Abstract
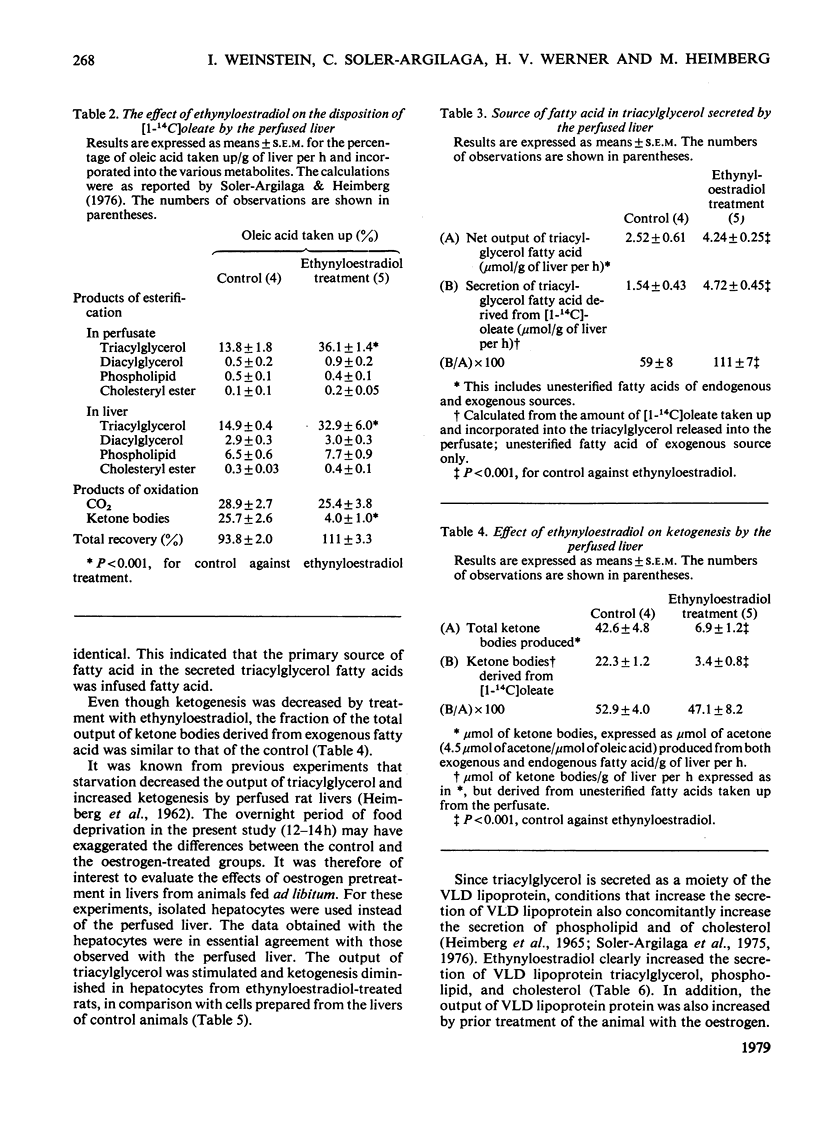
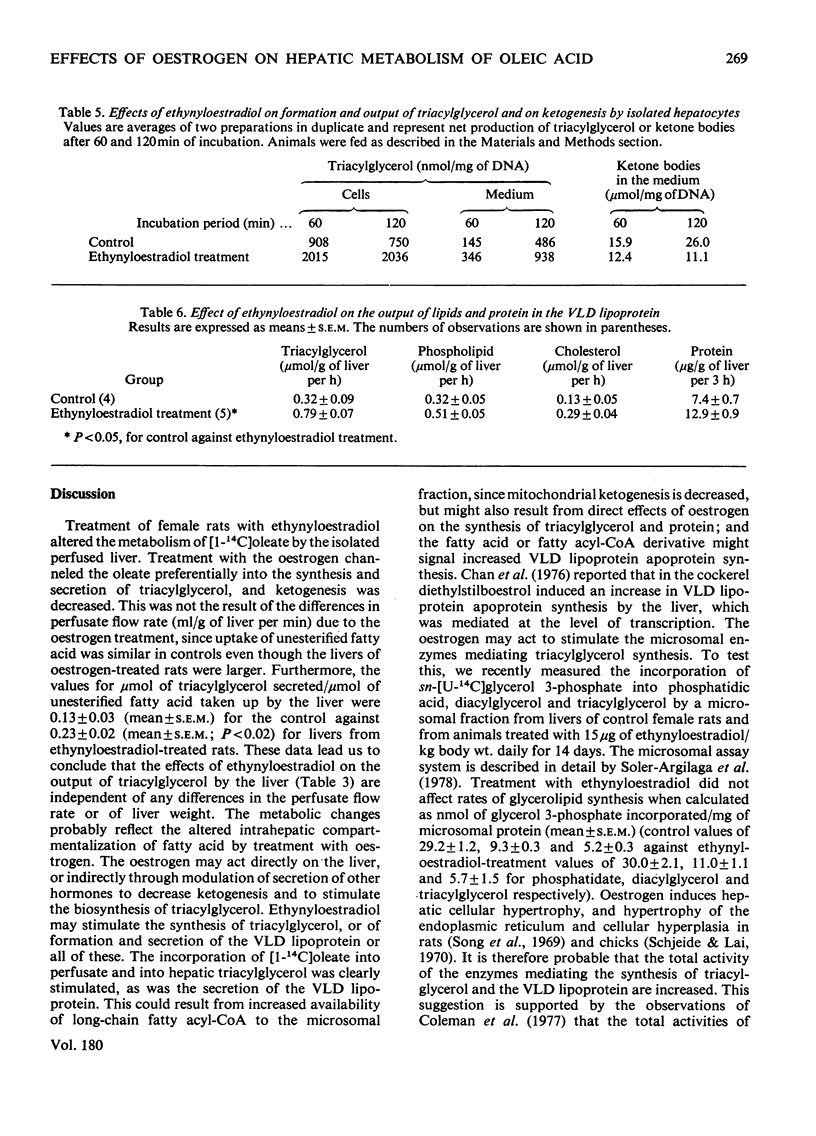
Normal female rats were given 15μg of ethynyloestradiol/kg body wt. for 14 days and were killed on day 15 after starvation for 12–14h. The livers were isolated and were perfused with a medium containing washed bovine erythrocytes, bovine serum albumin, glucose and [1-14C]oleic acid; 414μmol of oleate were infused/h during a 3h experimental period. The output of bile and the flow of perfusate/g of liver were decreased in livers from animals pretreated with ethynyloestradiol, whereas the liver weight was increased slightly. The rates of uptake and of utilization of [1-14C]oleate were measured when the concentration of unesterified fatty acid in the perfusate plasma was constant. The uptake of unesterified fatty acid was unaffected by pretreatment of the animal with oestrogen; however, the rate of incorporation of [1-14C]oleate into hepatic and perfusate triacylglycerol was stimulated, whereas the rate of conversion into ketone bodies was impaired by treatment of the rat with ethynyloestradiol. Pretreatment of the rat with ethynyloestradiol increased the output of very-low-density lipoprotein triacylglycerol, cholesterol, phospholipid and protein. The production of 14CO2 and the incorporation of radioactivity into phospholipid, cholesteryl ester and diacylglycerol was unaffected by treatment with the steroid. The net output of glucose by livers from oestrogen-treated rats was impaired despite the apparent increased quantities of glycogen in the liver. The overall effect of pretreatment with oestrogen on hepatic metabolism of fatty acids is the channeling of [1-14C]oleate into synthesis and increased output of triacylglycerol as a moiety of the very-low-density lipoprotein, whereas ketogenesis is decreased. The effect of ethynyloestradiol on the liver is apparently independent of the nutritional state of the animal from which the liver was obtained. It is pertinent that hepatocytes prepared from livers of fed rats that had been treated with ethynyloestradiol produced fewer ketone bodies and secreted more triacylglycerol than did hepatocytes prepared from control animals. In these respects, the effects of the steroid were similar in livers from fed or starved (12–14h) rats. Oestrogens may possibly inhibit hepatic oxidation of fatty acid, making more fatty acid available for the synthesis of triacylglycerol, or may stimulate the biosynthesis of triacylglycerol, or may be active on both metabolic pathways.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurell M., Cramér K., Rybo G. Serum lipids and lipoproteins during long-term administration of an oral contraceptive. Lancet. 1966 Feb 5;1(7432):291–293. doi: 10.1016/s0140-6736(66)90641-6. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Banik U. K., Revesz C., Herr F. Orally active oestrogens and progestins in prevention of pregnancy in rats. J Reprod Fertil. 1969 Apr;18(3):509–515. doi: 10.1530/jrf.0.0180509. [DOI] [PubMed] [Google Scholar]
- Beck P., Eaton R. P., Arnett D. M., Alsever R. N. Effect of contraceptive steroids on arginine-stimulated glucagon and insulin secretion in women: I-Lipid physiology. Metabolism. 1975 Sep;24(9):1055–1065. doi: 10.1016/0026-0495(75)90099-2. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberdorf F. A., Chernick S. S., Scow R. O. Effect of insulin and acute diabetes on plasma FFA and ketone bodies in the fasting rat. J Clin Invest. 1970 Sep;49(9):1685–1693. doi: 10.1172/JCI106386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L., Jackson R. L., O'Malley B. W., Means A. R. Synthesis of very low density lipoproteins in the cockerel. Effects of estrogen. J Clin Invest. 1976 Aug;58(2):368–379. doi: 10.1172/JCI108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Polokoff M. A., Bell R. M. Hypertriacylglycerolemia in the chick: effect of estrogen on hepatic microsomal enzymes of triacylglycerol and phospholipid synthesis. Metabolism. 1977 Oct;26(10):1123–1130. doi: 10.1016/0026-0495(77)90039-7. [DOI] [PubMed] [Google Scholar]
- Davis R. A., Showalter R., Kern F., Jr Reversal by triton WR-1339 of ethynyloestradiol-induced hepatic cholesterol esterification. Biochem J. 1978 Jul 15;174(1):45–51. doi: 10.1042/bj1740045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncombe W. G. The colorimetric micro-determination of long-chain fatty acids. Biochem J. 1963 Jul;88(1):7–10. doi: 10.1042/bj0880007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. P. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. J Lipid Res. 1973 May;14(3):312–318. [PubMed] [Google Scholar]
- Exton J. H., Corbin J. G., Park C. R. Control of gluconeogenesis in liver. IV. Differential effects of fatty acids and glucagon on ketogenesis and gluconeogenesis in the perfused rat liver. J Biol Chem. 1969 Aug 10;244(15):4095–4102. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GOODMAN D. S. Preparation of human serum albumin free of long-chain fatty acids. Science. 1957 Jun 28;125(3261):1296–1297. doi: 10.1126/science.125.3261.1296. [DOI] [PubMed] [Google Scholar]
- Gershberg H., Hulse M., Javier Z. Hypertriglyceridemia during treatment with estrogen and oral contraceptives. An alteration in hepatic function? Obstet Gynecol. 1968 Feb;31(2):186–189. doi: 10.1097/00006250-196802000-00006. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIMBERG M., WEINSTEIN I., KLAUSNER H., WATKINS M. L. Release and uptake of triglycerides by isolated perfused rat liver. Am J Physiol. 1962 Feb;202:353–358. doi: 10.1152/ajplegacy.1962.202.2.353. [DOI] [PubMed] [Google Scholar]
- Heimberg M., Weinstein I., Dishmon G., Fried M. Lipoprotein lipid transport by livers from normal and CCl-4-poisoned animals. Am J Physiol. 1965 Nov;209(5):1053–1060. doi: 10.1152/ajplegacy.1965.209.5.1053. [DOI] [PubMed] [Google Scholar]
- Heimberg M., Weinstein I., Kohout M. The effects of glucagon, dibutyryl cyclic adenosine 3',5'-monophosphate, and concentration of free fatty acid on hepatic lipid metabolism. J Biol Chem. 1969 Oct 10;244(19):5131–5139. [PubMed] [Google Scholar]
- Kissebah A. H., Harrigan P., Wynn V. Mechanism of hypertriglyceridaemia associated with contraceptive steroids. Horm Metab Res. 1973 May;5(3):184–190. doi: 10.1055/s-0028-1093969. [DOI] [PubMed] [Google Scholar]
- Kohout M., Kohoutova B., Heimberg M. The regulation of hepatic triglyceride metabolism by free fatty acids. J Biol Chem. 1971 Aug 25;246(16):5067–5074. [PubMed] [Google Scholar]
- Kudzma D. J., St Claire F., DeLallo L., Friedberg S. J. Mechanism of avian estrogen-induced hypertriglyceridemia: evidence for overproduction of triglyceride. J Lipid Res. 1975 Mar;16(2):123–133. [PubMed] [Google Scholar]
- LEFFLER H. H. Estimation of cholesterol in serum. Am J Clin Pathol. 1959 Apr;31(4):310–313. doi: 10.1093/ajcp/31.4.310. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matute M. L., Kalkhoff R. K. Sex steroid influence on hepatic gluconeogenesis and glucogen formation. Endocrinology. 1973 Mar;92(3):762–768. doi: 10.1210/endo-92-3-762. [DOI] [PubMed] [Google Scholar]
- Otway S., Robinson D. S. The use of a non-ionic detergent (Triton WR 1339) to determine rates of triglyceride entry into the circulation of the rat under different physiological conditions. J Physiol. 1967 May;190(2):321–332. doi: 10.1113/jphysiol.1967.sp008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade D. S., Eaton R. P. Modulation of fatty acid metabolism by glucagon in man. I. Effects in normal subjects. Diabetes. 1975 May;24(5):502–509. doi: 10.2337/diab.24.5.502. [DOI] [PubMed] [Google Scholar]
- Schillinger E., Gerhards E. Influence of hormonal contraceptives on carbohydrate and lipid metabolism in the rat. Biochem Pharmacol. 1973 Aug 1;22(15):1835–1844. doi: 10.1016/0006-2952(73)90043-9. [DOI] [PubMed] [Google Scholar]
- Soler-Argilaga C., Danon A., Wilcox H. G., Heimberg M. Effects of sex on formation and properties of plasma very low density lipoprotein in vivo. Lipids. 1976 Jul;11(7):517–525. doi: 10.1007/BF02532896. [DOI] [PubMed] [Google Scholar]
- Soler-Argilaga C., Heimberg M. Comparison of metabolism of free fatty acid by isolated perfused livers from male and female rats. J Lipid Res. 1976 Nov;17(6):605–615. [PubMed] [Google Scholar]
- Soler-Argilaga C., Russell R. L., Heimberg M. Enzymatic aspects of the reduction of microsomal glycerolipid biosynthesis after perfusion of the liver with dibutyryl adenosine-3',5'-monophosphate. Arch Biochem Biophys. 1978 Oct;190(2):367–372. doi: 10.1016/0003-9861(78)90289-8. [DOI] [PubMed] [Google Scholar]
- Soler-Argilaga C., Wilcox H. G., Heimberg M. The effect of sex on the quantity and properties of the very low density lipoprotein secreted by the liver in vitro. J Lipid Res. 1976 Mar;17(2):139–145. [PubMed] [Google Scholar]
- Song C. S., Rifkind A. B., Gillette P. N., Kappas A. Hormones and the liver. The effect of estrogens, progestins, and pregnancy on hepatic function. Am J Obstet Gynecol. 1969 Nov 1;105(5):813–847. [PubMed] [Google Scholar]
- Spector A. A., Steinberg D. The utilization of unesterified palmitate by Ehrlich ascites tumor cells. J Biol Chem. 1965 Oct;240(10):3747–3753. [PubMed] [Google Scholar]
- Spellacy W. N., Carlson K. L. Plasma insulin and blood glucose levels in patients taking oral contraceptives. A preliminary report of a prospective study. Am J Obstet Gynecol. 1966 Jun 15;95(4):474–478. doi: 10.1016/0002-9378(66)90137-2. [DOI] [PubMed] [Google Scholar]
- Stokes T., Wynn V. Serum-lipids in women on oral contraceptives. Lancet. 1971 Sep 25;2(7726):677–680. doi: 10.1016/s0140-6736(71)92247-1. [DOI] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J. 1972 Jan;126(2):295–311. doi: 10.1042/bj1260295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN HANDEL E., ZILVERSMIT D. B. Micromethod for the direct determination of serum triglycerides. J Lab Clin Med. 1957 Jul;50(1):152–157. [PubMed] [Google Scholar]
- Van Harken D. R., Dixon C. W., Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J Biol Chem. 1969 May 10;244(9):2278–2285. [PubMed] [Google Scholar]
- Watkins M. L., Fizette N., Heimberg M. Sexual influences on hepatic secretion of triglyceride. Biochim Biophys Acta. 1972 Sep 7;280(1):82–85. doi: 10.1016/0005-2760(72)90214-7. [DOI] [PubMed] [Google Scholar]
- Weinstein I., Seedman S., Veldhuis M. Effect of ethynyl estradiol on the secretion of hepatic triglyceride. Proc Soc Exp Biol Med. 1975 May;149(1):181–184. doi: 10.3181/00379727-149-38768. [DOI] [PubMed] [Google Scholar]
- Weinstein I., Seltzer M., Belitsky R. The interrelation of glucagon and gonadectomy upon hepatic triglyceride metabolism in rats. Biochim Biophys Acta. 1974 Apr 26;348(1):14–22. doi: 10.1016/0005-2760(74)90088-5. [DOI] [PubMed] [Google Scholar]
- Weinstein I., Soler-Argilaga C., Heimberg M. Effects of ethynyl estradiol on incorporation of (1-14C) oleate into triglyceride and ketone bodies by the liver. Biochem Pharmacol. 1977 Jan 1;26(1):77–80. doi: 10.1016/0006-2952(77)90136-8. [DOI] [PubMed] [Google Scholar]
- Weinstein I., Turner F. C., Soler-Argilaga C., Heimberg M. Effects of ethynyl estradiol on serum lipoprotein lipids in male and female rats. Biochim Biophys Acta. 1978 Sep 28;530(3):394–401. doi: 10.1016/0005-2760(78)90159-5. [DOI] [PubMed] [Google Scholar]
- Wilcox H. G., Woodside W. F., Breen K. J., Knapp H. R., Jr, Heimberg M. The effect of sex on certain properties of the very low density lipoprotein secreted by the liver. Biochem Biophys Res Commun. 1974 Jun 18;58(4):919–926. doi: 10.1016/s0006-291x(74)80231-7. [DOI] [PubMed] [Google Scholar]
- Woodside W. F., Heimberg M. Effects of anti-insulin serum, insulin, and glucose on output of triglycerides and on ketogenesis by the perfused rat liver. J Biol Chem. 1976 Jan 10;251(1):13–23. [PubMed] [Google Scholar]
- Wynn J. W., Doar J. W., Mills G. L. Some effects of oral contraceptives on serum-lipid and lipoprotein levels. Lancet. 1966 Oct 1;2(7466):720–723. doi: 10.1016/s0140-6736(66)92979-5. [DOI] [PubMed] [Google Scholar]
- Yang M. G., Sanger V. L., Mickelsen O. Feeding norethynodrel and mestranol to immature and adult female rats cross-section and length of long bones. Proc Soc Exp Biol Med. 1969 Apr;130(4):1146–1150. doi: 10.3181/00379727-130-33739. [DOI] [PubMed] [Google Scholar]


