Abstract
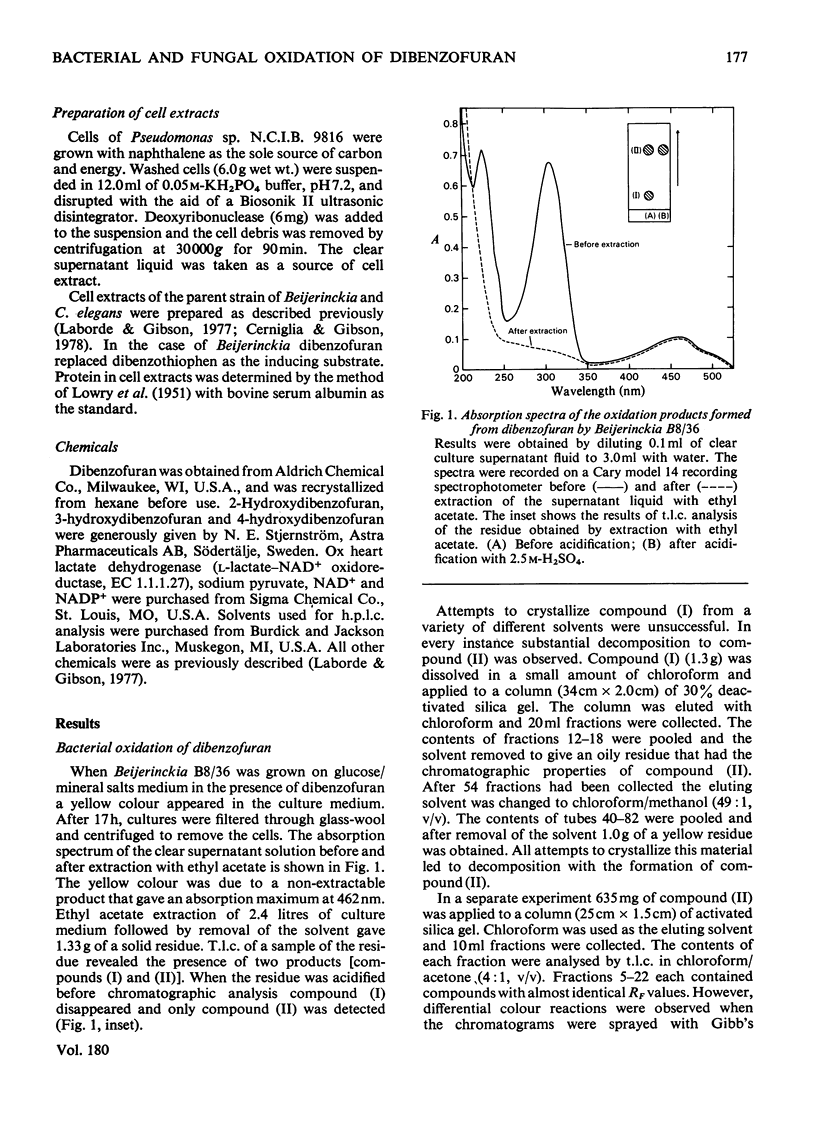
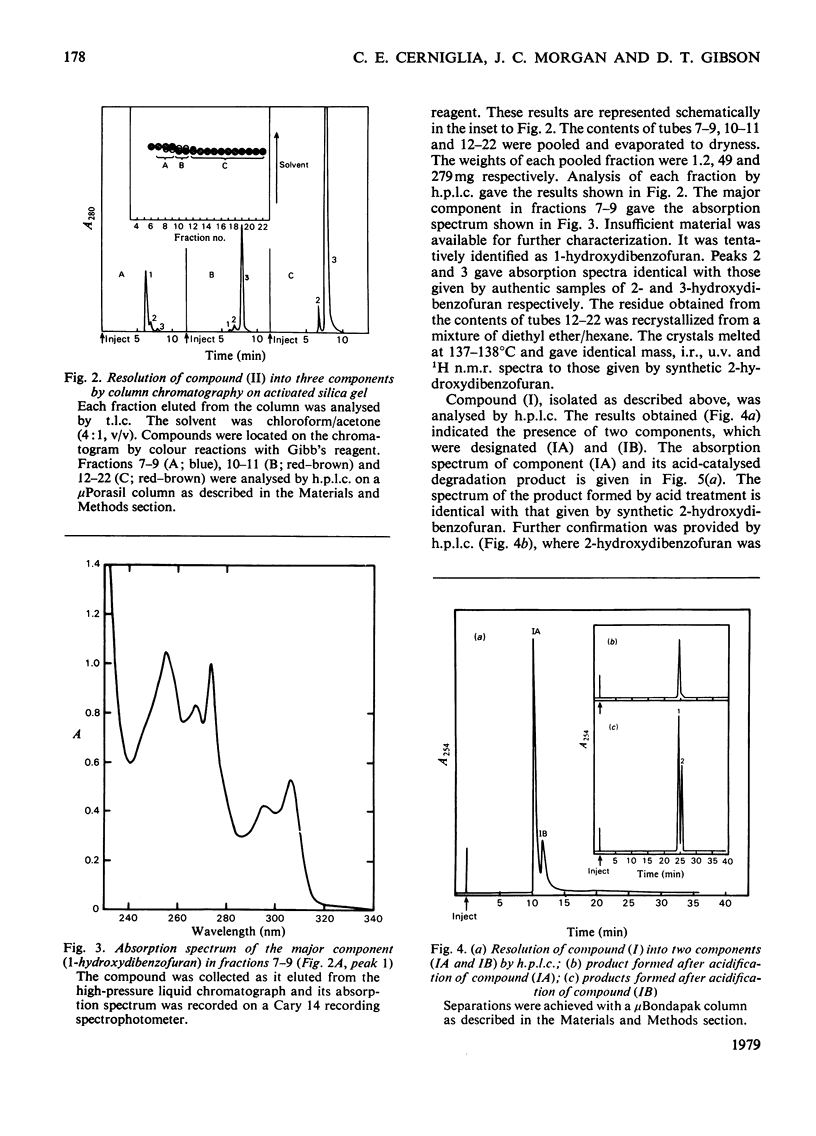
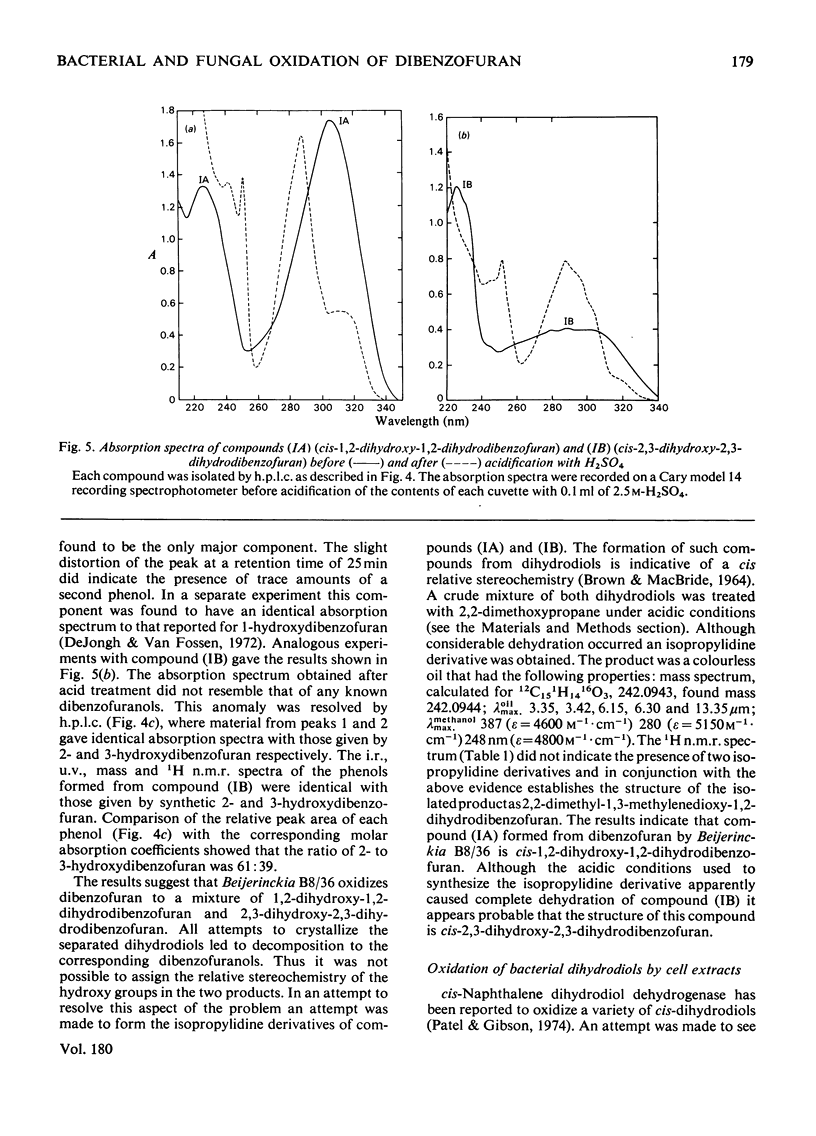
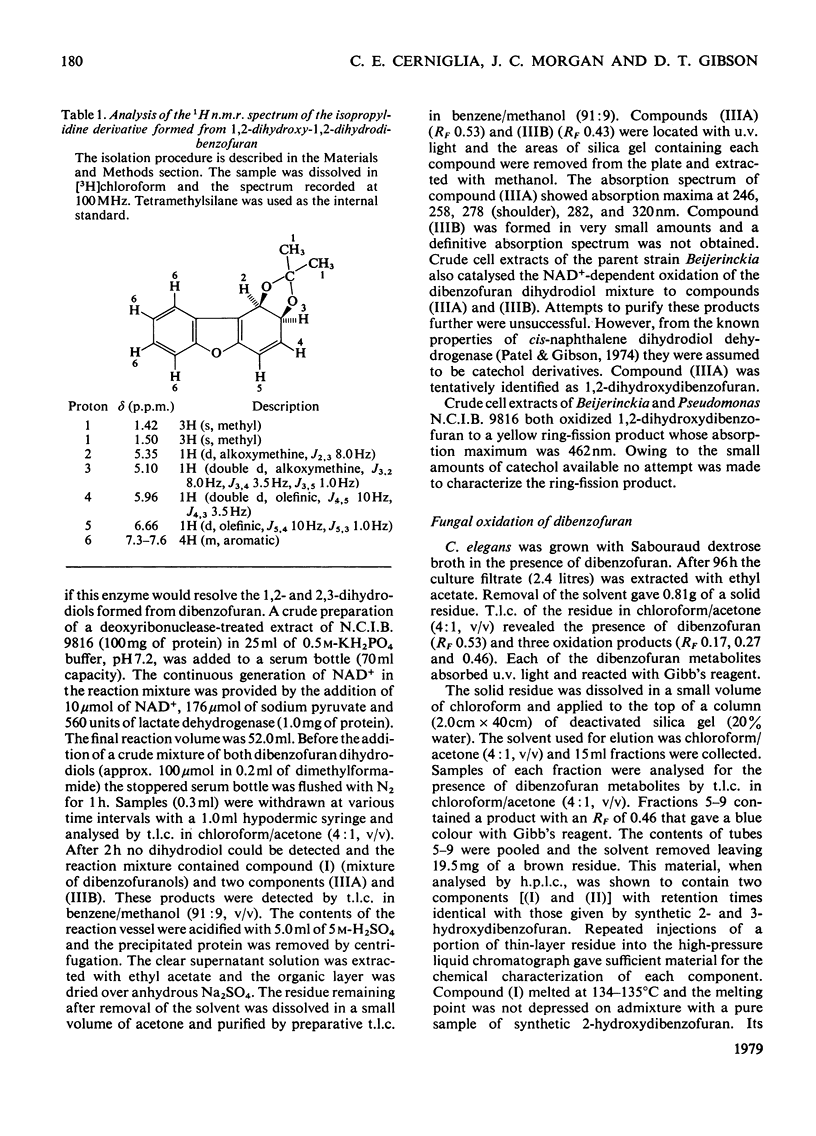
Cunninghamella elegans and a mutant strain (B8/36) of Beijerinckia both oxidized dibenzofuran to 2,3-dihydroxy-2,3-dihydrodibenzofuran. The bacterial metabolite was extremely unstable and, in the presence of acid, was rapidly converted into a mixture of 2- and 3-hydroxydibenzofuran. In contrast, the 2,3-dihydroxy-2,3-dihydrodibenzofuran formed by C. elegans was stable and only yielded 2- and 3-hydroxydibenzofuran when heated under acidic conditions. The results suggest that Beijerinckia B8/36 and C. elegans form the respective cis- and trans-isomers of 2,3-dihydroxy-2,3-dihydrodibenzofuran. C. elegans also oxidized dibenzofuran to 2- and 3-hydroxydibenzofuran under conditions that would not lead to the dehydration of the trans-dihydrodiol. These observations implicate the initial formation of dibenzofuran- 2,3-epoxide in the fungal oxidation of dibenzofuran. Beijerinckia B8/36 also produced a second unstable dihydrodiol that was tentatively identified as cis-1,2-dihydroxy-1,2-dihydrodibenzofuran. This compound gave 2-hydroxydibenzofuran as the major dehydration product and the cis relative stereochemistry was suggested by the isolation and characterization of an isopropylidine derivative. A preparation of cis-naphthalene dihydrodiol dehydrogenase and cell extracts of the parent strain of Beijerinckia oxidized both bacterial dihydrodiols to catechols. Cell extracts prepared from C. elegans catalysed an analogous oxidation of trans-2,3-dihydroxy-2,3-dihydrodibenzofuran to 2,3-dihydroxydibenzofuran. The latter product was also isolated and identified from culture filtrates. The results suggest that bacteria and fungi utilize different mechanisms to initiate the oxidation of dibenzofuran.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. H., Ollis W. D., Underwood J. G., Scrowston R. M. Constitution of the dibenzofuran, psi-rhodomyrtoxin, isolated from Rhodomyrtus macrocarpa Benth. J Chem Soc Perkin 1. 1969;18:2403–2408. doi: 10.1039/j39690002403. [DOI] [PubMed] [Google Scholar]
- Axcell B. C., Geary P. J. Purification and some properties of a soluble benzene-oxidizing system from a strain of Pseudomonas. Biochem J. 1975 Jan;146(1):173–183. doi: 10.1042/bj1460173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondesson G., Hedbom C., Högberg T., Magnusson O., Stjernström N. E., Carlson L. A. Potential hypolipidemic agents. 7. Synthesis and lipid-lowering properties of 2-(dibenzofuranyloxy)-2-methylpropionates and related compounds. J Med Chem. 1974 Jan;17(1):108–112. doi: 10.1021/jm00247a019. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Gibson D. T. Metabolism of naphthalene by Cunninghamella elegans. Appl Environ Microbiol. 1977 Oct;34(4):363–370. doi: 10.1128/aem.34.4.363-370.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Gibson D. T. Metabolism of naphthalene by cell extracts of Cunninghamella elegans. Arch Biochem Biophys. 1978 Feb;186(1):121–127. doi: 10.1016/0003-9861(78)90471-x. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Hebert R. L., Szaniszlo P. J., Gibson D. T. Fungal transformation of naphthalene. Arch Microbiol. 1978 May 30;117(2):135–143. doi: 10.1007/BF00402301. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Perry J. J. Crude oil degradation by microorganisms isolated from the marine environment. Z Allg Mikrobiol. 1973;13(4):299–306. doi: 10.1002/jobm.3630130403. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Jerina D. M., Witkop B. Arene oxides and the NIH shift: the metabolism, toxicity and carcinogenicity of aromatic compounds. Experientia. 1972 Oct 15;28(10):1129–1149. doi: 10.1007/BF01946135. [DOI] [PubMed] [Google Scholar]
- Ferris J. P., Fasco M. J., Stylianopoulou F. L., Jerina D. M., Daly J. W., Jeffrey A. M. Monooxygenase activity in Cunninghamella bainieri: evidence for a fungal system similar to liver microsomes. Arch Biochem Biophys. 1973 May;156(1):97–103. doi: 10.1016/0003-9861(73)90345-7. [DOI] [PubMed] [Google Scholar]
- Ferris J. P., MacDonald L. H., Patrie M. A., Martin M. A. Aryl hydrocarbon hydroxylase activity in the fungus Cunninghamella bainieri: evidence for the presence of cytochrome P-450. Arch Biochem Biophys. 1976 Aug;175(2):443–452. doi: 10.1016/0003-9861(76)90532-4. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Mahadevan V., Jerina D. M., Yogi H., Yeh H. J. Oxidation of the carcinogens benzo [a] pyrene and benzo [a] anthracene to dihydrodiols by a bacterium. Science. 1975 Jul 25;189(4199):295–297. doi: 10.1126/science.1145203. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Roberts R. L., Wells M. C., Kobal V. M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973 Jan 23;50(2):211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W. Arene oxides: a new aspect of drug metabolism. Science. 1974 Aug 16;185(4151):573–582. doi: 10.1126/science.185.4151.573. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Selander H., Yagi H., Wells M. C., Davey J. F., Mahadevan V., Gibson D. T. Dihydrodiols from anthracene and phenanthrene. J Am Chem Soc. 1976 Sep 15;98(19):5988–5996. doi: 10.1021/ja00435a035. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laborde A. L., Gibson D. T. Metabolism of dibenzothiophene by a Beijerinckia species. Appl Environ Microbiol. 1977 Dec;34(6):783–790. doi: 10.1128/aem.34.6.783-790.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch F. Mammalian epoxide hydrases: inducible enzymes catalysing the inactivation of carcinogenic and cytotoxic metabolites derived from aromatic and olefinic compounds. Xenobiotica. 1973 May;3(5):305–340. doi: 10.3109/00498257309151525. [DOI] [PubMed] [Google Scholar]
- Patel T. R., Gibson D. T. Purification and propeties of (plus)-cis-naphthalene dihydrodiol dehydrogenase of Pseudomonas putida. J Bacteriol. 1974 Sep;119(3):879–888. doi: 10.1128/jb.119.3.879-888.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. E., Gibson D. T. Purification and properties of cis-toluene dihydrodiol dehydrogenase from Pseudomonas putida. J Bacteriol. 1977 Jun;130(3):1117–1124. doi: 10.1128/jb.130.3.1117-1124.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]


