Abstract
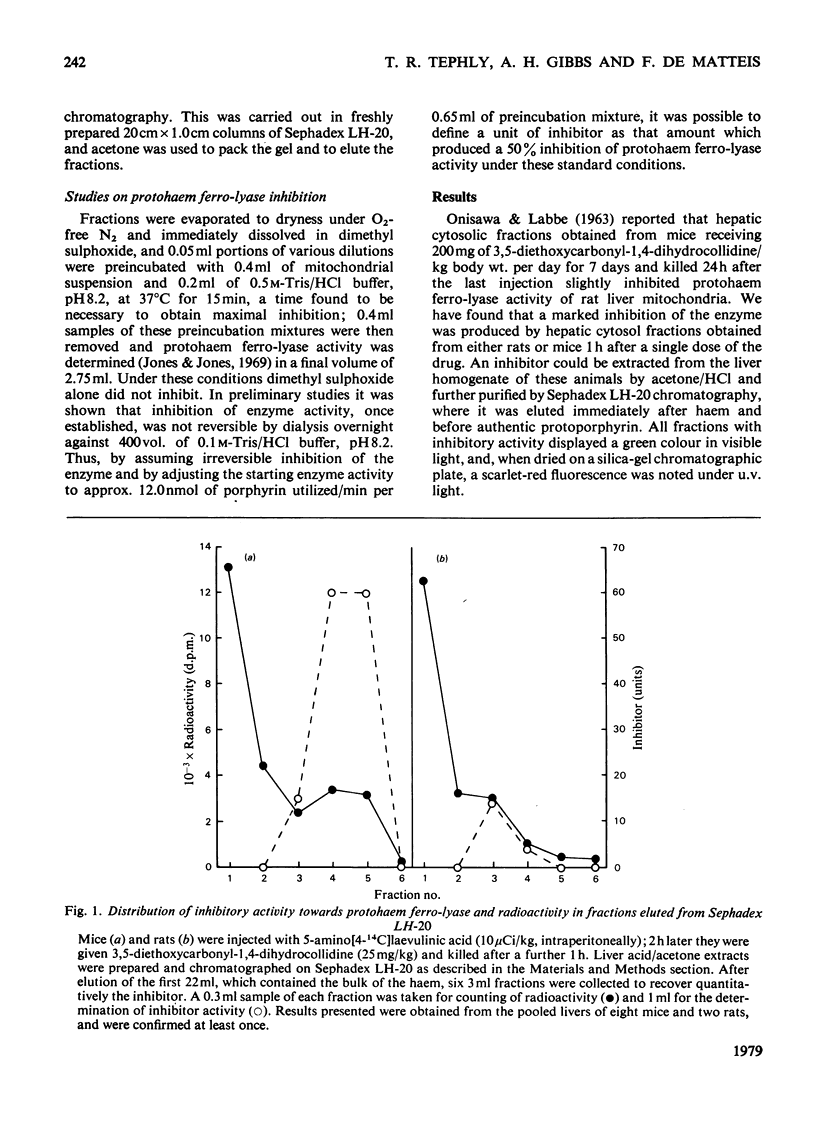
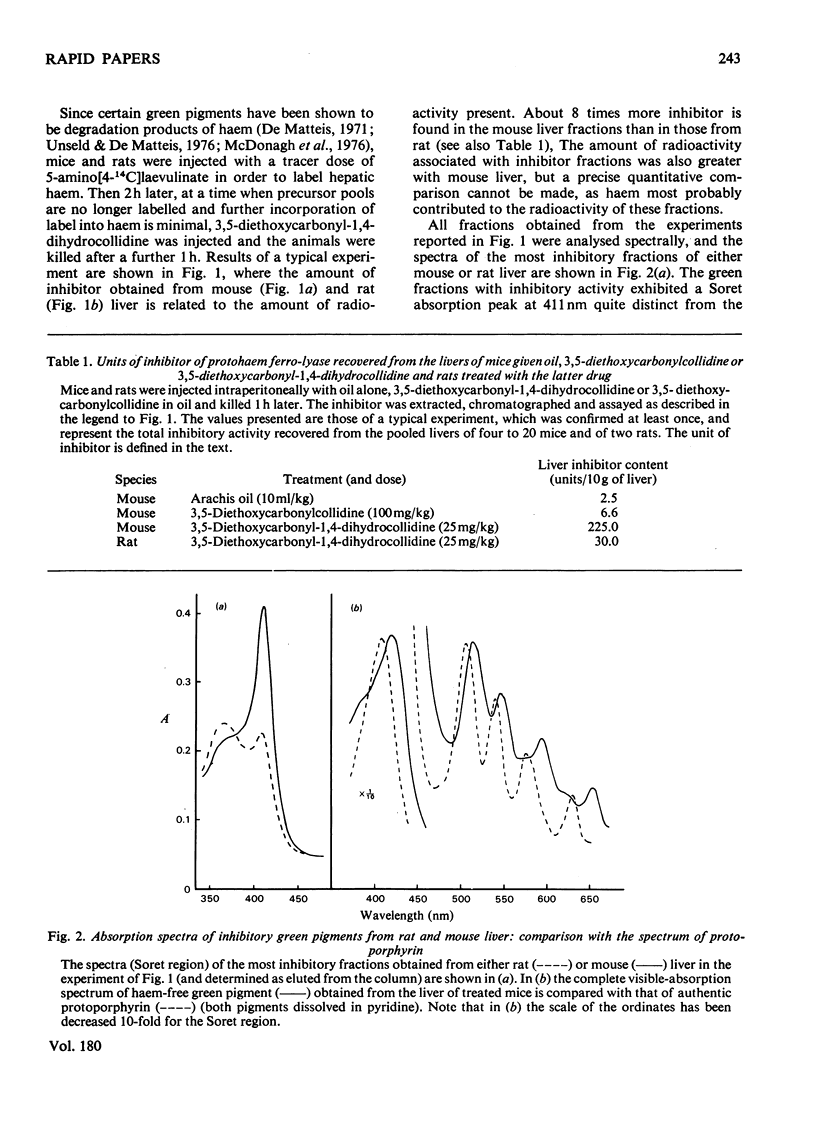
A green porphyrin-like pigment with inhibitory properties towards protohaem ferro-lyase activity was isolated from the livers of mice and rats after treatment with 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Mice, which are more sensitive to the porphyrogenic properties of the drug, produce more inhibitor. The non-porphyrogenic analogue 3,5-diethoxycarbonylcollidine does not cause accumulation of the pigment in the liver. The inhibitory substance is present in control liver at low but measurable concentrations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. Adenosine triphosphatase in the microsomal fraction from rat brain. Biochem J. 1962 Jun;83:527–533. doi: 10.1042/bj0830527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbritti G., De Matteis F. Effect of 3,5-diethoxycarbonyl-1,4-dihydrocollidine on degradation of liver haem. Enzyme. 1973;16(1):196–202. doi: 10.1159/000459381. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Abbritti G., Gibbs A. H. Decreased liver activity of porphyrin-metal chelatase in hepatic porphyria caused by 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Studies in rats and mice. Biochem J. 1973 Jul;134(3):717–727. doi: 10.1042/bj1340717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H. Stimulation of the pathway of porphyrin synthesis in the liver of rats and mice by griseofulvin, 3,5-Diethoxycarbonyl-1,4-dihydrocollidine and related drugs: evidence for two basically different mechanisms. Biochem J. 1975 Jan;146(1):285–287. doi: 10.1042/bj1460285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. Loss of haem in rat liver caused by the porphyrogenic agent 2-allyl-2-isopropylacetamide. Biochem J. 1971 Oct;124(4):767–777. doi: 10.1042/bj1240767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. The structural organization of haem synthesis in rat liver mitochondria. Biochem J. 1969 Jul;113(3):507–514. doi: 10.1042/bj1130507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh A. F., Pospisil R., Meyer U. A. Degradation of hepatic haem to porphyrins and oxophlorins in rats treated with 2-allyl-2-isopropylacetamide. Biochem Soc Trans. 1976;4(2):297–298. doi: 10.1042/bst0040297. [DOI] [PubMed] [Google Scholar]
- ONISAWA J., LABBE R. F. Effects of diethyl-1, 4-dihydro-2, 4,6-trimethylpyridine-3,5-dicarboxylate on the metabolism of porphyrins and iron. J Biol Chem. 1963 Feb;238:724–727. [PubMed] [Google Scholar]
- Tephly T. R., Hasegawa E., Baron J. Effect of drugs on heme synthesis in the liver. Metabolism. 1971 Feb;20(2):200–214. doi: 10.1016/0026-0495(71)90092-8. [DOI] [PubMed] [Google Scholar]


