Abstract
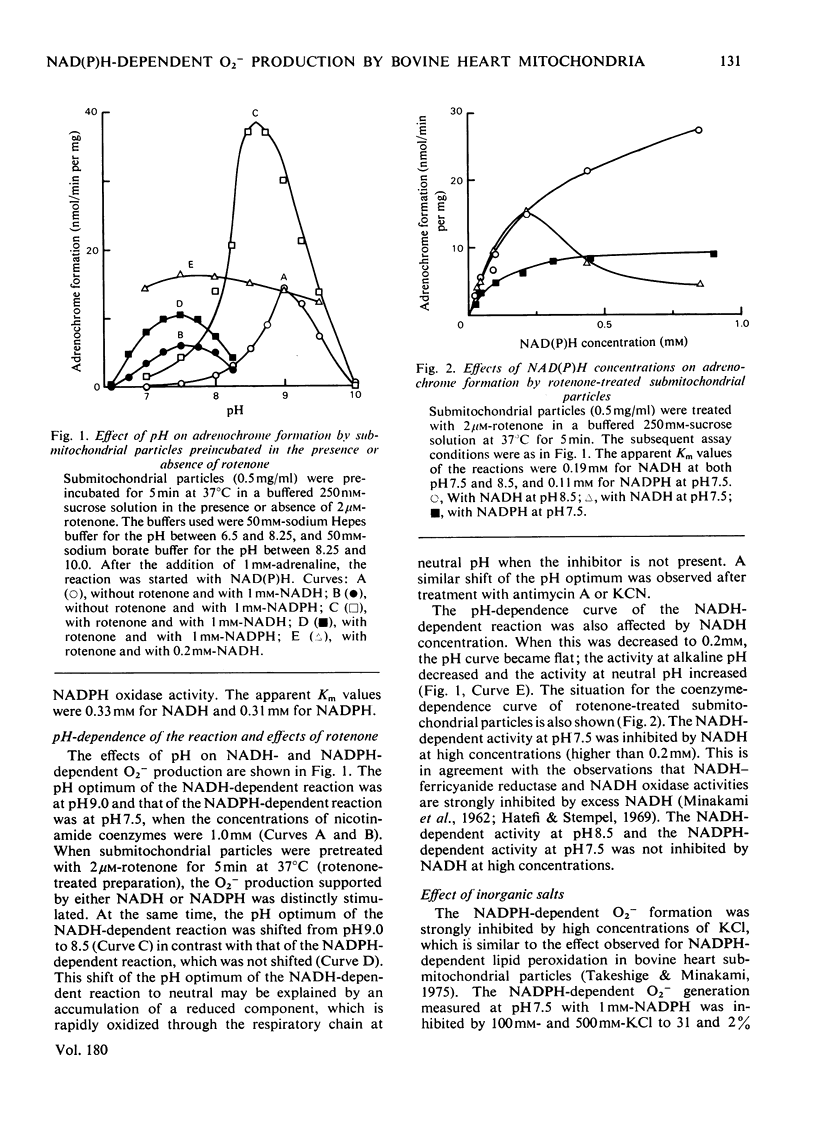
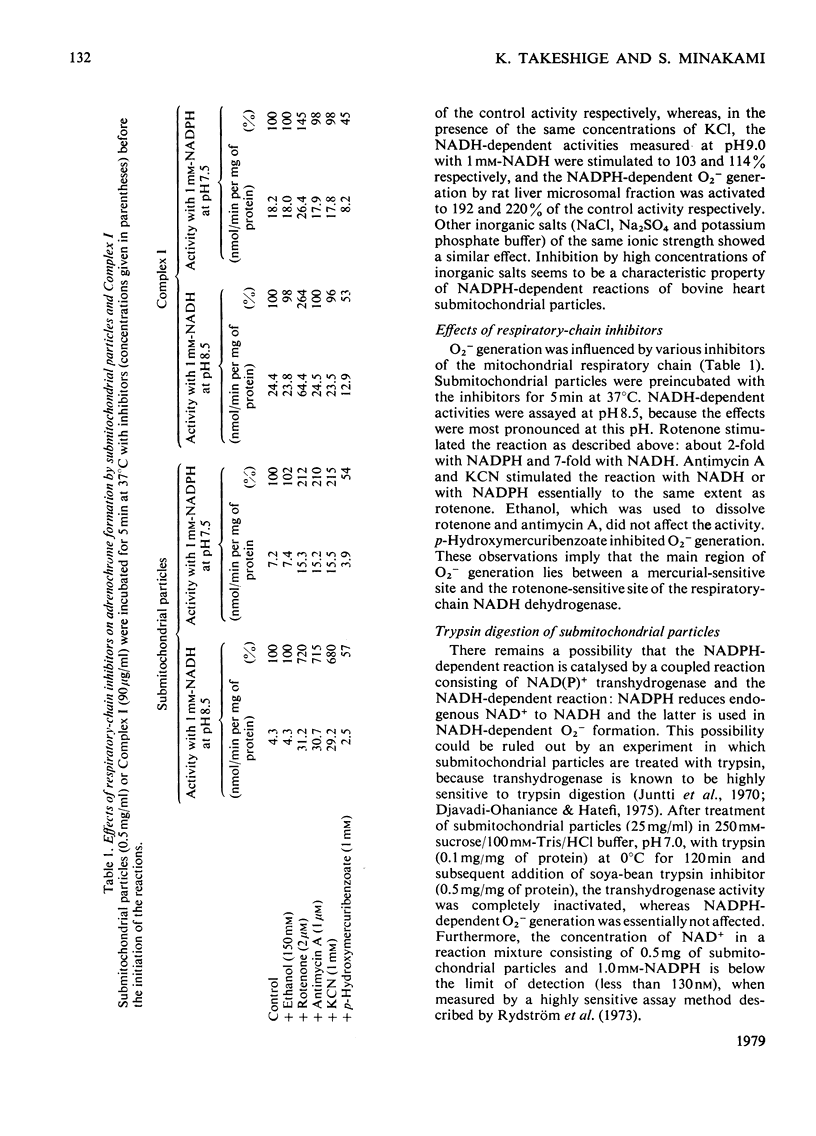
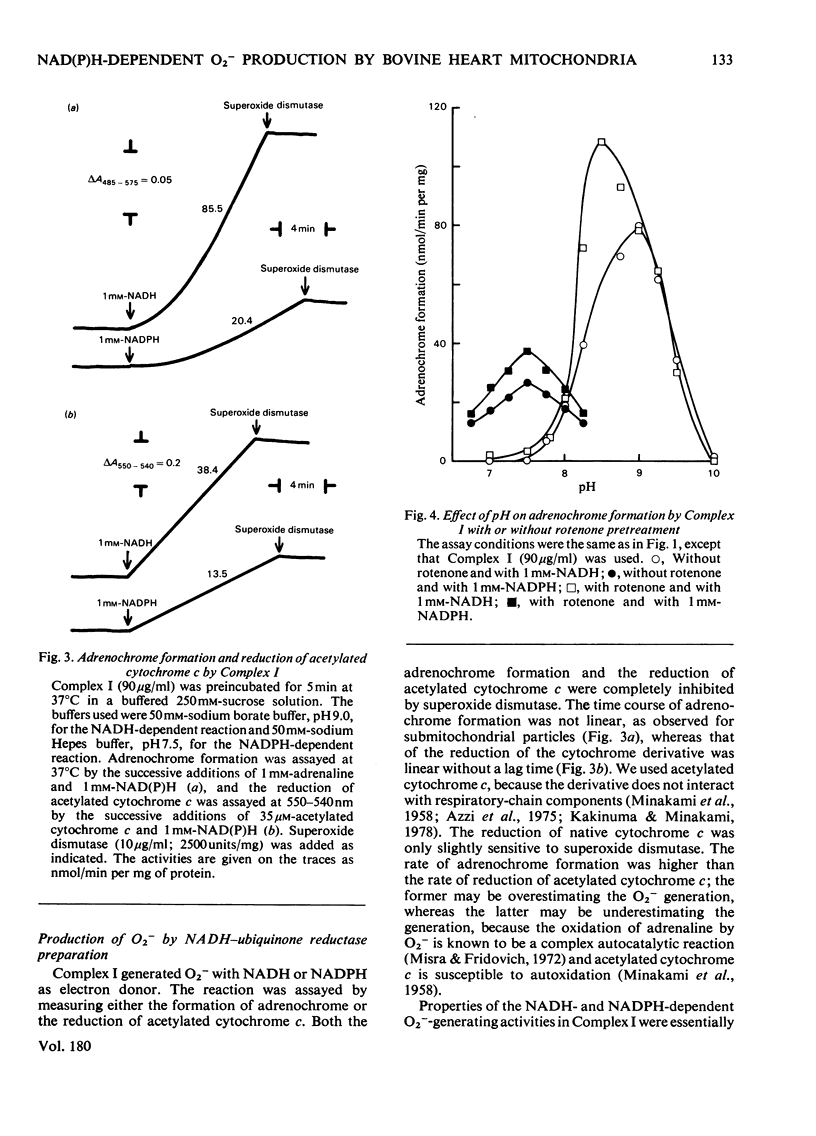
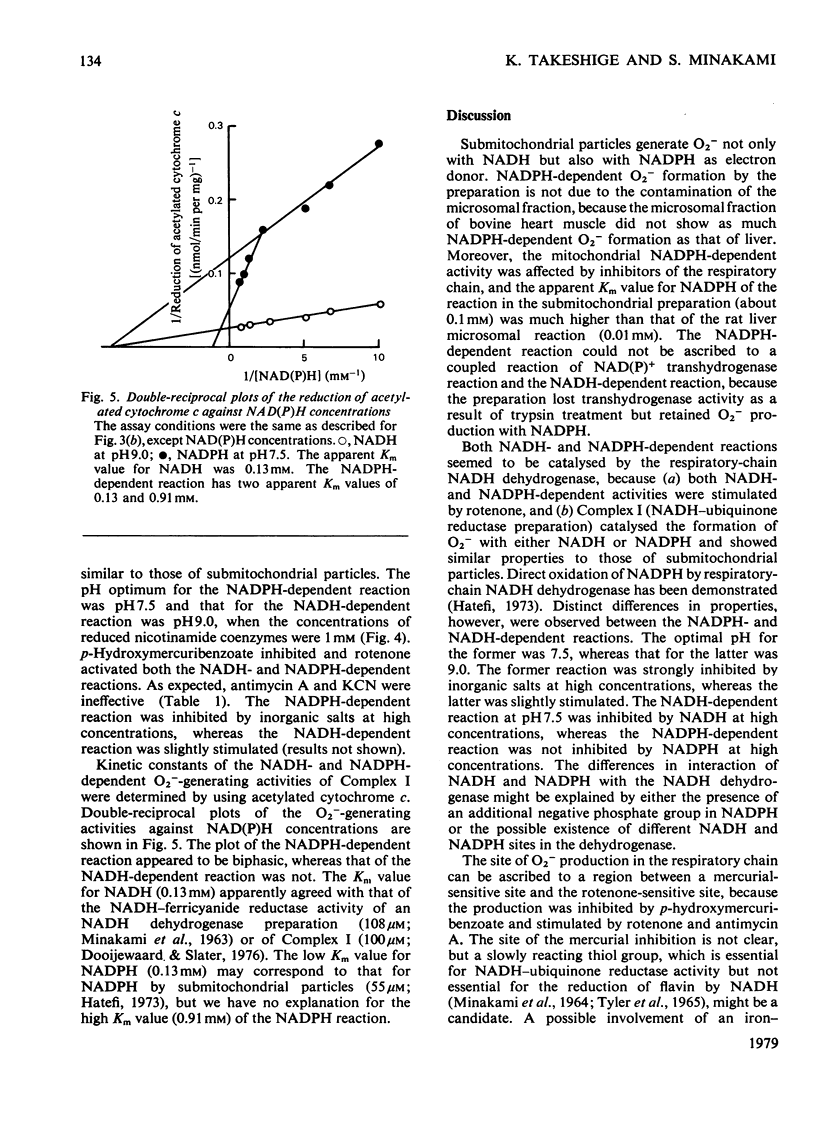
1. Both NADH and NADPH supported the oxidation of adrenaline to adrenochrome in bovine heart submitochondrial particles. The reaction was completely inhibited in the presence of superoxide dismutase, suggesting that superoxide anions (O2−) are responsible for the oxidation. The optimal pH of the reaction with NADPH was at pH7.5, whereas that with NADH was at pH9.0. The reaction was inhibited by treatment of the preparation with p-hydroxymercuribenzoate and stimulated by treatment with rotenone. Antimycin A and cyanide stimulated the reaction to the same extent as rotenone. The NADPH-dependent reaction was inhibited by inorganic salts at high concentrations, whereas the NADH-dependent reaction was stimulated. 2. Production of O2− by NADH–ubiquinone reductase preparation (Complex I) with NADH or NADPH as an electron donor was assayed by measuring the formation of adrenochrome or the reduction of acetylated cytochrome c which does not react with the respiratory-chain components. p-Hydroxymercuribenzoate inhibited the reaction and rotenone stimulated the reaction. The effects of pH and inorganic salts at high concentrations on the NADH- and NADPH-dependent reactions of Complex I were essentially similar to those on the reactions of submitochondrial particles. 3. These findings suggest that a region between a mercurialsensitive site and the rotenone-sensitive site of the respiratory-chain NADH dehydrogenase is largely responsible for the NADH- and NADPH-dependent O2− production by the mitochondrial inner membranes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aust S. D., Roerig D. L., Pederson T. C. Evidence for superoxide generation by NADPH-cytochrome c reductase of rat liver microsomes. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1133–1137. doi: 10.1016/0006-291x(72)90952-7. [DOI] [PubMed] [Google Scholar]
- Azzi A., Montecucco C., Richter C. The use of acetylated ferricytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem Biophys Res Commun. 1975 Jul 22;65(2):597–603. doi: 10.1016/s0006-291x(75)80188-4. [DOI] [PubMed] [Google Scholar]
- Boveris A., Cadenas E., Stoppani A. O. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem J. 1976 May 15;156(2):435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E., Boveris A., Ragan C. I., Stoppani A. O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977 Apr 30;180(2):248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- Djavadi-Ohaniance L., Hatefi H. Oxidation of NADPH by submitochondrial particles from beef heart in complete absence of transhydrogenase activity from NADPH to NAD. J Biol Chem. 1975 Dec 25;250(24):9397–9403. [PubMed] [Google Scholar]
- Dooijewaard G., Slater E. C. Steady-state kinetics of high molecular weight (type-I) NADH dehydrogenase. Biochim Biophys Acta. 1976 Jul 9;440(1):1–15. doi: 10.1016/0005-2728(76)90109-2. [DOI] [PubMed] [Google Scholar]
- GREEN S., MAZUR A., SHORR E. Mechanism of the catalytic oxidation of adrenaline by ferritin. J Biol Chem. 1956 May;220(1):237–255. [PubMed] [Google Scholar]
- Hatefi Y. Oxidation of reduced triphosphopyridine nucleotide by submitochondrial particles from beef heart. Biochem Biophys Res Commun. 1973 Feb 20;50(4):978–984. doi: 10.1016/0006-291x(73)91502-7. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Isolation and enzymatic properties of the mitochondrial reduced diphosphopyridine nucleotide dehydrogenase. J Biol Chem. 1969 May 10;244(9):2350–2357. [PubMed] [Google Scholar]
- Kakinuma K., Minakami S. Effects of fatty acids on superoxide radical generation in leukocytes. Biochim Biophys Acta. 1978 Jan 3;538(1):50–59. doi: 10.1016/0304-4165(78)90251-9. [DOI] [PubMed] [Google Scholar]
- Knowles A. F., Penefsky H. S. The subunit structure of beef heart mitochondrial adenosine triphosphatase. Isolation procedures. J Biol Chem. 1972 Oct 25;247(20):6617–6623. [PubMed] [Google Scholar]
- Loschen G., Azzi A., Richter C., Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974 May 15;42(1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- MINAKAMI S., RINGLER R. L., SINGER T. P. Studies on the respiratory chain-linked dihydrodiphosphopyridine nucleotide dehydrogenase. I. Assay of the enzyme in particulate and in soluble preparations. J Biol Chem. 1962 Feb;237:569–576. [PubMed] [Google Scholar]
- MINAKAMI S., SCHINDLER F. J., ESTABROOK R. W. HYDROGEN TRANSFER BETWEEN REDUCED DIPHOSPHOPYRIDINE NUCLEOTIDE DEHYDROGENASE AND THE RESPIRATORY CHAIN. I. EFFECT OF SULFHYDRYL INHIBITORS AND PHOSPHOLIPASE. J Biol Chem. 1964 Jun;239:2042–2048. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 May 25;247(10):3170–3175. [PubMed] [Google Scholar]
- Rich P. R., Bonner W. D. The sites of superoxide anion generation in higher plant mitochondria. Arch Biochem Biophys. 1978 May;188(1):206–213. doi: 10.1016/0003-9861(78)90373-9. [DOI] [PubMed] [Google Scholar]
- Rydström J., Hoek J. B., Ernster L. On the oxidation of reduced nicotinamide dinucleotide phosphate by submitochondrial particles from beef heart. Biochim Biophys Acta. 1973 Jun 28;305(3):694–698. doi: 10.1016/0005-2728(73)90095-9. [DOI] [PubMed] [Google Scholar]
- TYLER D. D., BUTOW R. A., GONZE J., ESTABROOK R. W. EVIDENCE FOR THE EXISTENCE AND FUNCTION OF AN OCCULT, HIGHLY REACTIVE SULPHYDRYL GROUP IN THE RESPIRATORY CHAIN DPNH DEHYDROGENASE. Biochem Biophys Res Commun. 1965 May 3;19:551–557. doi: 10.1016/0006-291x(65)90161-0. [DOI] [PubMed] [Google Scholar]
- Tyler D. D. A protective function of superoxide dismutase during respiratory chain activity. Biochim Biophys Acta. 1975 Sep 8;396(3):335–346. doi: 10.1016/0005-2728(75)90140-1. [DOI] [PubMed] [Google Scholar]


