Abstract
Introduction
Preexposure prophylaxis with monoclonal antibodies (mAbs) was developed in addition to COVID-19 vaccine for immunocompromised and those with insufficient immune response, among them patients with CLL. Omicron variant and its sublineages evolved mutations that escape mAbs neutralizing effect, yet the extent of which was not studied.
Methods
We evaluated anti-spike titers and neutralization activity of COVID-19 wild-type (WT), Delta, Omicron, BA.2, BA.4, and BA.5 before and after tixagevimab-cilgavimab (TGM/CGM) dose of 150/150 mg or 300/300 mg in patients with CLL.
Results
70 patients were tested 2 weeks before and 4 weeks after receiving TGM/CGM mAbs. After TGM/CGM, anti-spike ab level increased 170-folds from 13.6 binding antibody unit (BAU)/mL (IQR, 0.4–288) to 2,328 BAU/mL (IQR, 1,681–3,500). Neutralization activity increased in all variants and was 176-folds higher in WT and 55-folds higher in Delta compared to 10-folds higher in Omicron and its sublineages (BA.2 ×11, BA.4 ×4, BA.5 ×18). Over follow-up period of 3 months, 20 patients (29%) with CLL acquired COVID-19 infection, all recovered uneventfully. In a multivariate analysis, anti-spike antibody titer was found a significant predictor for post-TGM/CGM COVID-19 infection.
Conclusion
Efficacy of preexposure prophylaxis with TGM/CGM in patients with CLL is significantly reduced in era of Omicron and its sublineages BA.2, BA.4, and BA.5.
Keywords: Chronic lymphocytic leukemia, CLL, COVID-19, Tixagevimab-cilgavimab, Preexposure prophylaxis, COVID-19 variants
Introduction
Patients with CLL are vulnerable to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and are at high risk of severe disease and mortality compared to immunocompetent individuals [1–3]. Specifically, advanced age, comorbidity, hypogammaglobulinemia, and immunosuppressive therapy delay clearance of the virus and increase the risk of complications [4, 5]. Although mRNA vaccination is the optimal method of preexposure prophylaxis in the general population, patients with CLL may not maximally benefit from vaccination as they do not mount an adequate immune response [6]. After a two-dose mRNA vaccination, patients with CLL demonstrated lower seroconversion rates compared to the general population, with only 40–60% serologic response to spike protein [6–8]. In a previous study, we demonstrated that additional quarter of patients develop serologic response following a third booster vaccine [9]. Fully vaccinated patients with CLL also demonstrated impaired T-cell and humoral responses and are more likely to experience breakthrough infection [10]. Preexposure prophylaxis with monoclonal antibodies (mAbs) is a promising way to provide immediate immunity and circumvent impaired antibody production by immunosuppressed individuals. Tixagevimab and cilgavimab are two long-acting neutralizing mAbs obtained from plasma of convalescent patients that bound to different regions of the receptor-binding domain (RBD) of SARS-CoV-2 spike protein in a noncompetitive manner, thus increasing the chances of virus neutralization [11]. PROVENT, a phase 3 trial in unvaccinated adults, resulted in 70% risk reduction of COVID-19 infection after 3 months and 82% after 6 months following administration of TGM/CGM [11]. In 2021, the US Food and Drug Administration (FDA) has granted emergency use authorization (EUA) for TGM/CGM as preexposure prophylaxis in immune compromised high-risk population [12]. However, the trial was not conducted on immunocompromised patients’ cohort. Additionally, it was conducted when the Alpha and Delta variants were dominant, prior to the emergence of Omicron and its sublineages, which have since predominated in most parts of the world. Emerging at the end of 2021, the Omicron variant harbors multiple novel spike mutations, enabling humoral immunity evasion and higher transmissibility [13, 14]. The efficacy of TGM/CGM against Omicron subvariants is uncertain. In vitro studies suggest that TGM/CGM has less potent neutralizing activity against Omicron, and some of its sublineages completely escape neutralization [15, 16]. Double doses of the originally authorized TGM/CGM were recommended by the FDA to increase efficacy and prevent COVID-19 infection, based on laboratory neutralization studies and without clinical evidence for risk reduction. The emergence of rapidly spreading non-susceptible new SARS-CoV-2 variants led the FDA to withdraw EUA of TGM/CGM. In this study, we evaluated the efficacy of TGM/CGM preexposure prophylaxis on neutralization activity among patients with CLL during the omicron and its subvariants waves in Israel and compared it with neutralization activity versus older SARS-CoV-2 variants.
Methods
Study Design
This prospective study enrolled patients with CLL from two academic centers before and after receiving mAb TGM/CGM. The study was approved by the Institutional Review Board (IRB) and conducted in accordance with the Declaration of Helsinki. Eligibility criteria were diagnosis of CLL or small lymphocytic lymphoma. Demographic data, hematologic disease history, and prior COVID-19 mRNA vaccination were extracted from patients’ medical records. The primary objective was to evaluate the serologic response and neutralization effect before and after mAb TGM/CGM. This was assessed by anti-spike serology and neutralization activity assays to wild-type (WT) Delta, Omicron, and its sublineages BA.2, BA.4, and BA.5. Secondary objectives were to understand predictors of serologic response, evaluate the effect of previous COVID-19 mRNA vaccinations, assess TGM/CGM dose response, and follow short-time infection rates and safety outcomes. Patients underwent serologic assessments and viral neutralization 2 weeks before and 4 weeks after receiving standard or double-dose intramuscular 150/300 mg tixagevimab and 150/300 mg cilgavimab. SARS-CoV-2 infection was defined by clinical symptoms confirmed with positive reverse transcription-polymerase chain reaction assay on nasopharyngeal swabs. Patients with SARS-CoV-2 infection and hypoxemia (oxygen saturation ≤94% on room air) or need for oxygenation or ventilatory support were considered having severe disease.
Antibody Response and Neutralization Assay
Serologic response was assessed by titers of immunoglobulin G (IgG), aimed at the SARS-CoV-2 S protein receptor-binding domain. Patients’ serum samples were evaluated using commercial automatic chemiluminescent microparticle immunoassay SARS-CoV-2 IgG II Quant (Abbott Laboratories, Abbott Park, IL, USA), according to the manufacturer’s instructions. Serum samples were evaluated for Antibody levels were measured in binding antibody units (BAUs) as per the World Health Organization (WHO) standard measurements. Serologic response is considered positive at concentration of 21.4 BAU/mL and higher.
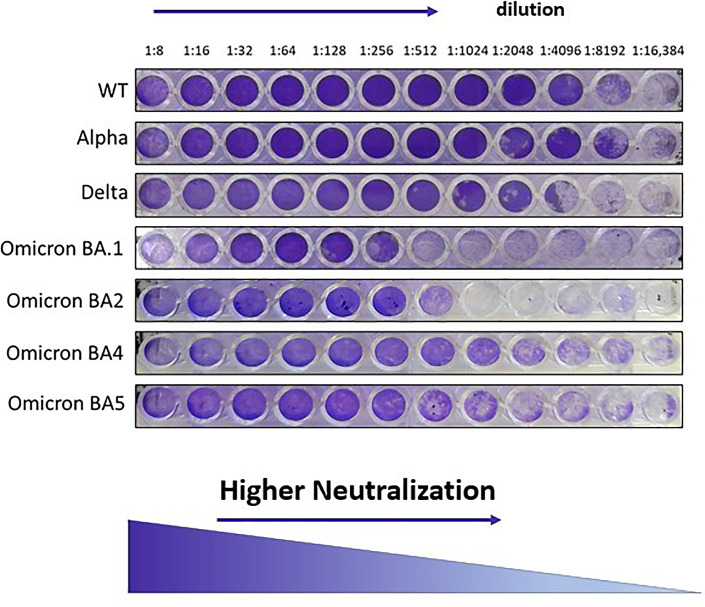
To test the neutralization capacity of the sera, a median tissue culture infectious dose (100 TCID50) of WT, Delta, Omicron (BA.1), BA.2, BA.4, and BA.5 SARS-CoV-2 isolates were incubated with serially diluted (1:8 to 1:16,384) inactivated serum (30 min, 56°C) in 96-well plates for 60 min at 33°C. Virus-serum mixtures were then added to VERO-E6 cells and incubated for 5 days at 33°C, after which cells were stained with gentian violet dye (1%). Neutralizing capacity was determined by the highest serum dilution at which no cytopathic effect was observed (shown in Fig. 1). The geometric mean titer (GMT) and its 95% confidence interval are reported for each variant by cohort.
Fig. 1.
SARS-CoV-2 micro-neutralization assay. Gentian violet stained and fixed the cell culture layer. Neutralizing dilution of each serum sample was determined by identifying the well with the highest serum dilution without observable cytopathic effect.
Statistical Methods
All statistical analysis and visualization were done using R statistical computing software (version 3.6.3). Non-normal variables were described as median with interquartile range (IQR). Pearson’s χ2 test was used for testing association between two large sample categorical variables, and Fisher’s exact test was used for testing association between small-sample categorical variables. Continuous variables were tested for normality using QQ-plots and Shapiro-Wilk’s tests. Some continuous variables were log-10 scaled prior to analysis to fit normality. Correlations between normally distributed variables were calculated using Pearson’s product-moment method, and correlation significance was calculated using F test. Paired t tests were used to compare two measures from the same patient. Univariate and multivariate binary logistic regression models were fitted to determine the influence of both categorical and continuous variables upon patients’ immune response. Variables which were significantly associated with response at a significance level p < 0.1 in the univariate models were included in the multivariate analysis. For all hypotheses testing, p value <0.05 was considered significant.
Results
Patients’ Disposition and Baseline Characteristics
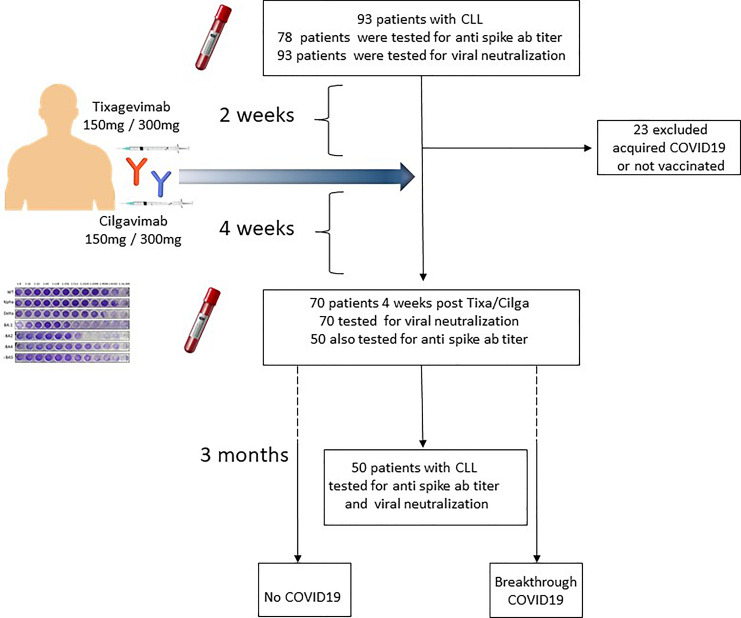
From February 2022 through June 2022, a total of 93 patients with CLL/small lymphocytic lymphoma were included in the study. All patients were tested for viral neutralization titer 2 weeks (range 1–36) before receiving TGM/CGM, and 78 of them were also tested for anti-spike antibodies (patient disposition, shown in Fig. 2). Baseline demographic and disease characteristics of 70 patients that were tested for viral neutralization before and after TGM/CGM are summarized in Table 1. The median age was 70 years (IQR, 65–75), and 42 (54%) were males. 85% of patients had hypogammaglobulinemia, with low IgG 58%, low IgA 46%, or low IgM 76%. Eighteen patients (27%) were treatment-naïve, 35 (50%) were on active therapy with ongoing Bruton’s tyrosine kinase inhibitor (20 [29%]) or B-cell lymphoma 2 (13 [19%]), and 11 of patients treated with anti-CD20 antibodies (50%) received it within the last 12 months prior to TGM/CGM. Fifteen patients (21%) were previously treated (off-therapy). Among the off-therapy patients, 10 (67%) were in remission (CR: n = 6; PR: n = 4), and 5 (33%) were in unconfirmed remission or in relapse.
Fig. 2.
Patient disposition.
Table 1.
Patient baseline characteristics
| Parameter | Patients with CLL (N = 70) |
|---|---|
| Age at Tixa/Cilga, median (IQR), years | 70 (65, 75) |
| Male sex, N (%) | 42 (54) |
| Binet stage,* N (%) | |
| A | 16 (61.5) |
| B | 6 (23.1) |
| C | 4 (15.4) |
| R-CIRS | |
| <6 | 42 (61.8) |
| ≥6 | 26 (38.2) |
| IGHV mutational status, N (%) | |
| Mutated | 10 (27.1) |
| Unmutated | 27 (72.9) |
| FISH, N (%) | |
| Normal | 11 (19) |
| Del(13q) | 18 (32) |
| Trisomy 12 | 11 (19) |
| Del(11q) | 7 (12) |
| Del(17p) and/or TP53 mut | 11 (19) |
| Disease/treatment status, N (%) | |
| Treatment-naive | 18 (26.5) |
| On-therapy | 35 (51.5) |
| Off-therapy in remission | 10 (16) |
| Off-therapy in relapse | 5 (6) |
| Laboratory parameters, median (IQR) | |
| Absolute lymphocyte count, 103/L | 3.3 [1.2, 16.06] |
| IgG, mg/dL | 675 [433, 866] |
| IgM, mg/dL | 21[35.45, ≤18.8] |
| IgA, mg/dL | 73 [36.3, 136.5] |
| Ongoing treatment, N (%) | |
| BTKi | 20 (57.2) |
| Venetoclax | 13 (37.1) |
| Other | 2 (5.7) |
| Time since last anti-CD20 antibody, N (%) | |
| <12 months | 11 (50) |
| ≥12 months | 11 (50) |
FISH, fluorescence in situ hybridization; Tixa/Cilga, tixagevimab/cilgavimab; R-CIRS, revised cumulative illness rating scale; Del, deletion; Mut, mutation; BTKi, Bruton’s tyrosine kinase.
*Treatment-naive patients and patients in relapse.
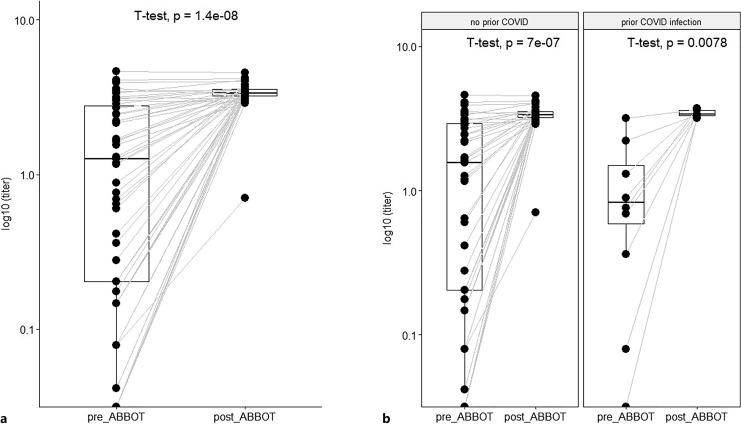
Four weeks (median 30 days, range 4–56 days) after TGM/CGM prophylaxis, 70 patients were tested for anti-spike titer, and 50 patients were tested also for SARS-CoV-2 neutralization activity that significantly increased in all variants. Twenty-three patients were not tested after TGM/CGM due to infection with COVID-19 during follow-up period or due to their preference. The anti-spike serology response after TGM/CGM increased 170-folds from baseline median of 13.6 BAU/mL (IQR, 0.4–288) 2 weeks before TGM/CGM to median of 328 BAU/mL (IQR, 1,681–3,500) 4 weeks after with only 43% (23/53) of patients having positive serology (>21.4 BAU/mL) before TGM/CGM to 96% (51/53) positive serology 4 weeks after TGM/CGM (shown in Fig. 3a). Sixteen patients (23%) had history of COVID-19 infection prior to TGM/CGM. Their anti-spike serology was not higher than that of patients without prior COVID-19 (shown in Fig. 3b).
Fig. 3.
a Anti-spike protein antibody titer before and after TGM/CGM among 70 CLL patients. T test found significantly higher titer after TGM/CGM (GMT = 21,577) as compared to baseline (GMT = 118). b Anti-spike serology increased after tixagevimab-cilgavimab to same extent in patients with and without prior COVID-19.
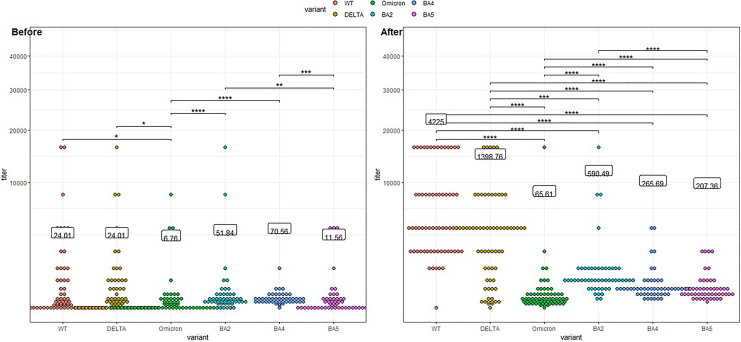
Viral neutralization GMT at baseline before patients received TGM/CGM was 24.01 compared to 6.76, four folds higher in WT and Delta variants than in Omicron, and neutralization of Omicron was significantly lower than its sublineages BA.2, BA.4, and BA.5 (shown in Fig. 4). Viral neutralization GMT after TGM/CGM was 4,335 in WT and 1,399 in Delta variants significantly higher than 65.6 in Omicron, 176-folds and 55-folds higher, respectively. The neutralization of the Omicron and sublineages were only 10-folds higher for Omicron, 11-folds higher for BA.2, 3.8-folds for BA.4, and 18-folds higher for BA.5. Anti-spike serology significantly correlated with viral neutralization GMT before and after TGM/CGM; however, after TGM/CGM administration, all correlations were weaker (shown in Fig. 5). Double dose of 300 mg tixagevimab and 300 mg cilgavimab was tested only in 2 patients and resulted in a significantly higher anti-spike titer than the standard dose, however, with no significant differences in neutralization of the virus (shown in Fig. 6a). COVID-19 disease before TGM/CGM was not a significant factor affecting anti-spike serology or viral neutralization before and after TGM/CGM (shown in Fig. 6b).
Fig. 4.
Relative neutralization activity for different SARS-CoV-2 variants before and after tixagevimab-cilgavimab. At baseline, before patients received TGM/CGM neutralization of WT and Delta were 4-folds higher than of Omicron and neutralization of Omicron was lower than its sublineages (BA.2, BA.4, BA.5). After TGM/CGM, neutralization was significantly higher for all variant with the highest cytopathic effect for the WT (176-folds higher) and Delta (×55) and only 10-folds higher for Omicron and its sublineages (BA.2 ×11, BA.4 ×4, BA.5 ×18). TGM/CGM was more effective against the WT (65-folds higher) and the Delta (20-folds higher) variants than the Omicron. TGM/CGM was significantly less effective against Omicron than to its subvariants.
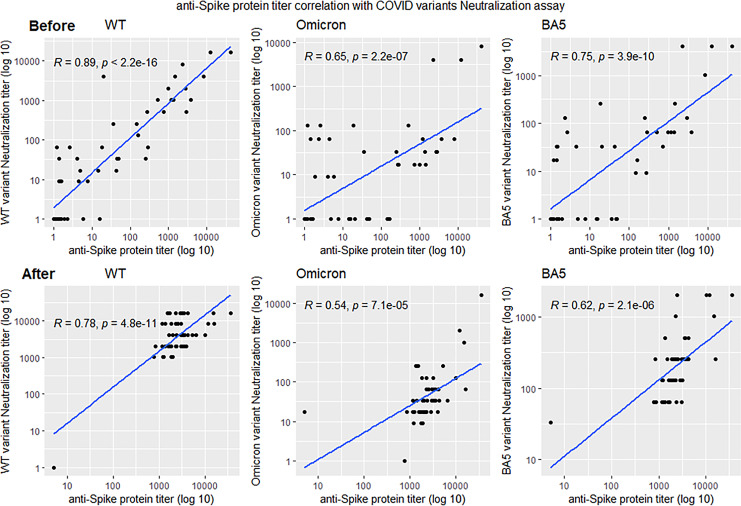
Fig. 5.
Correlation between anti-spike protein antibody titers and neutralization activity of SARS-CoV-2 variants. Anti-spike serology significantly correlated with viral neutralization before and after TGM/CGM. However, after TGM/CGM administration, all correlations were weaker.
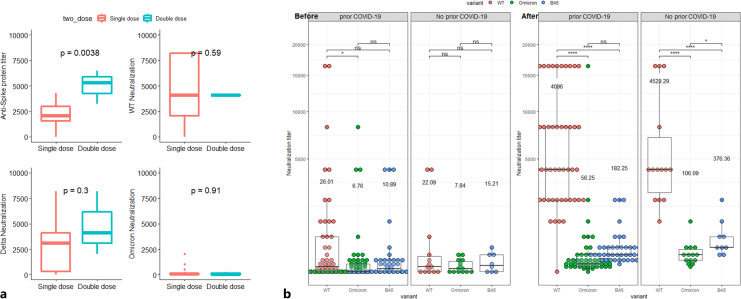
Fig. 6.
a Anti-spike antibody titer and neutralization activity before and after single versus double dose of tixagevimab-cilgavimab. b Previous COVID infection does not affect neutralization activity for COVID-19 variants before and after tixagevimab-cilgavimab infection. At baseline, significant difference was found only between WT and Omicron neutralization among patients who were not infected with SARS-CoV-2. After tixagevimab-cilgavimab, neutralization against WT was significantly higher than omicron and BA.5, both among patients infected and not infected with SARS-CoV-2.
Breakthrough Infection and Outcome
Among 70 patients with CLL that were tested before and after prophylaxis, there were no reports of severe complications after TGM/CGM nor specific adverse cardiovascular signals. Within 3 months after TGM/CGM, 20 patients (29%) acquired COVID-19 disease. At least 70% of them (14 reported) were treated with antiviral (remdesivir, paxlovid, or molnupiravir). Only 3 patients (1.5%) had severe disease, and all patients recovered without later on consequences. We evaluated the following factors in order to predict post-TGM/CGM COVID-19 infection including age, gender, CIRS score, lymphopenia, hypogamaglobulinemia, low IgG, IgA, IgM on treatment, post-TGM/CGM Delta naturalization quartile 1 (Q1), post-TGM/CGM Omicron naturalization quartile 1 (Q1), and previous COVID-19 infection. Univariate model found anti-spike titer, Delta neutralization, IgM titer, and CARES score significant predictors of post-TGM/CGM COVID-19 infection (p < 0.1). In a multivariate analysis, the only predictor of post-TGM/CGM COVID-19 was anti-spike titer in the first quartile (OR = 1.5, p = 0.01). (Table 2).
Table 2.
Multivariate logistic regression model
| Estimate | OR | p value | |
|---|---|---|---|
| CIRS score | −0.03 | 0.98 | 0.27 |
| Anti-spike Q1 | 0.41 | 1.51 | 0.01 |
| Low IgM | 0.22 | 1.25 | 0.17 |
| Delta variant Q1 | −0.29 | 0.75 | 0.30 |
Discussion
The Omicron and its five subvariants (BA.1, BA.2, BA.3, BA.4, and BA.5) harbor mutations in the spike protein that enhance transmissibility and enable escape from antibody neutralization. This study tested serologic response and neutralization of Omicron and its sublineages in immunocompromised patients with CLL before and after preexposure prophylaxis with TGM/CGM. Serology response increased 170-folds higher after TGM/CGM and was more efficacious in WT and Delta than Omicron BA.2, BA.4, and BA.5. Neutralization of Omicron and its sublineages were 10-fold higher after preexposure prophylaxis, however, much less effective than WT and Delta with 170- and 55-folds higher neutralization, respectively. In unvaccinated healthy population of the phase 3 PROVENT trial, TGM/CGM has been shown to reduce the risk of SARS-CoV-2 infection during the Alpha (B.1.1.7) and Delta (B.1.617.2) waves. Only 7% of patients had cancer or history of cancer and 3% were actively receiving immunosuppressive therapy [11]. Therefore, data on the efficacy of TGM/CGM during the Omicron wave are lacking, particularly in the immunocompromised.
Few studies have shown improved clinical response to TGM/CGM in terms of symptomatic disease, hospitalization, intensive care unit (ICU) admission, or death in healthy individuals and immunocompromised patients [17, 18]. A low rate of infections and severe illnesses reported among 1,112 immunocompromised patients treated with TGM/CGM [19]. A retrospective study of solid organ transplant recipients during the Omicron wave, not including BA.4 and BA.5, reported a lower risk of breakthrough SARS-CoV-2 infections of 5% in patients who received TGM/CGM for preexposure prophylaxis compared to 14% in vaccine-matched control group [20]. Hospitalizations and deaths due to SARS-CoV-2 infection were lower in the TGM/CGM group, but the study was underpowered to find differences in these outcomes. A study in kidney transplant recipients who received TGM/CGM compared to those who had high-titer anti-spike antibody responses to vaccination but did not receive TGM/CGM found no significant difference in the risk of symptomatic breakthrough Omicron infection [21]. On the other hand, a recent publication reported the reduced neutralizing activity of preexposure prophylaxis with TGM/CGM against the Omicron variant in kidney transplant recipients [22, 23]. A systematic review and meta-analysis of TGM/CGM in immunocompromised patients including more than 5,000 with hematological pathologies, showed clinical effectiveness against coronavirus breakthrough infections, COVID-19 hospitalization, ICU admission, all-cause mortality, and COVID-19-specific mortality; however, this systematic review was performed prior to the emergence of BQ1.1 and XBB.1.5 Omicron variants [17]. In vitro neutralizing studies against the most prevalent Omicron subvariants BA.1 and BA.1.1 that represented 95% of the circulating sequenced COVID-19 cases in the USA have led the FDA to amend the previously issued EUA. In February 2021, the FDA revised the authorized dosage regimen to an initial dose of 300 mg of tixagevimab and 300 mg of cilgavimab, delivered in two consecutive, sequential intramuscular injections. Few studies supported the FDA’s revised recommendation to use the higher dose for preexposure prophylaxis. In 416 kidney transplant recipients that received preexposure prophylaxis with cilgavimab-tixagevimab at the dose of 150 mg of each, antibody did not adequately protect against Omicron [24]. Thirty-nine (9.4%) developed COVID-19; of them, 14 (35.9%) required hospitalization, 3 patients (7.7%) admitted to ICU, and two died of COVID-19-related acute respiratory distress syndrome. Solid organ transplant recipients who received the 150/150 mg dose had a higher incidence of breakthrough infections compared to those who received the 300/300 mg dose [20]. In patients with hematologic malignancies, cilgavimab-tixagevimab failed to achieve meaningful neutralization of Omicron-RBD with a single 150 mg dose. Neutralization significantly increased above the positive cutoff after a single 300 mg dose, but it remained heterogeneous [25]. A preemptive treatment with 300/300 mg TGM/CGM for Omicron SARS-CoV-2 infection in a small and heterogeneous patient population with hematologic diseases prevented progression to severe COVID-19 [26]. In our study, in spite of higher level of antibody titer in 2 patients who received a double dose TGM/CGM, we did not find a difference in neutralization. In addition, we have not found an effect of prior COVID-19 infection as a factor associated with higher neutralization of Omicron and sublineages before or after TGM/CGM.
Mutation in the S codon substitutions and exposure to TGM/CGM may cause reduced effectiveness due to selection and emergence of novel resistant variants leading to breakthrough COVID-19 [27]. Though BA.4 and BA.5 escape neutralization by most therapeutic mAbs, cilgavimab neutralize Delta, BA.2, BA.4, and BA.5 with similar potency and remains potent up to 6 months [16]. The BA.5 variant remains sensitive to TGM/CGM, but the decay of the serum neutralizing activity in treated individuals is accelerated, compared with previously circulating variants [16]. A retrospective analysis of patients with B-cell malignancies who received TGM/CGM within the past three to 6 months may still be at risk of breakthrough COVID-19 infection. At a median of 3 months from TGM/CGM administration (mostly high dose), 27 (11%) patients experienced a confirmed COVID-19 breakthrough infection. As in our cohort, the dominant variant among the local population (85%) was BA.5 [28]. A BA.5 subvariant BQ.1.1 and BA.2 subvariant XBB have shown to have nine more changes in its RBD and immune evasion capabilities that are greater than those of earlier omicron variants, including BA.5 and BA.2. TGM/CGM has failed to neutralize both BA.5 and XBB [29, 30].
As of January 20, 2023, more than 90% of circulating SARS-CoV-2 variants in the USA, specifically Omicron BQ.1, BQ.1.1, XBB, and XBB.1.5 sublineages, were unlikely to be susceptible to the combined mAbs, TGM/CGM. The Food and Drug Administration (FDA) has withdrawn EUA for TGM/CGM and announced on January 26, 2023, that it is not currently authorized for preexposure prophylaxis against SARS-CoV-2 infection in the USA. At the time, we finalized the results of our study, and there were no approved neutralizing mAbs against SARS-CoV-2. Development of neutralizing antibodies is challenging, particularly due to selective pressure that rapidly produces resistant variants. More than 200 neutralizing mAbs have been studied, and 16 of them achieved late development [31]. With continues emergence of new subvariants producing an effective neutralizing mAb is an ongoing challenge.
The limitations of our study include the lack of control group of patients with CLL that have not received TGM/CGM preexposure prophylaxis; therefore, we cannot infer on the risk reduction of breakthrough infections and severe disease. Though we have shown significantly higher anti-spike titer with double-dose TGM/CGM without differences in neutralization of the virus, this was based only of a sample of 2 patients. In addition, we have not tested recently emerging more resistant subvariants including BQ.1/BQ.1.1, and XBB and its derivatives XBB.1 and XBB.1.5 that became more prevalent and have immune evasion capabilities that are greater than those of earlier Omicron variants and have led to the withdrawal of the FDA authorization of TGM/CGM for preexposure prophylaxis. Novel subvariants with enhanced transmissibility rates, derived from either BA.2 or BA.4/BA.5, rapidly emerged and became prevalent in November–December 2022. These subvariants have been shown to have relevant changes in their RBD and are less susceptible to TGM/CGM [32]. Current ongoing prospective trials comparing TGM/CGM to newer mAbs may shed light over its comparative efficacy to these variants.
A progress has been made with quick modifications and development of mRNA vaccines to new resistant variants, and the most recent fifth COVID-19 vaccine is now available. The continued evolution of omicron-resistant variants reinforces the need for new therapeutic mAbs as well for the immunocompromised and specifically for patients with CLL. Introducing neutralization studies in vitro and in patients’ population will allow improved response to prevalent and relevant variants.
The speed of variants’ emergence demonstrates the need for genomic surveillance of the circulating virus to define the use or discontinuation of drugs. The reduction of neutralizing activity in vitro does not always lead to a blockage of therapeutic activity in vivo [33]. Neutralizing mAbs are important defensive strategy against SARS-CoV-2 due to their safety profile and immediate immunity in immunosuppressed and patients with CLL. It is therefore essential that we have the know-how of quick development and production of new mAbs that can match and exceed resistant SARS-CoV-2 variants.
In summary, preexposure prophylaxis with TGM/CGM enhances neutralization activity to SARS-CoV-2 in immunosuppressed patients with CLL. However, its effectiveness is compromised in the face of evolving resistant Omicron subvariants. The experience gained from previous COVID-19 waves helps us prepare for the upcoming winter. In addition to preventive measures and updated vaccination to prevalent variants, newer effective mAbs are necessary in high-risk immunosuppressed patients.
Acknowledgments
The authors would like to thank the study coordinators with special thanks to Mrs. Halperin Rivka.
Statement of Ethics
This study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki, and it was approved by the Institutional Review Board and Ethics Committee of Sheba Medical Center (8314-21 SMC). All patients provided written informed consent to participate. This is a noninterventional cohort study, and collection and storage of data were performed by the investigators directly involved in the patients’ care using current techniques of ensuring privacy.
Conflict of Interest Statement
There are no conflicts of interest.
Funding Sources
This study has not been sponsored.
Author Contributions
O.B. designed, organized contributed patient’s data, and wrote the manuscript. T.T., A.A., and G.R. designed and organized contributed patient’s data. T.H. contributed patient data. R.G. performed all statistical analysis. L.K., N.A., and B.A. performed naturalization assay. F.F., I.L., and OG performed and analyzed serology tests. M.M. designed and performed the naturalization assay. All authors reviewed the final version of the manuscript and provided critical feedback.
Funding Statement
This study has not been sponsored.
Data Availability Statement
Research datasets for the present study would be available from the corresponding author upon reasonable request. The data are not publicly available because of privacy or ethical restrictions.
References
- 1. Mato AR, Roeker LE, Lamanna N, Allan JN, Leslie L, Pagel JM, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roeker LE, Eyre TA, Thompson MC, Lamanna N, Coltoff AR, Davids MS, et al. COVID-19 in patients with CLL: improved survival outcomes and update on management strategies. Blood. 2021;138(18):1768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chatzikonstantinou T, Kapetanakis A, Scarfò L, Karakatsoulis G, Allsup D, Cabrero AA, et al. COVID-19 severity and mortality in patients with CLL: an update of the International ERIC and Campus CLL study. Leukemia. 2021;35(12):3444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pagano L, Salmanton-García J, Marchesi F, Busca A, Corradini P, Hoenigl M, et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA). J Hematol Oncol. 2021;14(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benjamini O, Rokach L, Itchaki G, Braester A, Shvidel L, Goldschmidt N, et al. Safety and efficacy of the BNT162b mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Haematologica. 2022;107(3):625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roeker LE, Knorr DA, Thompson MC, Nivar M, Lebowitz S, Peters N, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;35(9):2703–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herishanu Y, Rahav G, Levi S, Braester A, Itchaki G, Bairey O, et al. Efficacy of a third BNT162b2 mRNA COVID-19 vaccine dose in patients with CLL who failed standard 2-dose vaccination. Blood. 2022;139(5):678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benjamini O, Gershon R, Bar-Haim E, Lustig Y, Cohen H, Doolman R, et al. Cellular and humoral response to the fourth BNT162b2 mRNA COVID-19 vaccine dose in patients with CLL. Eur J Haematol. 2023;110(1):99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of covid-19. N Engl J Med. 2022;386(23):2188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kertes J, Shapiro Ben David S, Engel-Zohar N, Rosen K, Hemo B, Kantor A, et al. Association between AZD7442 (Tixagevimab-Cilgavimab) administration and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, hospitalization, and mortality. Clin Infect Dis. 2023;76(3):e126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sheward DJ, Kim C, Ehling RA, Pankow A, Castro Dopico X, Dyrdak R, et al. Neutralisation sensitivity of the SARS-CoV-2 omicron (B.1.1.529) variant: a cross-sectional study. Lancet Infect Dis. 2022;22(6):813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hachmann NP, Miller J, Collier ARY, Ventura JD, Yu J, Rowe M, et al. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387(1):86–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Q, Guo Y, Iketani S, Nair MS, Li Z, Mohri H, et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608(7923):603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bruel T, Stéfic K, Nguyen Y, Toniutti D, Staropoli I, Porrot F, et al. Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4, and BA.5 in patients receiving monoclonal antibodies. Cell Rep Med. 2022;3(12):100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suribhatla R, Starkey T, Ionescu MC, Pagliuca A, Richter A, Lee LYW. Systematic review and meta-analysis of the clinical effectiveness of tixagevimab/cilgavimab for prophylaxis of COVID-19 in immunocompromised patients. Br J Haematol. 2023;201(5):813–23. [DOI] [PubMed] [Google Scholar]
- 18. Al-Obaidi MM, Gungor AB, Kurtin SE, Mathias AE, Tanriover B, Zangeneh TT. The prevention of COVID-19 in high-risk patients using tixagevimab-cilgavimab (evusheld): real-world experience at a large academic center. Am J Med. 2023;136(1):96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen Y, Flahault A, Chavarot N, Melenotte C, Cheminant M, Deschamps P, et al. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Clin Microbiol Infect. 2022;28(12):1654 e1–1654 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al Jurdi A, Morena L, Cote M, Bethea E, Azzi J, Riella LV. Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave. Am J Transplant. 2022;22(12):3130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bertrand D, Laurent C, Lemée V, Lebourg L, Hanoy M, Le Roy F, et al. Efficacy of anti-SARS-CoV-2 monoclonal antibody prophylaxis and vaccination on the Omicron variant of COVID-19 in kidney transplant recipients. Kidney Int. 2022;102(2):440–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benotmane I, Velay A, Gautier-Vargas G, Olagne J, Thaunat O, Fafi-Kremer S, et al. Pre-exposure prophylaxis with 300 mg Evusheld elicits limited neutralizing activity against the Omicron variant. Kidney Int. 2022;102(2):442–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bertrand D, Laurent C, Lemée V, Lebourg L, Hanoy M, Le Roy F, et al. Efficacy of tixagevimab/cilgavimab prophylaxis and vaccination on omicron variants (BA.1, BA.2, BA.5, and BQ.1.1) in kidney transplant recipients. Clin J Am Soc Nephrol. 2023;18(10):1343–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benotmane I, Velay A, Gautier-Vargas G, Olagne J, Obrecht A, Cognard N, et al. Breakthrough COVID-19 cases despite prophylaxis with 150 mg of tixagevimab and 150 mg of cilgavimab in kidney transplant recipients. Am J Transplant. 2022;22(11):2675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stuver R, Shah GL, Korde NS, Roeker LE, Mato AR, Batlevi CL, et al. Activity of AZD7442 (tixagevimab-cilgavimab) against Omicron SARS-CoV-2 in patients with hematologic malignancies. Cancer Cell. 2022;40(6):590–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Otiniano A, van de Wyngaert Z, Brissot E, Dulery R, Gozlan J, Daguenel A, et al. Tixagevimab/cilgavimab for Omicron SARS-CoV-2 infection in patients with haematologic diseases. Bone Marrow Transplant. 2022;58:340–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602(7898):671–5. [DOI] [PubMed] [Google Scholar]
- 28. Davis JA, Granger K, Roubal K, Smith D, Gaffney KJ, McGann M, et al. Efficacy of tixagevimab-cilgavimab in preventing SARS-CoV-2 for patients with B-cell malignancies. Blood. 2022;141(2):200–203. 10.1182/blood.2022018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Imai M, Ito M, Kiso M, Yamayoshi S, Uraki R, Fukushi S, et al. Efficacy of antiviral agents against omicron subvariants BQ.1.1 and XBB. N Engl J Med. 2023;388(1):89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arora P, Kempf A, Nehlmeier I, Schulz SR, Jäck HM, Pöhlmann S, et al. Omicron sublineage BQ.1.1 resistance to monoclonal antibodies. Lancet Infect Dis. 2023;23(1):22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Almeida Oliveira A, Praia Borges Freire D, Rodrigues de Andrade A, de Miranda Marques A, da Silva Madeira L, Moreno Senna JP, et al. The landscape of neutralizing monoclonal antibodies (nAbs) for treatment and prevention of COVID-19. J Pharm Innov. 2023:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, Zhou D, Ginn HM, Selvaraj M, et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185(14):2422–33 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ryu DK, Kang B, Noh H, Woo SJ, Lee MH, Nuijten PM, et al. The in vitro and in vivo efficacy of CT-P59 against Gamma, Delta and its associated variants of SARS-CoV-2. Biochem Biophys Res Commun. 2021;578:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research datasets for the present study would be available from the corresponding author upon reasonable request. The data are not publicly available because of privacy or ethical restrictions.