Abstract
Background
Polycystic Ovary Syndrome (PCOS) is one of the most common hormonal disorders in reproductive-age women caused by hyperinsulinemia. The portfolio Moderate-carbohydrate diet (PMCD) is a plant-based diet with a carbohydrate content of 40% and incorporates five cholesterol-lowering foods. While, the ketogenic diet is a high-fat diet with 70% fat, promoting a ketosis state. To the best of our knowledge, no study compared the therapeutic effects of these two diets in PCOS patients. Thus, this study aimed to compare the impact of PLCD and KD on anthropometric indices, metabolic status, and hormonal levels in overweight or obese women with PCOS.
Methods
This open-label, randomized clinical trial was conducted on forty-six PCOS women. 21 women in PMCD and 19 in the KD group completed the study. The anthropometric indices including body mass index (BMI) and fat body mass (FBM), metabolic markers (fasting blood glucose (FBG)) and plasma lipid profiles including low-density lipoprotein (LDL), triglycerides, and high-density lipoproteins (HDL) were measured. Reproductive hormones such as luteinizing hormone (LH), dehydroepiandrosterone sulfate (DHEA-s) and free testosterone were assessed at the baseline and after the intervention.
Results
However, after 8 weeks both diets demonstrated enhancement in anthropometric indices (BMI, FBM, lean body mass), metabolic status (FBG, insulin serum levels), and reproductive hormones such as LH, free testosterone, and DHEA-s. The mean difference in the KD improved more than the PMCD in the field of BMI reduction (MD (SD) 2.73 (0.351) vs. MD (SD) 1.71 (0.775)) and LH (MD 4.13 (1.375) vs.MD 2.46 (1.105)). Nevertheless, the lipid profile including LDL-C and triglycerides improved more in the PMCD compared to the KD (MD 33.95 (7.345) vs. MD 23.34 (14.136)) and (MD 38.20 (10.757) vs. MD 57.62 (21.688)) respectively. There were no significant changes in the Ferriman-Gallwey score within or between the two groups.
Conclusion
The findings revealed that both diets were effective in improving PCOS manifestations. However, the KD exhibited greater effectiveness in enhancing body measurements, metabolic factors, and reproductive hormone levels compared to the PMCD in obese PCOS women. Furthermore, PMCD could be more beneficial for PCOS women with lipide disorders.
Registration number of clinical trial
IRCT20200912048693N3, Trial registered 2022–12–14. https://www.irct.ir/trial/67548
Keywords: Polycystic ovary syndrome, Insulin resistance, Ketogenic diet, Portfolio low-carbohydrate diet
Introduction
Polycystic Ovary Syndrome (PCOS) is the most common endocrine-metabolic disorder among women of reproductive age, with a global prevalence of 20% [1]. PCOS is primarily caused by the overproduction of androgens from the ovaries and/or adrenal glands, although in certain women, it is associated with insulin resistance and metabolic imbalances [2]. The main clinical symptoms of this disease are hyperandrogenism, menstrual disturbances (oligomenorrhea, amenorrhea, prolonged irregular menstrual bleeding), alteration in the ovarian morphology, hirsutism, acne, and alopecia [3]. Observational studies have found a high prevalence of overweight and obesity in women with PCOS, with an estimated 75% being affected which is attributed to elevated androgen levels and an imbalance in the FSH/LH ratio [4]. Additionally, both normal-weight and overweight women with PCOS were found to exhibit android fat distribution [5]. This pattern of fat distribution, known as central obesity, is linked to metabolic syndrome (insulin resistance, high insulin levels, and abnormal lipid levels as well as ovarian hyperandrogenism which worsens the severity of PCOS [6]. Hence, there is a mutually instrumental relationship between obesity and PCOS. Moreover, significant alterations in carbohydrate, lipid, and amino acid metabolism with specific metabolomic such as a decrease in citric and lactic acid levels, isophosphatidylcholines, and glycerol phosphocholine, and increase in free fatty acids including carnitine, linoleic acid, and oleic acid have been identified among obese women with PCOS [7, 8]. Since these women suffer from low-grade chronic inflammation due to high levels of fat mass and insulin resistance, they are more susceptible to metabolic abnormalities such as hyperinsulinemia, impaired glucose metabolism, infertility, hypertension, and lipid profile disorders, which markedly increase the risk of chronic diseases like type 2 diabetes mellitus, cardiovascular disease, and metabolic syndrome [9–11]. PCOS diagnosis follows the Rotterdam criteria, requiring the presence of at least two out of three criteria: (I) clinical signs of hyperandrogenism (such as hirsutism, acne, seborrhea, and alopecia) and/or elevated levels of circulating androgens; (II) presence of ovarian cysts assessed by ultrasound examination; and (III) oligo-amenorrhea with oligo-or anovulation [12].
Although the treatment approaches for PCOS are based on the severity of complications, the cornerstone of PCOS treatment is based on a lifestyle change approach, with an emphasis on modifying diet and engaging in regular exercise [13]. A diet high in carbohydrates, especially those with a high glycemic index and high glycemic load nutrients, causes a sharp rise in blood glucose levels, which leads to an increase in insulin levels [14]. Thus, diets with a lower carbohydrate content, such as KD, indicate more beneficial effects in relieving PCOS compared to calorie-restricted diets [15]. However, no study has been conducted to identify the effective role of carbohydrate content in PCOS patient groups.
The Portfolio Moderate-carbohydrate diet (PMCD) is a plant-based diet consisting of 40% carbohydrates, 20% protein, and 40% fat [16].The portfolio diet is designed by excluding all animal products such as meat, fish, poultry, eggs, and dairy products and emphasizing on the consumption of five specific cholesterol-lowering foods and nutrients, including plant protein (mainly from soy products and pulses), viscous fiber (such as oats, barley, and certain fruits), nuts, phytosterols, and monosaturated fats [17, 18]. A systematic review has shown that adhering to a low carbohydrate diet (providing less than 45% of total energy from carbohydrates) while maintaining an energy deficit led to positive changes in body measurements, blood sugar levels, fasting insulin levels, insulin response during a 3-h oral glucose tolerance test, and reproductive hormones like FSH, LH, DHEA, SHBG, and free testosterone in women with PCOS [19].The beneficial effect of low-carbohydrate regimens in metabolic outcomes of PCOS is possibly due to the reduced levels of circulating glucose, insulin, insulin-like growth factor-1(IGF-1), and insulin-like growth factor binding protein 1(IGFBP1), which reduces the hyper-androgens among PCOS patients [20]. Numerous clinical studies have mentioned the association between PCOS and the cardiovascular risk profile of PCOS female patients, due to a lipid/glucose altered metabolism, hypertension, systemic inflammatory condition (assessable by markers such as VES, TNF-alpha, cytokines and C-reactive protein (hs-CRP) levels), and vascular injuries [21, 22]. Moreover, plant-based diets have high levels of polyphenols which have therapeutic potential in PCOS women by slowing the progression of inflammation and improving both insulin sensitivity and compensatory hyperinsulinemia [23]. Considering the lifelong and chronic status of this disease, PCOS should be considered as a real cardiovascular risk factor which affects the quality of life seriously [22]. Therefore, the treatment approaches for PCOS women should not only encompass alleviating PCOS manifestations, but also regard cardiovascular abnormalities. For instance, a randomized trial compared the effect of 3-month adherence to the low glycemic index vegan diet (LGIV) vs. the low-calorie (LC) weight loss diet among 18 PCOS and overweight women. The results of this study indicated that the participants in the LGIV diet group lost significantly more weight than the standard low-calory diet group [24]. A recent study analyzed three dietary-lifestyle patterns (western, plant foods, and plant foods and intensive physical activity) among 140 PCOS women. As it has been reported, higher adherence to the plant-based diet and physical activity were associated with higher chance of improving metabolic health status such as normal BMI, body fat more than 35%, lipide profile as well as reproductive hormone disorders among PCOS women [25]. Since fruits and vegetables are the main sources of carotenoids for instance flavanols, isoflavones, and quercetin, they could inhibit the secretion of testosterone and LH, and consequently ameliorate hyperandrogenism in PCOS women [26, 27]. A plant-based diet is rich in antioxidants like vitamin C which is associated with a significant reduction of the triglyceride serum level [28]. The antioxidants potentiate nitric oxide (NO) synthesis in cultured human endothelial cells, which has been identified as the mechanism that can preserve vessels from altered myogenic tone (vasoconstriction), atherosclerosis, and coagulation abnormalities [29].
Ketogenic diet (KD) is a dietary protocol characterized by high fat intake (70%), very low carbohydrate intake (less than 10%), and moderate protein levels (20%), resulting in the production of ketone bodies [30]. The KD was first used for treating retractor epilepsy [31]. However, recently many studies have demonstrated its beneficial effects in various numbers of diseases, including obesity, neurological disorders, type 2 diabetes mellitus, cancer, as well as PCOS [32–34]. A recent parallel randomized trial on 20 obese women with PCOS and liver dysfunction demonstrated that the KD considerably reduced the body weight, blood glucose, plasma estradiol, progesterone levels, and liver function markers compared to the control diet [35]. By reducing carbohydrate intake to less than 30 g per day for 3 to 5 days, the concentration of insulin in the body decreases. As a result, the glycogen stored in the liver and muscles is replaced by an increase in fatty acid oxidation derived from adipose tissue [36, 37]. This process produces ketone bodies such as acetoacetate, acetone, and B-hydroxybutyrate that serve as fuel for the body. Therefore, under the state of ketosis, the need for endogenous glucose production is reduced and replaced by fat oxidation. Adequate protein intake preserves the utilization of amino acids from lean muscle tissue so that they are not used as an energy source. Also, the reduction of fat mass reduces acyclic estrogen production and improves the ratio of LH to FSH by reducing the aromatization of androgens in adipose tissue [38].
Based on the above evidence, both the PMCD and the KD offer beneficial effects on PCOS manifestations due to their low carbohydrate content and consequently minimal insulin secretion. Moreover, these two diets have remarkable differences including the difference in the main source of energy (which is glucose in the PMCD vs. ketone bodies in the KD group) and the content of micro/macronutrients and fiber. To the best of our knowledge, there was no study comparing the therapeutic effects of these two diets in PCOS patients. Therefore, the objective of the present study was to compare the effects of the PMCD and the KD on the anthropometric indices (body weight, body mass index, waist circumstances, and hip circumstances), body compositions (fat mass and lean body mass), metabolic status (fasting blood glucose, insulin, insulin sensitivity, and lipid profile), reproductive hormone levels (luteinizing hormone, follicle-stimulating hormone, and free testosterone blood levels), dietary intake, and hirsutism.
Materials and methods
Study design
The entire protocol of this study has been previously published [39]. Briefly, this parallel randomized clinical trial took place at Fatahi Clinic of Kermanshah University of Medical Sciences (KUMS), Iran, from January 2023 to May 2023. The eligible participants were randomly assigned to follow either the PMCD group (N = 23) or the KD group (N = 23), using a computer-generated random sealed envelope. The protocol was approved by the Medical University of Kermanshah Research Ethics Board (IR.KUMS. REC.1401.404). Furthermore, the trial was registered at the Iranian Registry of Clinical Trials (IRCT20200912048693N3) on 2022 December 14. The procedures were carried out following the World Medical Association Declaration of Helsinki, the Guidelines of the International Conference on Harmonization on Good Clinical Practice, and we followed the Consolidated Standards of Reporting Trials (CONSORT). Before the commencement of the study, written informed consent was obtained from all participants. It was not possible to blind the participants due to the nature of the diet; however, there was no interaction between the two groups. Furthermore, to minimize bias, the outcome assessors and the data analysts were blinded. The primary outcome of this investigation was determining the effectiveness of the PMCD compared to the KD in improving PCOS disease manifestations by measuring weight loss before and after the intervention. The secondary outcomes were BMI, FBM, LBM, WC, HC, FBG, insulin level, HOMA-IR, HOMA-B, reproductive hormones (LH, FSH, DHEA, and free testosterone), lipid profile (total cholesterol, triglycerides, LDL, HDL), and Ferriman-Gallwey score before and after the intervention.
Sample size
The sample size for the study was calculated based on the formula used in a randomized clinical trial [40]. According to the formula, the standard deviation of weight in the dietary group of PMCD and KD would be equal to μ1 = 3.14 (σ1 = 2.42) and μ2 = 1.66 (σ2 = 0.8) respectively. In the following sample with α = 0.05, the sample size required to have 80% power to detect a difference of two units of change in weights would be 18 people in each group. Considering the possibility of 25% missing people in the follow-up, the sample size increases to 23 people in each group. Also, a type I error of 5% (α = 0.05) and a type II error of 20% (β = 0.2, power = 80%) were considered.
Participants
We recruited 46 overweight or obese women diagnosed with PCOS through a public advertisement in the medical centers, healthcare establishments, physician offices, pharmacies, and hospitals of Kermanshah. Participants were asked to first visit Fatahi Clinics where the screening was performed by a dietitian as well as an obstetrician-gynecologist to determine eligible patients according to the inclusion criteria. Written informed consent was provided after a detailed explanation of the study. Also, the demographic characteristics questionnaire, the physical activity questionnaire (IPAQ), as well as 3-day food records questionnaires of all participants were completed by a professional dietitian. Then the subjects were randomly allocated to receive either the PMCD or the KD by a random block method of 6 from the website https://www.sealedenvelope.com. The inclusion criteria were: diagnosis of PCOS according to the Rotterdam Criteria by an obstetrician-gynecologist, fertile age (18–45 years), and BMI ≥ 25 kg/m2. The exclusion criteria were: currently pregnancy or lactation, taking anti-psychotic or anti-seizure medication due to their induced effect on insulin resistance, following a specific nutritional diet or hypocaloric diet in the last 3 months, history of diabetes or other endocrine disorders (thyroid dysfunction and adrenal disorders), using medications that impact carbohydrate or lipid metabolism (oral contraceptive pills, antiepileptic, antipsychotics, statins, and fish oil), using fertility-enhancing or weight loss medications, history of hormonal therapy and/or insulin-sensitizers within the previous 2 months, and history of hepatic and renal disorders (alcoholic fatty liver disease (AFLD), non-alcoholic fatty liver disease (NAFLD)) hepatitis, hypertension, diagnosed anemia, severe respiratory disease (asthma and chronic bronchitis), and heart disease.
Anthropometric measurements
Body composition analysis and resting metabolic rate (RMR) were conducted using an electronic scale with bioimpedance analysis (Plus Avis 333 Body Analyzer, China). The analysis included measurements of fat body mass (FBM), lean body mass (LBM), total body water (TBW), total body protein (TBP), and total body mineral (TBM), conducted with subjects wearing light clothing and with shoes and socks removed. The measurements were taken to the nearest 0.1 kg. The resting metabolic rate (RMR) was reported in Kcal. Height was determined to the nearest 1 cm using a well-mounted portable stadiometer (Holtain Ltd, UK). Moreover, BMI was calculated in kg/ m2. The WC was measured after removing clothes from the abdomen, as the smallest circumference was between the lowest rib and the iliac crest on the midaxillary line. The HC was determined at the level of the widest circumference over the great trochanters, and the WHR was calculated as waist measures divided by hip measurement. All parameters were measured at baseline and at the end of the study, after 8 weeks of dietary intervention.
Biochemical analysis
At baseline and after 8 weeks of the study, 10 ml venous blood samples were drawn from each participant by a catheter. Blood samples were collected into BD vacutainer Tubes (SSTTM 2 advance, REE 397953) between 8: 00 a.m. and 9:00 a.m. after 10 to 12 h of overnight fasting and resting in bed during the early follicular phase (day 2–5) of menstrual cycle of each individual. The menstrual cycle was determined as the number of days from the onset of menstrual bleeding in one cycle to the first day of the next [41]. The participants' menstrual cycle at baseline was defined as the average menstrual cycle for 6 months before the intervention. Blood samples were immediately centrifuged at 4000 RPM using centrifuge J6-MC by Beckman. Then the obtained serum was aliquoted and stored at -80 °C until assay time. All samples were analyzed in the same analytical session for each test, using the same reagent lot and according to the manufacturer’s instructions with inter- and intra-assay coefficient variances (CVs) lower than 7%. Serum FBG was measured through enzymatic methods using the colorimetric technique, by commercial kits (Pars-Azmoon Co., Tehran, Iran) by an auto-analyzer (Hitachi-917, Tokyo, Japan). Serum insulin level was measured by chemiluminescence (IMMULITE 2000, SIEMENS). Total cholesterol, HDL, and LDL by enzymatic colorimetric in homogenous phase ((Roche Cobas e702, Roche Diagnostics, Mannheim, Germany), TGs by an enzymatic colorimetric method (Roche Cobas e702, Roche Diagnostics, Mannheim, Germany). Reproductive hormones including LH, FSH, DHEA, and free testosterone were evaluated by ELISA kits (Bioassay Technology Laboratory, Shanghai Korean Biotech, Shanghai City, China).
The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) index was calculated according to the formula (insulinemia mU/ml × glycemia mg/ dL/450). The homeostasis model assessment of β-cell dysfunction (HOMA-B) index was also calculated according to its formula (20 × fasting insulin (µU/ml)/ [fasting plasma glucose (mg/dl) – 63]). Moreover, the visceral adipose tissue (VAI) index and lipid accumulation product (LAP) are both common markers to estimate cardiometabolic risks in women with PCOS, which were calculated according to their formula. (VAI = WC (cm) /36.58 + (1.89 × BMI (Kg/m2)) × TG (mmol/l)/0.81 × 1.52/HDL (mmol/l) and LAP = (WC (cm) – 58) × TG (mmol/l)). All biochemical tests were assessed clinically at the Reference Laboratory of Kermanshah University of Medical Science before and after the study.
Diets
Participants were randomly stratified to the PMCD or the KD designed by a professional dietician to follow for 8 weeks. The PMCD is a plant-based diet with a macronutrient distribution of 40% carbohydrate, 20% protein, and 40% fat, excluding all animal products, for instance, meat, fish, poultry, eggs, and dairy. The Portfolio diet consisted of five food components added to the diet including 1) plant protein from soy products or dietary pulses such as beans, peas, chickpeas, and lentils; 2) viscous soluble fiber from oats, barley, psyllium, eggplant, okra, and certain fruit; 3) nuts from tree nuts or peanuts; 4) phytosterols; and 5) monounsaturated fats (MUFAs) in the form of olive, canola, or high oleic sunflower oils that indicated improvements of blood lipids [42]. The KD included 10% carbohydrates, 20% protein, and 70% fat. Carbohydrate intake was < 30 g per day, provided by a variety of vegetables as they are rich in fiber and have low amounts of carbohydrates [43]. Protein sources were fish, poultry, meat, and eggs. Furthermore, fat components were consumed in the form of olive oil, sesame, and oilseeds. Unsweetened drinks like herbal teas, tea, and infused coffee were allowed as they would not induce ketosis. Both groups received individual face-to-face counseling on their assigned diet at baseline and every two weeks by the same dietitian and similar protocol. Each diet was determined according to the prescribed daily calorie and macronutrient intake of individuals. Also, 500–700 kcal were removed from the total caloric intake of each person and then its macronutrient distribution was calculated based on the type of diet [44]. To increase the motivation of the patients to fully follow their diet, the registered dietician assured them to prescribe a stabilized diet at the end of the study. In addition, to compensate for the possible lack of micronutrients, all participants received a multivitamin-mineral supplement tablet (Dana, Multi daily Co., Tehran, Iran) every day.
Dietary, physical activity, and ketosis status assessments
Each patient was provided with at least ninety minutes of individualized face-to-face dietary counseling at the first session with a professional dietitian to educate their diet and encouraged to have aerobic physical activity, for instance, walking or jogging at least 60 min per day. Also, they were reminded to stop continuing the diet if they realized that they were pregnant or manifested serious complications. The dietitian was readily available to guide and advise individuals via phone, email, and other media during 8 weeks of intervention. To monitor participants' dietary adherence, the dietitian called each patient every two weeks to document their 3-day food records, two working days, and the weekend and send their food pictures. Then, the daily micro/macronutrient composition, calorie intake, fiber intake, and phytosterols of each diet were calculated by analyzing food data using Nutritionist IV software (First Databank, San Bruno, CA) to realize how participants follow their diets.
To monitor the patient’s physical activity, the physical activity questionnaire-short form (IPAQ-SF) was assessed every 2 weeks by the dietitian. The IPAQ-SF is a valid and reliable questionnaire with seven questions that measure a patient’s physical activity through the last 7 days [45]. Assessing the ketosis status was determined by the urinary keto sticks [46] (Kimia Pajohan Co., Tehran. Iran). Ketone assessment kits are a qualitative method for assessing ketosis situations. This kit contains dipsticks coated with chemicals that change color after reacting with ketone compounds. A dipstick is dipped in the urine sample. If the color was changed in less than one minute, it indicates the presence of ketone bodies in the urine. All participants were required to use urinary keto sticks two random times every week and send their pictures to the dietitian. If the participants did not follow the protocol of the study they were withdrawn from the clinical trial.
Ferriman-gallwey score
Hirsutism is considered one of the common clinical symptoms of PCOS and approximately 70% of PCOS women represent it [47]. It is evaluated by the Ferriman-Gallwey score. This scoring system evaluates nine androgen-dependent body parts such as the upper lip, chin, chest, upper abdomen, lower abdomen, upper arms, thigh, upper back, and lower back, with scores ranging from zero (no excessive terminal hair growth visible) to four (extensive hair growth visible) for each body part evaluated. A maximum score of 36 is possible [48]. In this study, Hirsutism was considered positive when the Ferryman-Gallwey score was > 8.
Statistical analysis
Statistical analyses were carried out according to intention to treat analyses. Data analysis was performed using SPSS Statistics (version 19, New York, USA). The distribution of data was expressed as mean (SD) for normally distributed quantitative data, and frequency (percent) for qualitative data. Kolmogorov–Smirnov test was used to check the normality of the data distribution. To compare the two groups at the baseline, independent sample tests and chi-squared tests were used. Assessments of differences within the group were made by paired-sample t-tests. Effect size mas measured based on Cohen’s d test. At the end of the study, the comparison of the two groups was conducted using covariance (ANCOVA) analysis one time without adjustment and the other time adjusted for the baseline parameters and confounders (age, BMI, weight, calorie intake, and physical activity level). A significance level of P < 0.05 was used to evaluate the statistical analyses.
Results
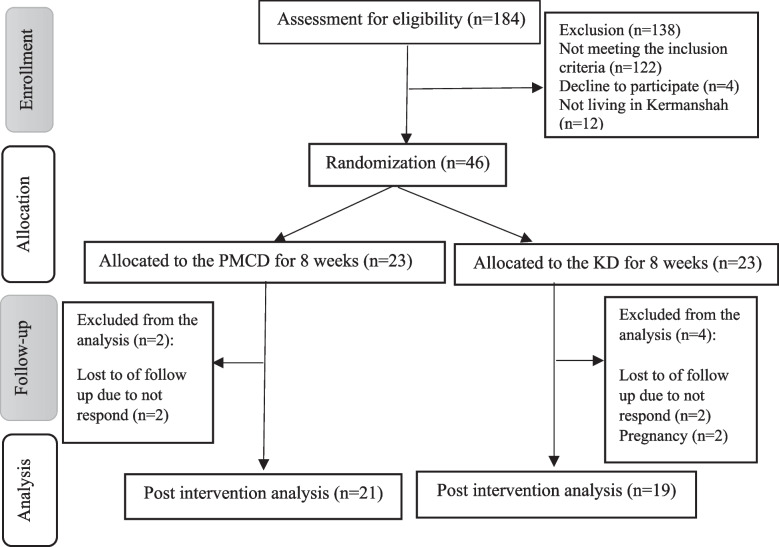
As depicted in Fig. 1, 46 women out of 184 individuals who responded to the recruitment advertisement met the eligibility criteria to take part in this study and were randomly assigned to the two diet groups. During 8 weeks of the intervention, 2 participants were excluded from the PMCD due to the loss of follow-up, while 4 women were excluded from the KD because of not responding and pregnancy. Overall, 21 and 19 women finished the study in the PMCD and the KD, respectively. Also, none of the participants of this study did not report any complications following the diets, which may be due to the short duration (8 weeks) of the trial.
Fig. 1.
CONSORT flow diagram of study design for portfolio low-carbohydrate and the ketogenic diet in women with polycystic ovary syndrome
Baseline characteristics
Following the CONSORT guidelines, the baseline data of individuals who were randomized is illustrated in Table 1. The SD of age and BMI of studied women were 30.13 (7.27) years and 29.47 (4.25) kg/m2 in the PMCD group and 30.30 (5.41) years and 29.21 (3.39) kg/m2 in the KD group with no significant differences (P = 0.927 and P = 0.496 respectively). Moreover, there were no significant differences in demographical data, such as height, WHR, total energy intake, physical activity, and marital status of participants in both groups. None of the participants were smokers. The qualitative result of the ketone sticks of all participants in the KD group was positive, while it was negative in all patients in the PMCD group during 8 weeks of intervention.
Table 1.
Baseline characteristics of women with PCOS
| Variables | PLCD (n = 23) | KD (n = 23) | P-value | |
|---|---|---|---|---|
| Age (year)* | 30.13 (7.27) | 30.30 (5.41) | 0.927† | |
| Height (cm)* | 163.26 (5.69) | 164.2 (7.59) | 0.631† | |
| BMI (Kg/m2)* | 29.47 (4.25) | 29.21 (3.39) | 0.496† | |
| WHR* | 0.914 (0.034) | 0.920 (0.026) | 0.769† | |
| Total energy intake (Kcal/day)* | 2401.04 (342.70) | 2531.78 (455.67) | 0.277† | |
| Physical activity (MET minute/week)* | 403.13 (43.79) | 391.49 (34.08) | 0.320† | |
| Single | Marital status** | 5 (21.7%) | 6 (26.1%) | 0.50§ |
| Married | 18 (78.3%) | 17 (73.9%) | ||
*Values are presented as mean (SD)
†Based on independent samples t-test
**Values are presented as number (%)
§Based on Fisher exact test
Abbreviations: PCOS polycystic ovary syndrome, WHR waist to hip ratio
Dietary intake
There were no significant differences in the baseline intake of calories, macronutrients, and micronutrients between the two groups (P > 0.05). After 8 weeks of intervention, the mean difference in carbohydrate intake decreased significantly in the KD group than the PMCD group (MD (SD) 280.67 (53.876) vs. MD (SD) 138.69 (63.438)). Although the energy intake in both diets did not have differences (P = 0.025), all macronutrients as well as micronutrients were remarkably different between the two diets due to their intrinsic distinct. The ketogenic diet has higher levels of cholesterol, SFAs, PUFA, MUFA, and TFA compared to the plant-based PMCD. While, the PMCD has a higher amount of total fiber and phytosterols than the ketogenic diet (Table 2).
Table 2.
Anthropometric index and body composition characteristics at baseline and after 8 weeks of intervention
| Variables | PLCD (n = 21) | KD (n = 19) | MD (CI%95) | P-value† | P-value | |
|---|---|---|---|---|---|---|
| Weight (kg) | Baseline | 78.49 (11.61) | 79.02 (12.34) | -0.52 | 0.88 | P < 0.05* |
| 8 weeks | 74.14 (11.29) | 73.38 (11.35) | 0.76 | 0.83 | P < 0.05§ | |
| MD (CI%95) | 4.35 (3.64, 5.56) | 5.64 (8.53, 7.55) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| BMI (kg/m2) | Baseline | 29.47 (4.25) | 29.21 (3.39) | 0.26 | 0.496 | P < 0.05* |
| 8 weeks | 27.76 (3.89) | 26.48 (3.56) | 1.28 | 0.286 | P < 0.05** | |
| MD (CI%95) | 1.71 (1.36, 2.07) | 2.90 (2.73, 3.07) | ||||
| p-Value | P < 0.05 | P < 0.05 | ||||
| WC (cm) | Baseline | 104.19 (10.89) | 105.62 (12.03) | -1.42 | 0.67 | P < 0.05* |
| 8 weeks | 101.49 (10.17) | 100.18 (12.34) | 1.30 | 0.71 | P < 0.05** | |
| MD (CI%95) | 2.70 (2.07, 3.34) | 5.44 (6.74, 8.10) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| HC (cm) | Baseline | 113.96 (10.68) | 114.65 (11.42) | -0.68 | 0.83 | P < 0.05* |
| 8 weeks | 110.73 (11.16) | 109.61 (12.88) | 1.12 | 0.76 | P < 0.05** | |
| MD (CI%95) | 3.44 (2.81, 4.07) | 7.31 (5.04, 9.57) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| WHR | Baseline | 0.914 (0.034) | 0.920 (0.026) | -0.006 | 0.769 | 0.338* |
| 8 weeks | 0.917 (0.039) | 0.914 (0.049) | 0.002 | 0.855 | 0.529** | |
| MD (CI%95) | -0.005 (-0.01, 0.003) | 0.004 (-0.014, 0.023) | ||||
| P-value | 0.243 | P < 0.05 | ||||
| FBM (kg) | Baseline | 29.86 (6.93) | 28.77 (7.04) | 1.09 | 0.598 | P < 0.05* |
| 8 weeks | 26.74 (6.26) | 24.42 (6.99) | 2.32 | 0.275 | P < 0.05** | |
| MD (CI%95) | 3.08 (2.06, 4.10) | 5.18 (4.26, 5.75) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| LBM (kg) | Baseline | 50.01 (5.50) | 50.24 (6.21) | -0.230 | 0.895 | P < 0.05* |
| 8 weeks | 48.61(5.31) | 48.62 (5.14) | -0.006 | 0.997 | P < 0.05** | |
| MD (CI%95) | 1.35 (0.67, 2.04) | 3.19 (2.64, 3.74) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| TBW (kg) | Baseline | 36.00 (3.97) | 36.18 (4.47) | -0.17 | 0.887 | P < 0.05* |
| 8 weeks | 35.01(4.07) | 35.16 (3.52) | -0.14 | 0.903 | P < 0.05** | |
| MD (CI%95) | 0.96 (0.51, 1.41) | 2.15(1.74, 2.55) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| TBP (kg) | Baseline | 9.50 (0.98) | 9.61 (1.13) | -0.10 | 0.730 | P < 0.05* |
| 8 weeks | 9.19(1.04) | 9.35 (1.02) | -0.16 | 0.622 | P < 0.05** | |
| MD (CI%95) | 0.30 (0.21, 0.40) | 0.56 (0.38, 0.74) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| TBM (kg) | Baseline | 4.49 (0.63) | 4.45 (0.67) | 0.03 | 0.841 | 0.541* |
| 8 weeks | 4.20 (0.71) | 4.34 (0.68) | -0.14 | 0.510 | 0.551** | |
| MD (CI%95) | 0.29 (0.17, 0.40) | 0.24 (0.15, 0.32) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| RMR (Kcal) | Baseline | 1300.00 (80.60) | 1305.86 (86.78) | -5.86 | 0.813 | 0.665* |
| 8 weeks | 1280.33(84.18) | 1298.74 (73.15) | -18.04 | 0.467 | 0.488** | |
| MD (CI%95) | 18.19 (3.67, 32.70) | 27.05 (4.26, 49.83) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
Data are expressed as mean (SD) and mean difference (95%CI)
†Indicate comparison between groups at baseline (Independent samples t-test for baseline)
*Indicate comparison between groups based on ANOVA without adjustment
**Indicate comparison between groups based on ANOVA adjusted for baseline measures and confounding factors (age, baseline BMI, physical activity, and, energy intake)
§Indicate comparison between groups based on ANOVA adjusted for baseline measures and confounding factors (age, physical activity, resting metabolite rate, and, energy intake)
Abbreviations: BMI body mass index, WC waist circumferences, HC hip circumferences, WHR waist to hip ratio, FBM fat body mass, LBM lean body mass, TBW total body water, TBP total body protein, TBM total body mineral, RMR resting metabolic rate
In addition, the KD contains more vitamin B12 and iodine than the PMCD due to its higher animal protein sources. Whereas, the PMCD contains more vitamin C, B9, calcium, iron, zinc, copper, sodium, potassium, magnesium, and phosphorus than the KD because of the soy and soy products as a source of plant protein. The main source of iron in the PMCD was non-hem, while it was mostly hem in the KD group (Table 3).
Table 3.
Metabolic and endocrine hormonal status at baseline and after 8 weeks of the intervention
| Variables | PLCD (n = 21) | KD (n = 19) | MD (CI%95) | P-value† | P-value | |
|---|---|---|---|---|---|---|
| FBG (mg/dl) | Baseline | 95.95 (4.60) | 96.21 (4.68) | -0.26 | 0.850 | P < 0.05* |
| 8 weeks | 90.90 (3.88) | 87.94 (2.67) | 2.95 | 0.030 | P < 0.05** | |
| MD (CI%95) | 5.00 (3.00, 6.99) | 8.84 (7.57, 10.10) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| Insulin (µU/ml) | Baseline | 24.74 (3.83) | 27.57 (3.17) | -2.83 | 0.009 | P < 0.05* |
| 8 weeks | 17.50 (3.48) | 14.45 (3.10) | 3.04 | 0.006 | P < 0.05** | |
| MD(CI%95) | 7.35 (5.76, 8.93) | 13.44(12.34, 14.55) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| HOMA-IR | Baseline | 5.87 (1.05) | 6.56 (0.92) | -0.68 | 0.023 | P < 0.05* |
| 8 weeks | 3.93 (0.82) | 3.15 (0.75) | 0.78 | 0.003 | P < 0.05** | |
| MD(CI%95) | 1.96 (1.58, 2.35) | 3.53 (3.24, 3.81) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| HOMA-B | Baseline | 15.17 (2.32) | 16.85 (2.62) | -1.67 | 0.027 | P = 0.071* |
| 8 weeks | 12.71 (2.79) | 11.78 (2.40) | 0.92 | 0.269 | P = 0.075** | |
| MD(CI%95) | 2.57 (1.07, 4.06) | 4.94 (3.92, 5.96) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| TC (mg/dl) | Baseline | 224.21 (33.37) | 230.21 (17.55) | -6.00 | 0.449 | P < 0.05* |
| 8 weeks | 185.09 (16.81) | 193.94 (15.47) | -8.85 | 0.092 | P = 0.030** | |
| MD(CI%95) | 44.38 (36.10, 52.65) | 38.15 (34.36, 41.95) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| TG (mg/dl) | Baseline | 266.86 (32.85) | 272.04 (34.72) | -5.17 | 0.606 | P < 0.05* |
| 8 weeks | 228.66 (37.29) | 214.42 (31.97) | 14.24 | 0.205 | P < 0.05** | |
| MD(CI%95) | 40.71(35.81, 45.61) | 61.42 (50.96, 71.87) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| LDL-C (mg/dl) | Baseline | 133.95 (13.69) | 134.86 (14.75) | -0.91 | 0.829 | P < 0.05* |
| 8 weeks | 100.00 (11.89) | 111.52 (11.22) | -11.52 | 0.003 | P < 0.05** | |
| MD(CI%95) | 33.80 (30.46, 37.15) | 21.52 (14.71, 28.33) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| HDL-C (mg/dl) | Baseline | 50.21(10.18) | 48.04 (8.91) | 2.17 | 0.445 | P < 0.05* |
| 8 weeks | 65.04 (8.91) | 59.52 (9.54) | 5.52 | 0.052 | P < 0.05** | |
| MD(CI%95) | -13.71 (-15.46, -11.96) | -10.42 (-12.71, -8.13) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| LAP (cm. mmol/L) | Baseline | 138.43 (32.44) | 146.90 (45.75) | -8.46 | 0.047 | P < 0.05* |
| 8 weeks | 111.30 (26.43) | 102.53 (38.25) | 8.77 | 0.040 | P < 0.05** | |
| MD(CI%95) | 28.37 (23.81, 32.93) | 44.37 (59.15, 45.43) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| VAI | Baseline | 4.86 (1.17) | 5.36 (1.26) | -0.39 | 0.25 | P = 608.0* |
| 8 weeks | 3.20 (60.0) | 3.37 (76.0) | -0.16 | 0.44 | P = 628.0** | |
| MD(CI%95) | 1.66 (1.33, 1.98) | 1.99 (1.60, 2.37) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| LH (mIU/ml) | Baseline | 12.15 (1.82) | 12.10 (1.95) | 0.04 | 0.93 | P < 0.05* |
| 8 weeks | 9.69 (0.97) | 7.97 (2.38) | 1.71 | 0.00 | P < 0.05** | |
| MD (CI%95) | 2.48 (1.98, 2.98) | 4.38 (3.72, 5.05) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| FSH (mIU/ml) | Baseline | 4.39 (2.17) | 4.06 (1.30) | 0.32 | 0.85 | P < 0.05* |
| 8 weeks | 4.63 (2.14) | 4.74 (1.41) | -0.10 | 0.53 | P < 0.05** | |
| MD(CI%95) | -0.24 (-0.54, -0.35) | -0.68 (-0.69, -0.40) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| Testosterone (ng/ml) | Baseline | 0.61 (0.13) | 0.67 (0.10) | -0.05 | 0.12 | P < 0.05* |
| 8 weeks | 0.48 (0.16) | 0.49 (0.09) | -0.01 | 0.79 | P < 0.05** | |
| MD(CI%95) | 0.12 (0.07, 0.17) | 0.18 (0.15, 0.20) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| DHEA-s(µg/ml) | Baseline | 2.09 (0.59) | 2.34 (0.55) | -0.24 | 0.15 | P = 0.071* |
| 8 weeks | 1.86 (0.55) | 1.93 (0.52) | -0.07 | 0.64 | P = 0.075** | |
| MD(CI%95) | 0.23 (0.17, 0.34) | 0.41 (-0.69, -0.40) | ||||
| P-value | P < 0.05 | P < 0.05 | ||||
| Ferriman Gallway Score | Baseline | 12.34 (2.91) | 12.82 (2.44) | -0.47 | 0.55 | P = 0.771* |
| 8 weeks | 11.52 (2.18) | 12.05 (2.30) | -0.52 | 0.457 | ||
| MD(CI%95) | 0.71 (-1.9, 1.62) | 0.80 (1.01, 1.08) | ||||
| P-value | 0.118 | 0.12 | ||||
Data are expressed as mean (SD) and mean difference (95%CI)
†Indicate comparison between groups at baseline (Independent samples t-test for baseline)
*Indicate comparison between groups based on ANOVA without adjustment
**Indicate comparison between groups based on ANOVA adjusted for baseline measures and confounding factors (age, baseline BMI, physical activity, and, energy intake)
§Indicate comparison between groups based on ANOVA adjusted for baseline measures and confounding factors (age, physical activity, resting metabolite rate, and, energy intake)
Abbreviations: FBS fasting blood glucose, HOMA-IR homeostatic model assessment of insulin resistance, HOMA_B homeostasis model assessment of beta cell function, TC total cholesterol, TG triglycerides, HDL high-density lipoprotein, LDL-C low-density lipoprotein, LAP lipid accumulation products, VAI visceral adipose index, LH luteinizing hormone, FSH follicle-stimulating hormone, DHEA-s dehydroepiandrosterone sulfate
Anthropometric index and body composition
As shown in Table 4, there were no notable disparities in the baseline anthropometric measurements between the two groups at the beginning of the study. After 8 weeks of intervention, significant changes were observed in anthropometric parameters including weight, BMI, WC, HC, FBM, LBM, TBW, TBP, TBM, and RMR within groups (P < 0.05). However, when comparing the two groups while adjusting for baseline values and changes in age, energy intake, RMR, and physical activity levels, it was found that the KD group showed a significant weight reduction (MD (SD) 5.64 (1.012) vs. MD (SD) 4.35 (2.107)), improvement in BMI (MD 2.73 (0.351) vs. MD 1.71 (0.775)), FBM (MD (SD) 4.35 (1.163) vs. MD (SD) 3.12 (0.862)), LBM (MD (SD) 1.62 (1.507) vs.MD (SD) 1.40 (1.132)), TBW, TBP, WC, and HC compared to the PMCD group. However, there were no significant differences in WHR, TBM, and RMR between the two groups. Although following both diets for 8 weeks was associated with a significant weight reduction of 4.35 kg in the PMCD vs. 5.64 kg in the KD, the distribution of that was not the same in the two diets. For instance, in the PMCD fat body mass (FBM), total body water (TBW) loss, and total body protein (TBP) reduction were 3.12, 0.96, and 0.30 kg whereas it was 4.35, 1.02, and 0.26 kg in the KD group, respectively. The reduction of FBM in the KD was more due to fat oxidation and ketone body production. However, there were no significant differences in the TBP due to moderate protein intake (20%) with the emphasis on consumption of high protein value (HPV) sources of protein in both groups.
Table 4.
Energy and macronutrient intake at baseline and after 8 weeks of intervention
| PLCD (n = 21) | KD (n = 19) | MD (CI%95) | P-value† | ||
|---|---|---|---|---|---|
| Energy (Kcal/day) | Baseline | 2401.04 (342.70) | 2531.78 (455.67) | -130.73 | 0.277 |
| 8 weeks | 1630.95 (150.39) | 1828.95 (333.88) | 197.99 | 0.019 | |
| MD (CI%95) | 723.47 (562.23, 884.71) | 642.84 (524.01, 761.67) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Carbohydrate (gr/day) | Baseline | 294.78 (62.76) | 303.00 (51.51) | -8.21 | 0.630 |
| 8 weeks | 156.09 (19.53) | 23.33 (2.42) | 132.75 | 0.00 | |
| MD(CI%95) | 131.14 (102.27, 160.02) | 277.72 (251.75, 303.68) | |||
| p-Value | P < 0.05 | P < 0.05 | |||
| Protein (gr/day) | Baseline | 68.0 (6.78) | 67.65 (6.66) | 0.34 | 0.862 |
| 8 weeks | 109.49 (10.45) | 118.61 (6.81) | -9.12 | 0.003 | |
| MD(CI%95) | -42.11 (-48.01, -36.21) | -51.93 (-56.91, -46.94) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Fat (gr/day) | Baseline | 106.48 (9.30) | 107.45 (11.03) | -0.96 | 0.749 |
| 8 weeks | 64.37 (13.67) | 139.46 (21.93) | -75.09 | 0.00 | |
| MD(CI%95) | 41.20 (33.93, 48.47) | -32.71 (-39.58, -25.83) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Total cholesterol (gr/day) | Baseline | 155.39 (41.32) | 163.86 (44.91) | -8.47 | 0.509 |
| 8 weeks | 3.63 (0.53) | 576.11 (142.17) | -572.47 | 0.00 | |
| MD(CI%95) | 146.27 (128.99, 163.54) | -417.8 (-487.9, -347.8) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Baseline | 25.03 (5.71) | 23.89 (5.30) | 1.15 | 0.481 | |
| Total Fiber (gr/day) | 8 weeks | 38.97 (4.57) | 12.87(1.67) | 26.10 | 0.00 |
| MD(CI%95) | -14.58 (-17.73, -11.43) | 10.24 (7.79, 12.68) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| MUFA (gr/day) | Baseline | 41.58 (3.56) | 42.96 (3.56) | -1.37 | 0.196 |
| 8 weeks | 20.40 (5.31) | 48.51 (4.08) | -28.11 | 0.00 | |
| MD(CI%95) | 20.75 (17.64, 23.86) | -6.02 (-7.90, -4.13) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| PUFA (gr/day) | Baseline | 12.70 (1.97) | 13.00 (1.91) | -0.30 | 0.593 |
| 8 weeks | 51.14 (2.84) | 52.25 (2.81) | -10.68 | 0.00 | |
| MD(CI%95) | -38.44 (-15.07, -12.24) | -39.25 (-24.91, -23.13) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| SFAs (gr/day) | Baseline | 51.14 (2.84) | 52.25 (2.81) | -1.10 | 0.192 |
| 8 weeks | 11.82 (6.12) | 41.25 (2.24) | -29.43 | 0.00 | |
| MD(CI%95) | 39.00 (35.76, 42.23) | 10.73 (8.94, 12.52) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| TFA (gr/day) | Baseline | 0.079 (0.016) | 0.080 (0.018) | -0.001 | 0.804 |
| 8 weeks | 0.005 (0.008) | 0.156 (0.021) | -0.15 | 0.00 | |
| MD(CI%95) | 0.07 (0.06, 0.08) | -0.07 (-0.09, -0.06) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Phytosterols (gr/day) | Baseline | 73.77 (4.08) | 75.54 (3.73) | -1.7 | 0.136 |
| 8 weeks | 162.42(39.39) | 1.55 (42.0) | 160.87 | 0.00 | |
| MD(CI%95) | -88.75 (-106.72, -70.78) | 73.86 (71.95, 75.77) | |||
| P-value | P < 0.05 | P < 0.05 | |||
Data are expressed as mean (SD) and mean difference (95%CI)
†Indicate comparison between groups at baseline (Independent samples t-test for baseline)
*Indicate comparison between groups based on ANOVA without adjustment
Abbreviations: MUFA mono-unsaturated fatty acids, PUFA poly-unsaturated fatty acids, SFA saturated fatty acids, TFA trans fatty acids
Metabolic and reproductive hormonal status
According to Table 5, both the PMCD and KD groups led to significant enhancements in the metabolic status (FBG, serum insulin levels, HOMA-IR, HOMA-B, and lipid profile) and reproductive hormone levels (LH, FSH, free-testosterone, and DHEA) of participants at the beginning and end of the study. The intragroup analysis showed a significant reduction in the mean difference of glycemic and lipid profiles such as FBG, insulin, HOMA-IR, HOMA-B, total cholesterol, triglyceride, LDL, LAP, and VAI in the two groups (P < 0.05). After adjustment for the confounding variables, a significant difference was observed between the two groups regarding FBG, insulin, HOMA-IR, TC, triglyceride, LDL, HDL, and LAP parameters (P < 0.05). Besides, the PMCD decreased the level of total cholesterol (MD (SD) 39.12 (18.186) vs. MD (SD) 36.27 (7.869) and LDL (MD (SD) 33.95 (7.345) vs. MD (SD) 23.34 (14.136)), while increasing the level of HDL (MD (SD) -14.83 (3.848) vs. MD (SD) -11.48 (4.753)) more than the KD group.
Table 5.
Micronutrient intake at baseline and after 8 weeks of the intervention
| PLCD (n = 21) | KD (n = 19) | MD (CI%95) | P-value† | ||
|---|---|---|---|---|---|
| Vitamin C (mg/day) | Baseline | 253.93 (5.28) | 256.79 (6.83) | -2.86 | 0.119 |
| 8 weeks | 162.49 (6.02) | 88.73 (4.64) | 73.76 | 0.00 | |
| MD (CI%95) | 91.19 (87.74, 94.64) | 167.70 (163.6, 171.80) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Vitamin B9 (mg/day) | Baseline | 329.13 (8.87) | 332.10 (4.83) | -2.96 | 0.166 |
| 8 weeks | 339.74 (8.42) | 192.12 (5.01) | 147.62 | 0.00 | |
| MD(CI%95) | -10.68 (-15.44, -5.91) | 139.49 (136.34, 142.64) | |||
| p-Value | P < 0.05 | P < 0.05 | |||
| Vitamin B12 (mic/day) | Baseline | 1.85 (0.12) | 1.91 (0.13) | -0.06 | 0.105 |
| 8 weeks | 0.18(0.03) | 6.65 (1.54) | -6.46 | 0.00 | |
| MD(CI%95) | 1.67 (1.60, 1.73) | -4.72 (-5.44, -4.00) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Calcium (mg/day) | Baseline | 458.93 (21.17) | 469.04 (14.15) | -10.11 | 0.064 |
| 8 weeks | 634.93 (13.15) | 241.77 (13.22) | 293.15 | 0.00 | |
| MD(CI%95) | -176.65 (-189.25, -164.05) | 127.05(116.34, 137.77) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Iron (mg/day) | Baseline | 9.82 (0.67) | 9.37 (0.77) | 0.44 | 0.043 |
| 8 weeks | 24.05 (2.32) | 11.51 (0.99) | 12.54 | 0.00 | |
| MD(CI%95) | -14.18 (-15.25, -13.10) | -2.17 (-2.80, -1.54) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Zinc (mg/day) | Baseline | 6.57 (0.75) | 6.75 (0.64) | -0.17 | 0.393 |
| 8 weeks | 13.21 (0.89) | 10.15 (0.66) | 3.06 | 0.00 | |
| MD(CI%95) | -6.56 (-7.06, -6.06) | -3.40 (-3.85, -2.95) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Copper (mg/day) | Baseline | 1.96 (0.58) | 2.03 (0.55) | -0.06 | 0.70 |
| 8 weeks | 3.42 (0.57) | 2.21 (0.48) | 1.21 | 0.00 | |
| MD(CI%95) | -1.42 (-1.82, -1.02) | -0.18 (-0.46, 0.08) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Sodium (mg/day) | Baseline | 1989.52 (6.8) | 1991.38 (8.05) | -1.85 | 0.405 |
| 8 weeks | 6051.53 (11.91) | 1690.40 (5.83) | 4361.13 | 0.00 | |
| MD(CI%95) | -4061.89 (-4067.65, -4056.12) | 301.07 (296.21, 305.93) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Potassium (mg/day) | Baseline | 4621.36 (8.56) | 4626.70 (5.18) | -5.33 | 0.014 |
| 8 weeks | 5124.27 (6.72) | 1982.02 (7.12) | 3142.24 | 0.00 | |
| MD(CI%95) | -502.38 (-506.69, -498.07) | 2644.70 (2640.21, 2649.19) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Magnesium (mg/day) | Baseline | 246.11 (8.51) | 251.26 (7.12) | -5.15 | 0.031 |
| 8 weeks | 620.04 (8.23) | 337.53 (68.62) | 282.51 | 0.00 | |
| MD(CI%95) | -374.65 (-381.13, -368.18) | -86.57 (-118.01, -55.13) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Phosphors (mg/day) | Baseline | 1031.08 (6.43) | 1038.61 (5.32) | -7.52 | 0.00 |
| 8 weeks | 1613.63 (7.19) | 1309.09 (9.17) | 304.54 | 0.00 | |
| MD(CI%95) | -582.94 (-587.85, -578.03) | -270.66 (-274.95, -266.36) | |||
| P-value | P < 0.05 | P < 0.05 | |||
| Iodine (mg/day) | Baseline | 72.55 (6.20) | 74.43 (6.53) | -1.87 | 0.324 |
| 8 weeks | 23.46 (3.77) | 86.05 (15.06) | -62.58 | 0.00 | |
| MD(CI%95) | 49.29 (45.65, 52.92) | -11.34 (-17.63, -5.04) | |||
| P-value | P < 0.05 | P < 0.05 |
Note: Data are expressed as mean (SD) and mean difference (95%CI)
†Indicate comparison between groups at baseline (Independent samples t-test for baseline)
*Indicate comparison between groups based on ANOVA without adjustment
In addition, the intragroup differences in the levels of LH, FSH, free testosterone hormones, and DHEA was statistically significant (P < 0.05). On the other hand, the mean difference between the two groups (PMCD vs. KD) for LH was (MD (SD) 2.46 (1.105) vs. MD (SD) 4.13 (1.375)) with the effect size of 2.247 vs. 3.191 and (MD (SD) 0.23 (0.185) vs. MD (SD) 0.41 (0.139)) with the effect size of 1.417 vs. 3.304 for DHEA-s and after adjustment for main confounding factors they were statistically significant (P < 0.05).
However, the level of HOMA-B (P = 0.075), VAI (P = 0.628), FSH (P = 0.171), and free testosterone (P = 0.142) did not show a significant change in comparison between groups. At the end of the study, there was no statistically significant difference in the Freeman Galloway score in the PMCD (P = 0.118) and the KD (0.121) groups.
Discussion
The present study is the first intervention that compares two calorie-restricted diets, the PMCD and the KD among overweight or obese women with PCOS. The findings of this study reveal that both diets have beneficial effects on weight loss, reducing FBM, LBM, TBP, WC, and HC. In addition, both diets showed improvements in metabolic status such as FBG, insulin sensitivity, and lipid profile as well as reproductive hormones (LH, FSH, free testosterone, and DHEA) in the participants. However, the ketogenic diet was probably more effective through weight loss, reduced body composition (from fat mass), improved blood glucose status, and reproductive hormones such as LH and DHEA-s. Nevertheless, there was a more significant improvement in lipid profiles including TG, LDL-C, and HDL-C in the PMCD group compared to the KD group. However, no significant improvement was observed within and between the two groups for the Ferriman-Gallway score. Additionally, two married women from the KD group got pregnant despite many previous unsuccessful attempts.
PCOS is recognized as one of the most common endocrinopathies affecting women of reproductive age, causing infertility and linked to a wide range of chronic cardiovascular and metabolic issues, resulting in 0.43 million disability-adjusted life-years (DALY) [49]. PCOS is a multifactorial disease, that not only includes genetic agents, but also environmental, metabolic, and endocrine factors [50]. The high prevalence of overweight, obesity, and central fat with an estimation of 75% among PCOS women indicates the important role of environmental factors which are directly associated with lifestyle habits [51]. Excessive adipose tissue particularly abdominal adipocytes as an endocrine organ, increases the production of estrogen and estrone [52]. In the hypothalamus, the additional levels of estrogen and estrone lead to more secretion of GnRH, and consequently increase preferential production of LH as well as hyperandrogenism [53]. Although the etiology of PCOS has not been exactly clarified, studies hypothesized that insulin resistance (IR) have a positive correlation with both total and central fat which induces an increased risk of higher androgens [54]. Therefore, obesity and IR are two crucial aspects of causes of PCOS. According to recent guidelines from the American Society for Reproductive Medicine (ASRM), lifestyle adjustments, such as diet control, exercise, and weight management, are recommended as the first-line therapy for PCOS [55]. Moreover, studies have demonstrated that a 5–10% weight loss in overweight or obese women with PCOS can improve ovarian function and increase pregnancy rates [56]. Subsequently, numerous studies have reported that hypocaloric diets are effective in both desirable weight reduction and alleviating PCOS manifestations [57, 58]. Further investigations have shown that a low-carbohydrate diet could have benefits for women with PCOS, regardless of the severity of their symptoms. These benefits include improvements in endocrine and metabolic parameters, fertility, and weight loss which is due to lower insulin secretion and increased fat oxidation when compared to the conventional calorie-restricted diets [6].
The results of our study align with Paoli and colleagues that following a Ketogenic-Mediterranean diet with extracts for 12 weeks, in 14 overweight women with PCOS, significantly improved anthropometric parameters, glycemic status, lipid profiles, and reproductive hormones [59]. Another trial on 17 overweight and obese women diagnosed with PCOS represented that following a hypocaloric-ketogenic diet (carbohydrate intake 40–50 gr/day) for 45 days ameliorates anthropometric index, insulin sensitivity, and androgen production. It appears that the observed improvements are due to the reduction of carbohydrates and the induction of therapeutic ketosis due to the diet, which leads to changes in the ratio of LH, FSH, LH/FSH, and an increase in SHBG [60]. Also, a recent study compared the KD with a standard, balanced hypocaloric diet like the Mediterranean diet (MD) for a short period. The study found that both diets showed significant improvements in anthropometric (weight, WHR, and BMI) and biochemical parameters (FBG, HOMA-IR, LH/FSH, total testosterone). However, the KD resulted in a greater reduction in all the parameters compared to the MD [61].
Different mechanisms have been identified that the KD could alleviate the symptoms of PCOS. Less than 30 g/day of carbohydrate consumption results in decreasing insulinemia, an improvement in insulin sensitivity, and a reduction in androgen production [62, 63]. The beneficial effects of the KD on obesity are explained by the reduction in insulin secretion which enhances fat catabolism by decreased de novo lipogenesis activation, increased fatty acid oxidation, and ketone body production which would result in weight loss mainly from adipose tissue [64]. Furthermore, following the KD feeding, the activation of AMPK (adenosine-monophosphate-activated protein kinase) may be facilitated by low glucose levels, extremely low levels of insulin, as well as the activation of pathways involved in hepatic lipid oxidation which is consistent with the decrease in body weight and increase in energy expenditure [65, 66]. Researches have been illustrated that in the nutritional ketosis status (the condition that the concentration of blood ketone is more than 0.5 mmol/L), the secretion of the hunger hormone “ghrelin” decreased which results in lower food intake. It is also notable that this feeling of hunger is accompanying with fat reduction and consequently weight loss, which lasts only as long as the individuals remain in ketosis situation [67]. Thus, it is believed that ketosis is the primarily responsible for the appetite-suppressing effects observed with a KD [68]. Also, the reduction of fat mass leads to reduce the acyclic production of estrogens deriving from the aromatization in the adipose tissue from the androgen excess, which improves the LH to FSH ratio [60]. Additionally, the noticeable effect of the KD in PCOS is through the AMPK and silent mating type information regulation 2 homolog 1 (SIRT1) activation which improved glucose homeostasis and insulin sensitivity [69]. Charlot et al. demonstrated that a low-carbohydrate but high-fat diet (LCHFD) compared to the Western diet (with the same number of calories) was more successful in preventing weight gain, maintaining blood glucose and insulin levels, as well as reducing triglyceride accumulation in the liver of mice [63].
In the present study, the main weight loss in the KD was related to fat mass reduction. It might be due to higher protein intake (20% of total calories) compared to the previous studies (15–18%). Furthermore, WHR was significantly reduced in the KD more than in the PMCD. However, in both diets there was a significant reduction in the carbohydrate intake (from 300 g/day to 150 g/day in the PMCD group and 23 g/day in the KD), the level of insulin and insulin sensitivity (HOMA-IR) decreased significantly more in the KD group compared to the PMCD. The reduction of weight from fat mass and the lower level of serum insulin was accompanied by improvement in reproductive hormones (significant decreased in LH and DHEA-s) and consequently improvement of PCOS. Observing these significant changes in metabolic and reproductive hormone results in a short time (8 weeks) of intervention may be due to significant changes in macronutrient intake before and during the study. For instance, before the study patients consumed approximately 106 gr/day fat while through 8 weeks of the study, participants consumed 64 gr/day and 139 gr/day fat in the PMLCD and the KD respectively. This variation was seen in other macronutrients intake (carbohydrate and protein) as well. Moreover, through the first 3-day food recorder people announced more consumption of junk foods and less fruits, vegetables, and protein sources.
Although both diets indicated improvement in lipide profiles such as TC, TG, LDL-C, and HDL-C, the PMCD showed more positive effects due to possessing higher levels of antioxidants, soluble fiber, and phytosterols with lower amounts of saturated fatty acids and trans fatty acids. Since animal products (as a rich source of vitamin B12, and heme Iron) were removed from the PMCD diet, it might cause a deficit in these nutrients for a long time. Therefore, a daily multivitamin-mineral was prescribed to prevent deficit complications. PCOS women often have an atherogenic lipid profile, which includes high levels of LDL, total cholesterol, and triglycerides, along with low levels of HDL [70]. Thus, the PMCD might be beneficial for particularly those women with lipid profile disorders.
One of the common disorders reported by women with PCOS is hirsutism. This disorder is often associated with excessive androgen levels, including nonclassical adrenal hyperplasia, androgen-secreting tumors, and PCOS [71]. One of the important tools for clinical diagnosis and evaluation of this disorder is the Freeman-Galloway score [72]. In the present study, there were no significant changes in the Ferriman Gallwey score progression, possibly due to the short study duration which was inadequate to observe hair growth accurately. Since the average life cycle of a hair follicle is about 6 months it takes over three months to see measurable effects of the hair cycle and growth, which vary depending on the body area [73].
According to similar interventions, these two diets did not have serious adverse or harmful effects in a short time. However, the ketogenic diet has demonstrated some adverse events (AEs) such as fatigue, headache, irritability, dehydration, hypoglycemia, lethargy, hyperuricemia, and gestational side effects (diarrhea, nausea, and vomiting) for a short time and serious adverse effects (SAEs) including hypercalcemia, hypokalemia, lipid profile change, urolithiasis, gallstone, and hair loss for long-term intervention. Furthermore, due to the lack of sufficient clinical evidence of the long-term safety of KD in chronic disease, the recommendation to follow this type of diet in the long term should be made with caution.
However, both diets indicated improvements in the serum levels of FBG, insulin, and HOMA-IR due to their low carbohydrate content, the KD might be a better option for improving PCOS symptoms in a short time due to the production of ketone bodies and greater effectiveness on reproductive hormones. However, considering the fewer side effects and the fact that it is easier to follow the PMCD than the KD for women with PCOS, it seems that until the achievement of reliable scientific evidence in this regard, it is recommended to follow PMCD with fewer side effects and longer duration in real life.
The strength of the present study was the precise evaluation of dietary adherence in both groups. We utilized urinary keto strips twice a week at random to confirm the induction of ketosis in the patients' bodies in the KD group. Also, despite the short duration of the trial (8 weeks), we observed positive effects of both diets on participants. The adherence to both diets was high and only two people from each group were withdrawn from the study. In addition, none of the participants reported any complications while following the diets and after one-month follow-up from the end of the study.
The study encountered several limitations. First, the inability to assess inflammatory markers such as C-reactive protein (CRP), tumor necroses factor-α (TNF-a), and interleukin-6 due to limited financial support. Several studies have shown that the balance in the levels of inflammatory markers is important in maintaining the proper functioning of the ovaries [74], The imbalance in the levels of anti-inflammatory and pro-inflammatory cytokines may aggravate the condition of ovarian dysfunction. In this context, an increase in the levels of CRP and some cytokines such as IL-6, interleukin 18 (IL-18), and TNF-α have been suggested in polycystic ovary disease, which can be related to the development of chronic inflammation as a risk factor for endothelial dysfunction, atherosclerosis, coronary heart disease, IR, and abdominal obesity [75, 76]. Secondly, blinding of participants was not possible in this study due to the nature of the diet. However, to minimize bias, the outcome assessors and data analysts were blinded. Another limitation of this study was the short study duration. Considering that following a low-carbohydrate diet such as a ketogenic diet in the long term could have various difficulties and side effects for participants, which could cause a higher dropout rate. Based on similar studies [60], 8 weeks was considered for conducting the study. Furthermore, we did not examine how different diets affect the gut microbiota which should be considered as an important effector on the metabolic status of women with PCOS. Also, it is recommended to assess the liver enzymes including alanine aminotransferase (ALT) as well as aspartate aminotransferase (AST) through prescribing such diets to find out the effects of them on the liver’s function of the PCOS women. The small sample size and lack of potential external validity were other limitations of this study. However, the sample size was designed to have sufficient power (80%) to detect a difference of 2 units of change in weights as the primary outcome. Also, considering that all participants of this study were from Kermanshah city, to achieve better external validity, more studies should be done on samples from other geographical regions.
Conclusion
The results of our study have revealed that both PMCD and KD diets, which are characterized by their low carbohydrate content, could have a significant positive impact on the outcomes of individuals with PCOS over 8-weeks. In addition, the study showed that inducing ketone status by following KD, had a significant positive effect on various health parameters, including anthropometric indices such as body weight and BMI, glycemic and metabolic status, as well as reproductive hormones such as LH and DHEA-s. However, the PMCD indicates more beneficial effects on lipid profiles including LDL-C, and HDL-C. Since PMCD compared to KD has shown more favorable effects on lipid profile, which is an important risk factor for chronic heart diseases, it can be prioritized as a useful treatment option for PCOS women with higher cardiovascular risk factors. The findings of this clinical trial showed that following low carbohydrate diets such as KD and PMCD can be considered an effective and short-term adjuvant treatment in overweight or obese women with PCOS. However, due to the individual differences, the complexity of designing, and implementing such nutritional interventions in different disorders, it is recommended to perform these nutritional interventions under the supervision of registered dietitian with the cooperation of the treatment team. Overall, due to the potential side effects of following KD in the long term, it is recommended to conduct more clinical trials with larger sample sizes and longer durations (6 to 12 months) to assess potential side effects or interaction of dietary interventions with other therapies like medications. Further research should be conducted on the cellular mechanisms of these diets in women with PCOS.
Acknowledgements
We would like to acknowledge all of the participants and their time and effort. Also, the Kermanshah University of Medical Sciences is gratefully acknowledged. We would like to thank Shima Moradi as the researcher in this study.
Abbreviations
- BMI
Body mass index
- DHEA
Dehydroepiandrosterone sulfate
- FBM
Fat body mass
- FBG
Fasting blood glucose
- FSH
Follicle-stimulating hormone
- HDL
High-density lipoprotein
- HOMA-IR
Homeostasis model assessment-insulin resistance
- HOMA-B
Homeostasis model assessment of beta cell function
- HC Hip
Circumference
- KD
Ketogenic diet
- LH
Luteinizing hormone
- LAP
Lipid accumulation products
- LBM
Lean body mass
- LDL
Low-density lipoprotein
- PCOS
Polycystic ovary syndrome
- MUFAs
Mono-unsaturated fatty acids
- PMCD
Portfolio low-carbohydrate diet
- PUFA
Poly-unsaturated fatty acids
- RD
Registered dietitian
- RMR
Resting metabolic rate
- SFA
Saturated fatty acids
- SHBG
Sex hormone-binding globulin
- TBP
Total body protein
- TBM
Total body mineral
- TBW
Total body water
- TC
Total cholesterol
- TFA
Trans fatty acids
- TG
Triglycerides
- VAI
Visceral adipose index
- WC
Waist circumference
- WHC
Waist to hip circumference
Authors’ contributions
MS wrote the manuscript, coordinated the study, and designed the diets plan. AS, JM, and AJM are the investigators; they designed the study and interpreted the results. YS analyzed and interpreted the results and provided statistical advice. All authors edited and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data is provided within the manuscript.
Declarations
Ethics approval and consent to participate
This trial was approved by the Ethics Committee of Kermanshah University of medical sciences with code No. IR.KUMS.REC.1401.404 and conducted in accordance with the Declaration of Helsinki. In addition, all women were given written inforsmed consent before participating in the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amir Saber, Email: amir.saber@kums.ac.ir.
Jalal Moludi, Email: jmoludi@yahoo.com.
References
- 1.Khan R, Rehman R, Alam F. Situation analysis of polycystic ovary syndrome in Western Asia. Polycystic Ovary Syndrome: Elsevier; 2024. p. 207–15. [Google Scholar]
- 2.Ding H, Zhang J, Zhang F, Zhang S, Chen X, Liang W, et al. Resistance to the insulin and elevated level of androgen: a major cause of polycystic ovary syndrome. Front Endocrinol. 2021;12:741764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye W, Xie T, Song Y, Zhou L. The role of androgen and its related signals in PCOS. J Cell Mol Med. 2021;25(4):1825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashtary-Larky D, Bagheri R, Bavi H, Baker JS, Moro T, Mancin L, et al. Ketogenic diets, physical activity and body composition: a review. Br J Nutr. 2022;127(12):1898–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alharbi A, Al-Sowayan NS. The effect of ketogenic-diet on health. Food Nutr Sci. 2020;11(4):301–13. [Google Scholar]
- 6.Barrea L, Marzullo P, Muscogiuri G, Di Somma C, Scacchi M, Orio F, et al. Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr Res Rev. 2018;31(2):291–301. [DOI] [PubMed] [Google Scholar]
- 7.Teede HJ, Hutchison S, Zoungas S, Meyer C. Insulin resistance, the metabolic syndrome, diabetes, and cardiovascular disease risk in women with PCOS. Endocrine. 2006;30(1):45–53. [DOI] [PubMed] [Google Scholar]
- 8.Vonica CL, Farcas AD, Roman G, Muresan AA, Fodor A, Cernea S, et al. Metabolomic biomarkers of polycystic ovary syndrome related-obesity: a review of the literature. Revista Romana de Medicina de Laborator. 2020;28(3):241–55. [Google Scholar]
- 9.Dumesic DA, Abbott DH, Sanchita S, Chazenbalk GD. Endocrine–metabolic dysfunction in polycystic ovary syndrome: An evolutionary perspective. Current opinion in endocrine and metabolic research. 2020;12:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Molecular metabolism. 2020;35:100937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo F, Gong Z, Fernando T, Zhang L, Zhu X, Shi Y. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huddleston HG, Dokras A. Diagnosis and treatment of polycystic ovary syndrome. JAMA. 2022;327(3):274–5. [DOI] [PubMed] [Google Scholar]
- 13.Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. 2021;106(3):e1071–83. [DOI] [PubMed] [Google Scholar]
- 14.Suyoto PS, de Rijk MG, de Vries JH, Feskens EJ. The Effect of Meal Glycemic Index and Meal Frequency on Glycemic Control and Variability in Female Nurses Working Night Shifts: A Two-Arm Randomized Cross-Over Trial. J Nutr. 2024;154(1):69–78. [DOI] [PubMed] [Google Scholar]
- 15.McGrice M, Porter J. The effect of low carbohydrate diets on fertility hormones and outcomes in overweight and obese women: a systematic review. Nutrients. 2017;9(3):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volek JS, Yancy WS Jr, Gower BA, Phinney SD, Slavin J, Koutnik AP, et al. Expert consensus on nutrition and lower-carbohydrate diets: An evidence- and equity-based approach to dietary guidance. Front Nutr. 2024;11:1376098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiavaroli L, Nishi SK, Khan TA, Braunstein CR, Glenn AJ, Mejia SB, et al. Portfolio dietary pattern and cardiovascular disease: a systematic review and meta-analysis of controlled trials. Prog Cardiovasc Dis. 2018;61(1):43–53. [DOI] [PubMed] [Google Scholar]
- 18.Glenn AJ, Lo K, Jenkins DJ, Boucher BA, Hanley AJ, Kendall CW, et al. Relationship between a plant-based dietary portfolio and risk of cardiovascular disease: findings from the Women’s Health Initiative prospective cohort study. J Am Heart Assoc. 2021;10(16):e021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porchia LM, Hernandez-Garcia SC, Gonzalez-Mejia ME, López-Bayghen E. Diets with lower carbohydrate concentrations improve insulin sensitivity in women with polycystic ovary syndrome: a meta-analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2020;248:110–7. [DOI] [PubMed] [Google Scholar]
- 20.García-Gómez E, Gómez-Viais YI, Cruz-Aranda MM, Martínez-Razo LD, Reyes-Mayoral C, Ibarra-González L, et al. The Effect of Metformin and Carbohydrate-Controlled Diet on DNA Methylation and Gene Expression in the Endometrium of Women with Polycystic Ovary Syndrome. Int J Mol Sci. 2023;24(7):6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wekker V, Van Dammen L, Koning A, Heida K, Painter R, Limpens J, et al. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update. 2020;26(6):942–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scicchitano P, Dentamaro I, Carbonara R, Bulzis G, Dachille A, Caputo P, et al. Cardiovascular Risk in Women With PCOS. International journal of endocrinology and metabolism. 2012;10(4):611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietrasińska O, Wiśniewska K, Okręglicka K. Eating habits among women with polycystic ovary syndrome (PCOS) on a vegetarian vs non-vegetarian diet. Family Med Prim Care Rev. 2022;24(3):258–62. [Google Scholar]
- 24.Turner-McGrievy GM, Davidson CR, Wingard EE, Billings DL. Low glycemic index vegan or low-calorie weight loss diets for women with polycystic ovary syndrome: a randomized controlled feasibility study. Nutr Res. 2014;34(6):552–8. [DOI] [PubMed] [Google Scholar]
- 25.Bykowska-Derda A, Kaluzna M, Ruchała M, Ziemnicka K, Czlapka-Matyasik M. The significance of plant-based foods and intense physical activity on the metabolic health of women with PCOS: a priori dietary-lifestyle patterns approach. Appl Sci. 2023;13(4):2118. [Google Scholar]
- 26.Hu X, Li X, Deng P, Zhang Y, Liu R, Cai D, et al. The consequence and mechanism of dietary flavonoids on androgen profiles and disorders amelioration. Crit Rev Food Sci Nutr. 2023;63(32):11327–50. [DOI] [PubMed] [Google Scholar]
- 27.Irmak E, Sanlier NT, Sanlier N. Could polyphenols be an effective treatment in the management of polycystic ovary syndrome? Int J Vitam Nutr Res. 2024;94(5–6):422–33. [DOI] [PubMed] [Google Scholar]
- 28.Wong SK, Chin K-Y, Ima-Nirwana S. Vitamin C: a review on its role in the management of metabolic syndrome. Int J Med Sci. 2020;17(11):1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Islam SU, Ahmed MB, Ahsan H, Lee Y-S. Recent molecular mechanisms and beneficial effects of phytochemicals and plant-based whole foods in reducing LDL-C and preventing cardiovascular disease. Antioxidants. 2021;10(5):784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGaugh E, Barthel B. A Review of Ketogenic Diet and Lifestyle. Mo Med. 2022;119(1):84–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Díez-Arroyo C, García-García M, Soto-Méndez MJ, Molina-Montes E, Gil-Campos M, Gil Á, et al. Effect of the ketogenic diet as a treatment for refractory epilepsy in children and adolescents: a systematic review of reviews. Nutr Rev. 2024;82(4):487–502. [DOI] [PubMed] [Google Scholar]
- 32.Barry D, Ellul S, Watters L, Lee D, Haluska R Jr, White R. The ketogenic diet in disease and development. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2018;68:53–8. [DOI] [PubMed] [Google Scholar]
- 33.Muscogiuri G, El Ghoch M, Colao A, Hassapidou M, Yumuk V, Busetto L. European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes Facts. 2021;14(2):222–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dyńka D, Kowalcze K, Ambrozkiewicz F, Paziewska A. Effect of the ketogenic diet on the prophylaxis and treatment of diabetes mellitus: a review of the meta-analyses and clinical trials. Nutrients. 2023;15(3):500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Bai WP, Jiang B, Bai LR, Gu B, Yan SX, et al. Ketogenic diet in women with polycystic ovary syndrome and liver dysfunction who are obese: A randomized, open-label, parallel-group, controlled pilot trial. J Obstet Gynaecol Res. 2021;47(3):1145–52. [DOI] [PubMed] [Google Scholar]
- 36.Valenzano A, Polito R, Trimigno V, Di Palma A, Moscatelli F, Corso G, et al. Effects of very low calorie ketogenic diet on the orexinergic system, visceral adipose tissue, and ROS production. Antioxidants. 2019;8(12):643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monda V, Polito R, Lovino A, Finaldi A, Valenzano A, Nigro E, et al. Short-term physiological effects of a very low-calorie ketogenic diet: effects on adiponectin levels and inflammatory states. Int J Mol Sci. 2020;21(9):3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boison D. New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol. 2017;30(2):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Najafabadi MS, Moludi J, Salimi Y, Saber A. A comparison of the portfolio low-carbohydrate diet and the ketogenic diet in overweight and obese women with polycystic ovary syndrome: study protocol for a randomized controlled trial. Trials. 2023;24(1):509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabrizi FPF, Farhangi MA, Vaezi M, Hemmati S. The effects of spinach-derived thylakoid supplementation in combination with calorie restriction on anthropometric parameters and metabolic profiles in obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Nutr J. 2020;19(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmalenberger KM, Tauseef HA, Barone JC, Owens SA, Lieberman L, Jarczok MN, et al. How to study the menstrual cycle: Practical tools and recommendations. Psychoneuroendocrinology. 2021;123:104895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glenn AJ, Boucher BA, Kavcic CC, Khan TA, Paquette M, Kendall CWC, et al. Development of a portfolio diet score and its concurrent and predictive validity assessed by a food frequency questionnaire. Nutrients. 2021;13(8):2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masood W, Annamaraju P, Khan Suheb MZ, Uppaluri KR. Ketogenic Diet. 2023 Jun 16. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. PMID: 29763005. [PubMed]
- 44.Drapeau V, Jacob R, Panahi S, Tremblay A. Effect of energy restriction on eating behavior traits and psychobehavioral factors in the low satiety phenotype. Nutrients. 2019;11(2):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moghaddam MB, Aghdam FB, Jafarabadi MA, Allahverdipour H, Nikookheslat SD, Safarpour S. The Iranian Version of International Physical Activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci J. 2012;18(8):1073–80. [Google Scholar]
- 46.Huang J, Yeung AM, Bergenstal RM, Castorino K, Cengiz E, Dhatariya K, et al. Update on measuring ketones. J Diabetes Sci Technol. 2024;18(3):714–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spritzer PM, Marchesan LB, Santos BR, Fighera TM. Hirsutism, normal androgens and diagnosis of PCOS. Diagnostics. 2022;12(8):1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bazarganipour F, Ziaei S, Montazeri A, Faghihzadeh S, Frozanfard F. Psychometric properties of the Iranian version of modified polycystic ovary syndrome health-related quality-of-life questionnaire. Hum Reprod. 2012;27(9):2729–36. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Wu Q, Hao Y, Jiao M, Wang X, Jiang S, et al. Measuring the global disease burden of polycystic ovary syndrome in 194 countries: Global Burden of Disease Study 2017. Hum Reprod. 2021;36(4):1108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sukul S, Ramesh PS, Agasti N. Understanding polycystic ovary syndrome: a multifactorial endocrine disorder. J Clin Diagn Res. 2021;15(10):BE01–5.
- 51.Ashraf S, Aslam R, Bashir I, Majeed I, Jamshaid M. Environmental determinants and PCOS symptoms severity: A cross-sectional study. Health Care Women Int. 2022;43(1–3):98–113. [DOI] [PubMed] [Google Scholar]
- 52.Bril F, Ezeh U, Amiri M, Hatoum S, Pace L, Chen Y-H, et al. Adipose tissue dysfunction in polycystic ovary syndrome. J Clin Endocrinol Metab. 2024;109(1):10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azziz R. Adiposity in polycystic ovary syndrome: excess versus dysfunction. Fertil Steril. 2021;116(1):87–8. [DOI] [PubMed] [Google Scholar]
- 54.Shang Y, Zhou H, Hu M, Feng H. Effect of diet on insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2020;105(10):3346–60. [DOI] [PubMed] [Google Scholar]
- 55.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Human reproduction (Oxford, England). 2018;33(9):1602–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fong SL, Douma A, Verhaeghe J. Implementing the international evidence-based guideline of assessment and management of polycystic ovary syndrome (PCOS): how to achieve weight loss in overweight and obese women with PCOS? Journal of Gynecology Obstetrics and Human Reproduction. 2021;50(6):101894. [DOI] [PubMed] [Google Scholar]
- 57.Kulshreshtha B, Sharma N, Pant S, Sharma L, Pahuja B, Singh P. Isocaloric diet is as effective as the hypocaloric diet in ameliorating symptoms in PCOS patients. Int J Diabetes Dev Ctries. 2023;44:356–65. [Google Scholar]
- 58.Che X, Chen Z, Liu M, Mo Z. Dietary interventions: a promising treatment for polycystic ovary syndrome. Ann Nutr Metab. 2021;77(6):313–23. [DOI] [PubMed] [Google Scholar]
- 59.Paoli A, Mancin L, Giacona MC, Bianco A, Caprio M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cincione RI, Losavio F, Ciolli F, Valenzano A, Cibelli G, Messina G, et al. Effects of mixed of a ketogenic diet in overweight and obese women with polycystic ovary syndrome. Int J Environ Res Public Health. 2021;18(23):12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cincione IR, Graziadio C, Marino F, Vetrani C, Losavio F, Savastano S, et al. Short-time effects of ketogenic diet or modestly hypocaloric Mediterranean diet on overweight and obese women with polycystic ovary syndrome. J Endocrinol Invest. 2023;46(4):769–77. [DOI] [PubMed] [Google Scholar]
- 62.Makievskaya CI, Popkov VA, Andrianova NV, Liao X, Zorov DB, Plotnikov EY. Ketogenic diet and ketone bodies against ischemic injury: Targets, mechanisms, and therapeutic potential. Int J Mol Sci. 2023;24(3):2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charlot A, Bringolf A, Mallard J, Charles AL, Niederhoffer N, Duteil D, et al. Hypercaloric low-carbohydrate high-fat diet protects against the development of nonalcoholic fatty liver disease in obese mice in contrast to isocaloric Western diet. Front Nutr. 2024;11:1366883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nasser S, Solé T, Vega N, Thomas T, Balcerczyk A, Strigini M, et al. Ketogenic diet administration to mice after a high-fat-diet regimen promotes weight loss, glycemic normalization and induces adaptations of ketogenic pathways in liver and kidney. Mol Metab. 2022;65:101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson JC, Mattar SG, Greenway FL, Lindquist RJ. Measuring ketone bodies for the monitoring of pathologic and therapeutic ketosis. Obes Sci Pract. 2021;7(5):646–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007;292(6):E1724–39. [DOI] [PubMed] [Google Scholar]
- 67.Valinejad A, Khodaei K. Does exercise during a ketogenic diet effectively alter appetite sensation, appetite-regulating hormones, and body composition? Exp Biol Med. 2022;247(21):1898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roekenes J, Martins C. Ketogenic diets and appetite regulation. Curr Opin Clin Nutr Metab Care. 2021;24(4):359–63. [DOI] [PubMed] [Google Scholar]
- 69.Draznin B, Wang C, Adochio R, Leitner J, Cornier M-A. Effect of dietary macronutrient composition on AMPK and SIRT1 expression and activity in human skeletal muscle. Horm Metab Res. 2012;44(09):650–5. [DOI] [PubMed] [Google Scholar]
- 70.Livadas S, Macut D, Bothou C, Kuliczkowska-Płaksej J, Vryonidou A, Bjekic-Macut J, et al. Insulin resistance, androgens, and lipids are gradually improved in an age-dependent manner in lean women with polycystic ovary syndrome: insights from a large Caucasian cohort. Hormones. 2020;19:531–9. [DOI] [PubMed] [Google Scholar]
- 71.Oliveira TF, Comim FV. Understanding hirsutism in PCOS. Expert Rev Endocrinol Metab. 2024;19(2):103–10. [DOI] [PubMed] [Google Scholar]
- 72.Cook H, Brennan K, Azziz R. Reanalyzing the modified Ferriman-Gallwey score: is there a simpler method for assessing the extent of hirsutism? Fertil Steril. 2011;96(5):1266–70.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grymowicz M, Rudnicka E, Podfigurna A, Napierala P, Smolarczyk R, Smolarczyk K, et al. Hormonal effects on hair follicles. Int J Mol Sci. 2020;21(15):5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aboeldalyl S, James C, Seyam E, Ibrahim EM, Shawki HE-D, Amer S. The role of chronic inflammation in polycystic ovarian syndrome—a systematic review and meta-analysis. Int J Mol Sci. 2021;22(5):2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rudnicka E, Kunicki M, Suchta K, Machura P, Grymowicz M, Smolarczyk R. Inflammatory Markers in Women with Polycystic Ovary Syndrome. Biomed Res Int. 2020;2020(1):4092470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abraham Gnanadass S, Divakar Prabhu Y, Valsala GA. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): an update. Arch Gynecol Obstet. 2021;303(3):631–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript.