Abstract
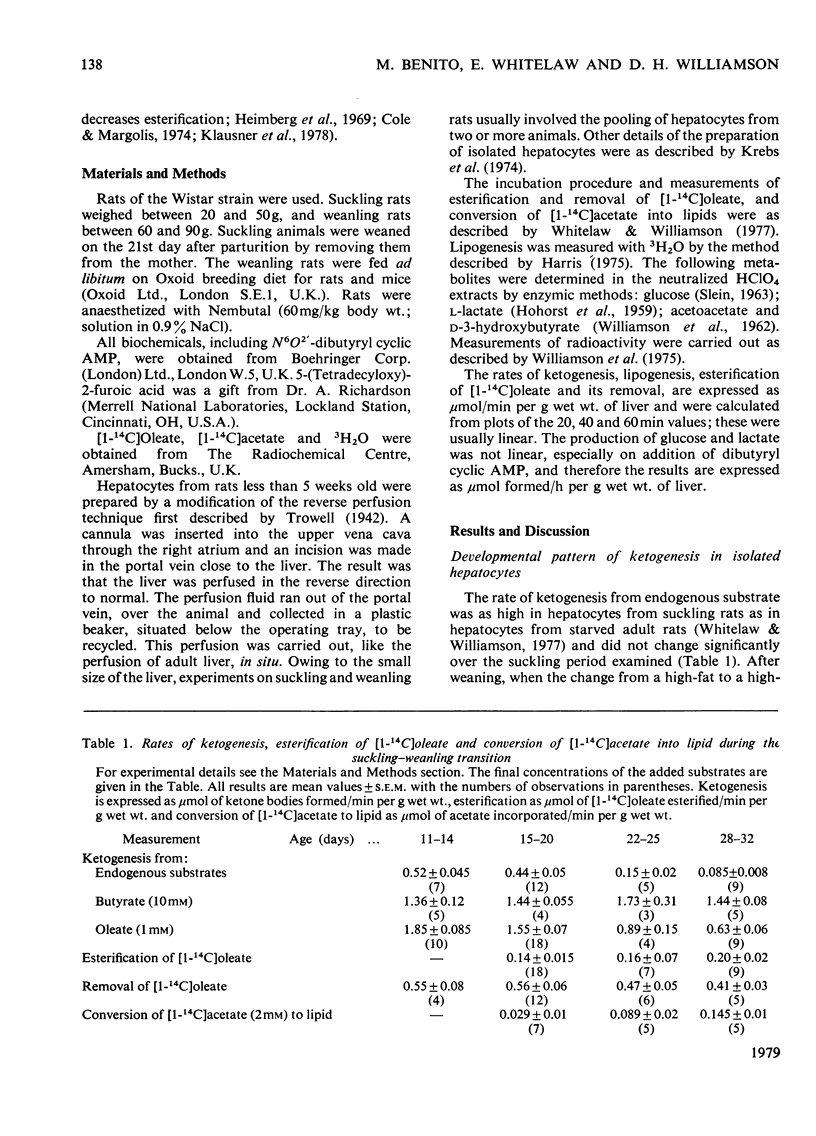
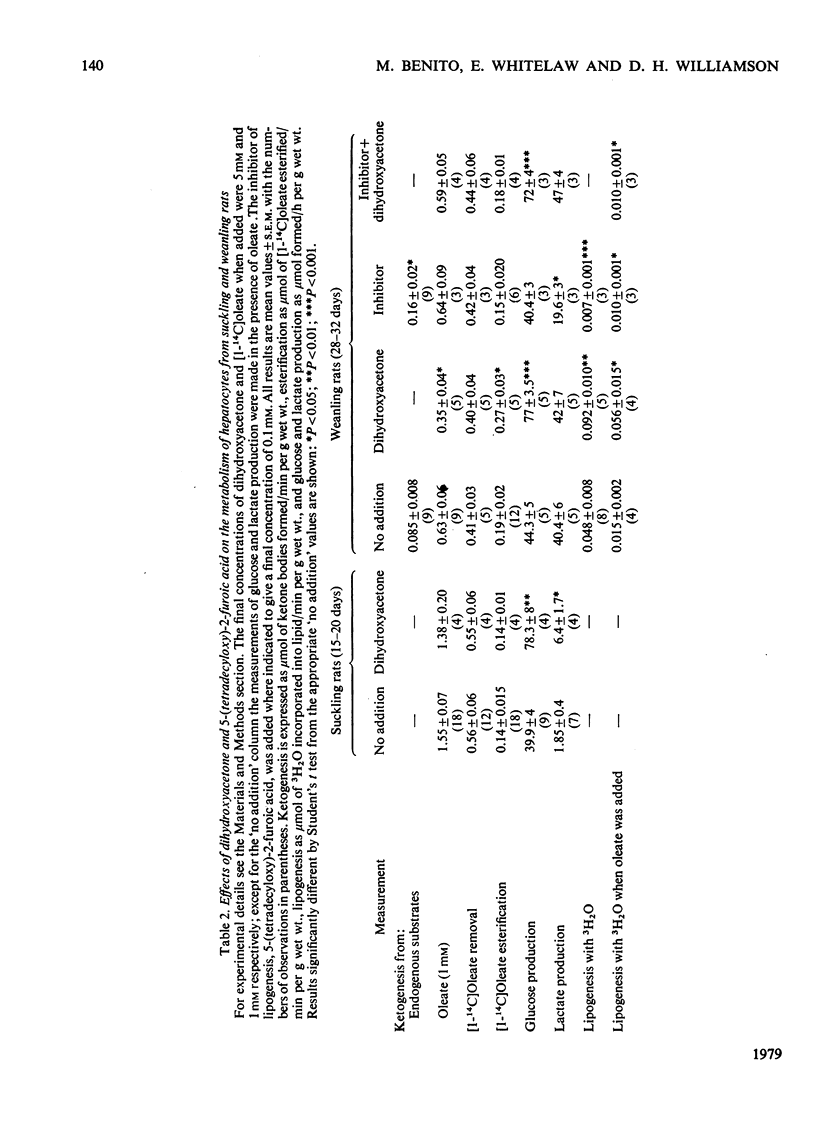
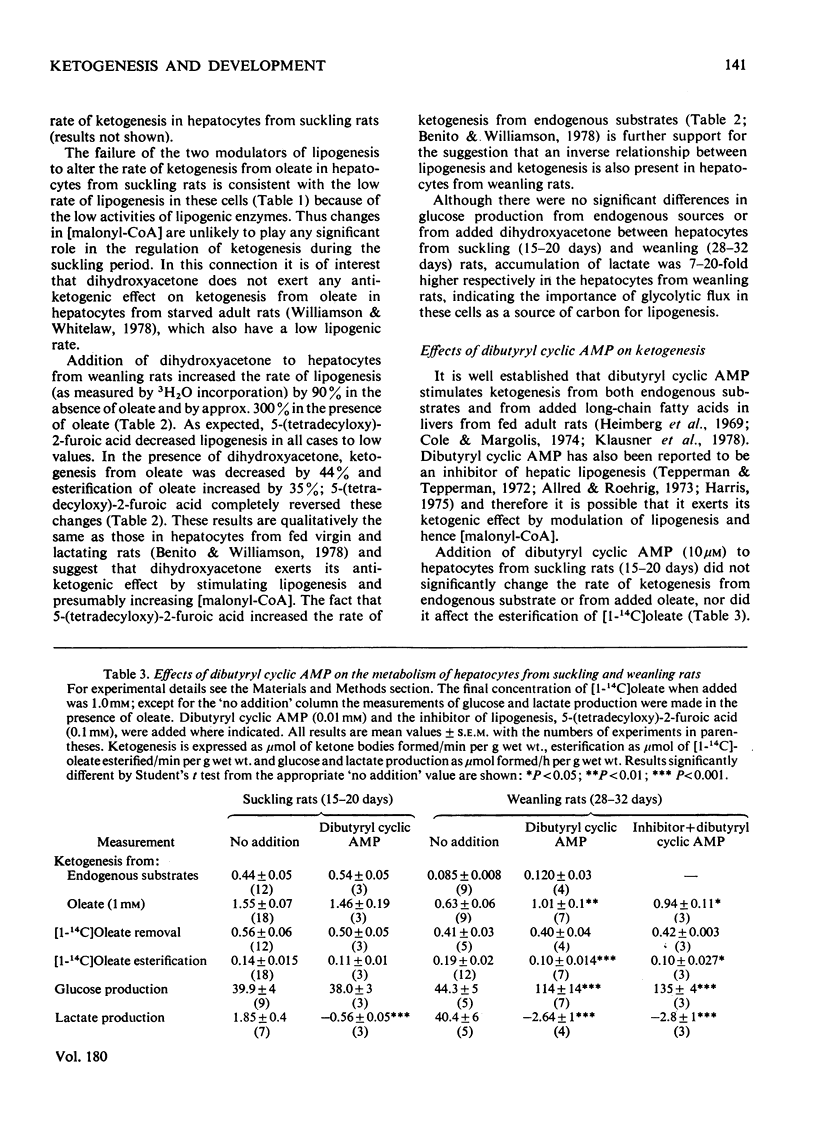
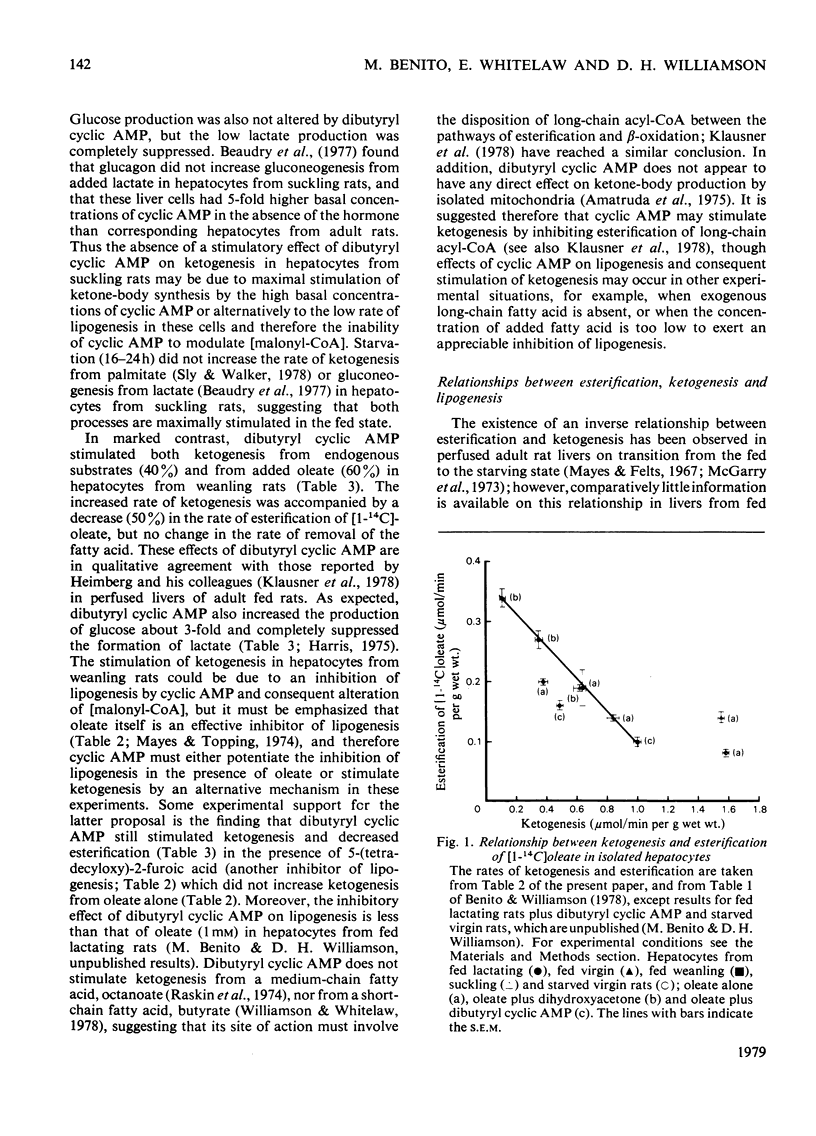
The rates of ketogenesis from endogenous substrates, butyrate or oleate, have been measured in isolated hepatocytes from suckling and weanling rats. Ketogenesis from endogenous substrate and from oleate decreased on weaning, whereas the rate from butyrate remained unchanged. It is concluded that the major site of regulation of ketogenesis during this period of development involves the disposal of long-chain fatty acyl-CoA between the esterification and beta-oxidation pathways. Modulators of lipogenesis [dihydroxyacetone and 5-(tetradecyloxy)-2-furoic acid] did not alter the rate of ketogenesis in hepatocytes from suckling rats, and it is suggested that this is due to the low rate of lipogenesis in these cells. Hepatocytes from fed weanling rats have a high rate of lipogenesis and evidence is presented for a reciprocal relationship between ketogenesis and lipogenesis, and ketogenesis, and esterification in these cells. Dibutyryl cyclic AMP stimulated ketogenesis from oleate in hepatocytes from fed weanling rats, even in the presence of an inhibitor of lipogenesis [5-(tetradecyloxy)-2-furoic acid], but not in cells from suckling rats. It is suggested that cyclic AMP may act via inhibition of esterification and that in hepatocytes from suckling rats ketogenesis is already maximally stimulated by the high basal concentrations of cyclic AMP [Beaudry, Chiasson & Exton (1977) Am. J. Physiol. 233, E175--E180].
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas M., Bremer J. Short-chain fatty acid activation in rat liver. A new assay procedure for the enzymes and studies on their intracellular localization. Biochim Biophys Acta. 1968 Oct 22;164(2):157–166. doi: 10.1016/0005-2760(68)90142-2. [DOI] [PubMed] [Google Scholar]
- Allred J. B., Roehrig K. L. Inhibition of rat liver acetyl coenzyme A carboxylase by N 6 ,O 2' -dibutyryl cyclic adenosine 3':5'-monophosphate in vitro. J Biol Chem. 1973 Jun 10;248(11):4131–4133. [PubMed] [Google Scholar]
- Amatruda J. M., Margolis S., Lockwood D. H. Regulation of ketone body production from (14C)palmitate in rat liver mitochondria: effects of cyclic nucleotides and unlabeled fatty acids. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1337–1345. doi: 10.1016/0006-291x(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Beaudry M. A., Chiasson J. L., Exton J. H. Gluconeogenesis in the suckling rat. Am J Physiol. 1977 Sep;233(3):E175–E180. doi: 10.1152/ajpendo.1977.233.3.E175. [DOI] [PubMed] [Google Scholar]
- Benito M., Williamson D. H. Evidence for a reciprocal relationship between lipogenesis and ketogenesis in hepatocytes from fed virgin and lactating rats. Biochem J. 1978 Oct 15;176(1):331–334. doi: 10.1042/bj1760331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. A., Margolis S. Stimulation of ketogenesis by dibutyryl cyclic AMP in isolated rat hepatocytes. Endocrinology. 1974 May;94(5):1391–1396. doi: 10.1210/endo-94-5-1391. [DOI] [PubMed] [Google Scholar]
- Cook G. A., King M. T., Veech R. L. Ketogenesis and malonyl coenzyme A content of isolated rat hepatocytes. J Biol Chem. 1978 Apr 25;253(8):2529–2531. [PubMed] [Google Scholar]
- DYMSZA H. A., CZAJKA D. M., MILLER S. A. INFLUENCE OF ARTIFICIAL DIET ON WEIGHT GAIN AND BODY COMPOSITION OF THE NEONATAL RAT. J Nutr. 1964 Oct;84:100–106. doi: 10.1093/jn/84.2.100. [DOI] [PubMed] [Google Scholar]
- Dahlquist G., Persson U., Persson B. The activity of D- -hydroxybutyrate dehydrogenase in fetal, infant and adult rat brain and the influence of starvation. Biol Neonate. 1972;20(1):40–50. doi: 10.1159/000240444. [DOI] [PubMed] [Google Scholar]
- Drahota Z., Hahn P., Kleinzeller A., Kostolánská A. Acetoacetate formation by liver slices from adult and infant rats. Biochem J. 1964 Oct;93(1):61–65. doi: 10.1042/bj0930061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRITZ I. B. Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev. 1961 Jan;41:52–129. doi: 10.1152/physrev.1961.41.1.52. [DOI] [PubMed] [Google Scholar]
- Foster P. C., Bailey E. Changes in hepatic fatty acid degradation and blood lipid and ketone body content during development of the rat. Enzyme. 1976;21(5):397–407. doi: 10.1159/000458889. [DOI] [PubMed] [Google Scholar]
- Foster P. C., Bailey E. Changes in the activities of the enzymes of hepatic fatty acid oxidation during development of the rat. Biochem J. 1976 Jan 15;154(1):49–56. doi: 10.1042/bj1540049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- Harris R. A. Studies on the inhibition of hepatic lipogenesis by N-6,O-2'-dibutyryl adenosine 3',5'-monophosphate. Arch Biochem Biophys. 1975 Jul;169(1):168–180. doi: 10.1016/0003-9861(75)90330-6. [DOI] [PubMed] [Google Scholar]
- Heimberg M., Weinstein I., Kohout M. The effects of glucagon, dibutyryl cyclic adenosine 3',5'-monophosphate, and concentration of free fatty acid on hepatic lipid metabolism. J Biol Chem. 1969 Oct 10;244(19):5131–5139. [PubMed] [Google Scholar]
- Kariya T., Wille L. J. Inhibition of fatty acid synthesis by RMI 14,514 (5-tetradecyloxy-2-furoic acid). Biochem Biophys Res Commun. 1978 Feb 28;80(4):1022–1024. doi: 10.1016/0006-291x(78)91347-5. [DOI] [PubMed] [Google Scholar]
- Klausner H. J., Soler-Argilaga C., Heimberg M. Effects of dibutyryl adenosine 3',5'-monophosphate on hepatic metabolism of free fatty acids. Metabolism. 1978 Jan;27(1):13–25. doi: 10.1016/0026-0495(78)90119-1. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Sokoloff L. Changes in D(--)-beta-hydroxybutyric dehydrogenase activity during brain maturation in the rat. J Biol Chem. 1967 Sep 10;242(17):3880–3883. [PubMed] [Google Scholar]
- Kopec B., Fritz I. B. Comparison of properties of carnitine palmitoyltransferase I with those of carnitine palmitoyltransferase II, and preparation of antibodies to carnitine palmitoyltransferases. J Biol Chem. 1973 Jun 10;248(11):4069–4074. [PubMed] [Google Scholar]
- Krebs H. A., Hems R. Fatty acid metabolism in the perfused rat liver. Biochem J. 1970 Sep;119(3):525–533. doi: 10.1042/bj1190525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood E. A., Bailey E. The course of ketosis and the activity of key enzymes of ketogenesis and ketone-body utilization during development of the postnatal rat. Biochem J. 1971 Aug;124(1):249–254. doi: 10.1042/bj1240249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Regulation of fat metabolism of the liver. Nature. 1967 Aug 12;215(5102):716–718. doi: 10.1038/215716a0. [DOI] [PubMed] [Google Scholar]
- Mayes P. A., Topping D. L. Regulation of hepatic lipogenesis by plasma free fatty acids: simultaneous studies on lipoprotein secretion, cholesterol synthesis, ketogenesis and gluconeogenesis. Biochem J. 1974 Apr;140(1):111–114. doi: 10.1042/bj1400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Hormonal control of ketogenesis. Biochemical considerations. Arch Intern Med. 1977 Apr;137(4):495–501. [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. Characteristics of fatty acid oxidation in rat liver homogenates and the inhibitory effect of malonyl-CoA. Biochim Biophys Acta. 1978 Sep 28;530(3):305–313. doi: 10.1016/0005-2760(78)90150-9. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Meier J. M., Foster D. W. The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J Biol Chem. 1973 Jan 10;248(1):270–278. [PubMed] [Google Scholar]
- Page M. A., Krebs H. A., Williamson D. H. Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem J. 1971 Jan;121(1):49–53. doi: 10.1042/bj1210049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek E., Cook G. A., Cornell N. W. Inhibition by 5-(tetradecyloxy)-2-furoic acid of fatty acid and cholesterol synthesis in isolated rat hepatocytes. Lipids. 1977 Oct;12(10):814–818. doi: 10.1007/BF02533270. [DOI] [PubMed] [Google Scholar]
- Raskin P., McGarry J. D., Foster D. W. Independence of cholesterol and fatty acid biosynthesis from cyclic adenosine monophosphate concentration in the perfused rat liver. J Biol Chem. 1974 Oct 10;249(19):6029–6032. [PubMed] [Google Scholar]
- Ribereau-Gayon G. Inhibition of mitochondrial tricarboxylate anion translocation and of liver fatty acid synthesis by a new hypolipidemic agent. FEBS Lett. 1976 Mar 1;62(3):309–312. doi: 10.1016/0014-5793(76)80082-8. [DOI] [PubMed] [Google Scholar]
- Robles-Valdes C., McGarry J. D., Foster D. W. Maternal-fetal carnitine relationship and neonatal ketosis in the rat. J Biol Chem. 1976 Oct 10;251(19):6007–6012. [PubMed] [Google Scholar]
- Shah J., Bailey E. Changes in the activities of the enzymes of hepatic ketogenesis in the rat between late fetal life and weaning. Enzyme. 1977;22(1):35–40. doi: 10.1159/000458505. [DOI] [PubMed] [Google Scholar]
- Sly M. R., Walker D. G. A comparison of lipid metabolism in hepatocytes isolated from fed and starved neonatal and adult rats. Comp Biochem Physiol B. 1978;61(4):501–506. doi: 10.1016/0305-0491(78)90042-1. [DOI] [PubMed] [Google Scholar]
- Taylor C. B., Bailey E., Bartley W. Changes in hepatic lipigenesis during development of the rat. Biochem J. 1967 Nov;105(2):717–722. doi: 10.1042/bj1050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tildon J. T., Cone A. L., Cornblath M. Coenzyme A transferase activity in rat brain. Biochem Biophys Res Commun. 1971 Apr 2;43(1):225–231. doi: 10.1016/s0006-291x(71)80111-0. [DOI] [PubMed] [Google Scholar]
- Trowell O. A. Urea formation in the isolated perfused liver of the rat. J Physiol. 1942 Mar 31;100(4):432–458. doi: 10.1113/jphysiol.1942.sp003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw E., Williamson D. H. Effects of lactation of ketogenesis from oleate or butyrate in rat hepatocytes. Biochem J. 1977 Jun 15;164(3):521–528. doi: 10.1042/bj1640521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., McKeown S. R., Ilic V. Interactions of glucose, acetoacetate and insulin in mammary-gland slices of lactating rats. Biochem J. 1975 Aug;150(2):145–152. doi: 10.1042/bj1500145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Veloso D., Ellington E. V., Krebs H. A. Changes in the concentrations of hepatic metabolites on administration of dihydroxyacetone or glycerol to starved rats and their relationship to the control of ketogenesis. Biochem J. 1969 Sep;114(3):575–584. doi: 10.1042/bj1140575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y. Y., Streuli V. L., Zee P. Relative utilization of fatty acids for synthesis of ketone bodies and complex lipids in the liver of developing rats. Lipids. 1977 Apr;12(4):367–374. doi: 10.1007/BF02533640. [DOI] [PubMed] [Google Scholar]


