. Noninvasive assessment of liver fibrosis. Hepatology. 2011;53(6):2107–10. [DOI] [PubMed] [Google Scholar]
- 32.Zhang E, Wartelle-Bladou C, Lepanto L, Lachaine J, Cloutier G, Tang A. Cost-utility analysis of nonalcoholic steatohepatitis screening. Eur Radiol. 2015;25:3282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterology: WJG. 2014;20(23):7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Idilman IS, Keskin O, Celik A, Savas B, Halil Elhan A, Idilman R, et al. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiol. 2016;57(3):271–8. [DOI] [PubMed] [Google Scholar]
- 35.Cantero I, Elorz M, Abete I, Marin BA, Herrero JI, Monreal JI, et al. Ultrasound/Elastography techniques, lipidomic and blood markers compared to Magnetic Resonance Imaging in non-alcoholic fatty liver disease adults. Int J Med Sci. 2019;16(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gastaldelli A, Kozakova M, Højlund K, Flyvbjerg A, Favuzzi A, Mitrakou A, et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology. 2009;49(5):1537–44. [DOI] [PubMed] [Google Scholar]
- 37.Calori G, Lattuada G, Ragogna F, Garancini MP, Crosignani P, Villa M, et al. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology. 2011;54(1):145–52. [DOI] [PubMed] [Google Scholar]
- 38.Wu J, Tian S, Li H, Xu Z, Li S, Chen Y-l, et al. Population-specific cut-off points of fatty liver index: a study based on the National Health and Nutrition Examination Survey data. BMC Gastroenterol. 2022;22(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee Y-h, Bang H, Park YM, Bae JC, Lee B-W, Kang ES, et al. Non–laboratory-based self-assessment screening score for non-alcoholic fatty liver disease: development, validation and comparison with other scores. PLoS ONE. 2014;9(9):e107584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dehnavi Z, Razmpour F, Naseri MB, Nematy M, Alamdaran SA, Vatanparast HA et al. Fatty liver index (FLI) in predicting non-alcoholic fatty liver disease (NAFLD). Hepat Monthly. 2018;18(2).
- 41.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–5. [DOI] [PubMed] [Google Scholar]
- 42.Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocr. 2014;35(1):8–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han S-i, Komatsu Y, Murayama A, Steffensen KR, Nakagawa Y, Nakajima Y, et al. Estrogen receptor ligands ameliorate fatty liver through a nonclassical estrogen receptor/Liver X receptor pathway in mice. Hepatology. 2014;59(5):1791–802. [DOI] [PubMed] [Google Scholar]
- 44.Yang B-L, Wu W-C, Fang K-C, Wang Y-C, Huo T-I, Huang Y-H, et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large-scale cross-sectional study in Taiwan. PLoS ONE. 2015;10(3):e0120443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting this study’s findings are available from the Noncommunicable Disease Research Center of Fasa University of Medical Sciences. Still, restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. The data are, however, available from the corresponding author upon reasonable request and with the permission of the Noncommunicable Disease Research Center of Fasa University of Medical Sciences.