Abstract
The open reading frame III of Borna disease virus (BDV) codes for a protein with a mass of 16 kDa, named p16 or BDV-M. p16 was described as an N-glycosylated protein in several previous publications and therefore was termed gp18, although the amino acid sequence of p16 does not contain any regular consensus sequence for N glycosylation. We examined glycosylation of p16 and studied its membrane topology using antisera raised against peptides, which comprise the N and the C termini. Neither an N- nor a C-terminal peptide is cleaved from p16 during maturation. Neither deglycosylation of p16 by endoglycosidases nor binding of lectin to p16 was detectable. Introduction of typical N-glycosylation sites at the proposed sites of p16 failed in carbohydrate attachment. Flotation experiments with membranes of BDV-infected cells on density gradients revealed that p16 is not an integral membrane protein, since it can be dissociated from membranes. Our experimental data strongly suggest that p16 is a typical nonglycosylated matrix protein associated at the inner surface of the viral membrane, as is true for homologous proteins of other members of the Mononegavirales order.
Borna disease virus (BDV) is the etiological agent of Borna disease, a rare, progressive meningoencephalitis caused by an immunopathological reaction in the central nervous tissue that affects horses, sheep, and other animal species. It is also suspected to contribute to human psychiatric disorders. BDV is an enveloped virus characterized by a nonsegmented negative-strand RNA genome that belongs to the family Bornaviridae in the order Mononegavirales (2, 5, 9). Unlike other viruses of this order, BDV replicates and transcribes in the nuclei of infected cells (1, 6, 7). Virus particles are formed in small numbers in vivo and in vitro, which may reflect the low synthesis rate of membrane proteins (23, 25, 26). Fine structure and morphogenesis of BDV analyzed by electron microscopy revealed spherical, approximately 90- to 130-nm enveloped virus particles, which contain spikes of 7 nm in length. The virions are reproduced by budding on the cell surface (16, 22, 37). The BDV genome consists of an 8.9-kb RNA, which includes at least six open reading frames (ORFs). ORF I codes for the nucleoprotein p40 (NP), ORF II codes for the phosphoprotein p24 (P), ORF III codes for p16 (M), also known as a matrix-like glycoprotein, gp18 (2, 15, 31), ORF IV codes for p57, also known as glycoprotein gp94 (G) (12, 23, 25, 26), ORF V codes for p180/190, the phosphorylated polymerase (L) (34), and an ORF overlapping with the P gene codes for protein p10 (X), which is associated with the viral phospho- and nucleoproteins (8, 18, 27, 35, 36). Several BDV strains and isolates from various parts of the world were characterized (28, 29). A comparison of their nucleotide and deduced amino acid sequences revealed amino acid identities from 84 to 95.5% among all gene products (20, 29).
The ORF III of the BDV genome encodes a polypeptide with a calculated mass of 16.2 kDa, which is considered the putative matrix protein (see Fig. 1) (5). BDV-M was the first glycoprotein, gp18, described for BDV, although it contains no consensus N-glycosylation motif (N-X-S/T) within its polypeptide chain. Instead, the sequences N-I-Y and L-N-S-L-S at the positions 74 to 76 and 87 to 91, respectively, were discussed as alternative N-glycosylation motives (2, 15). Originally, a 14.5-kDa BDV-specific protein was characterized from persistently BDV-infected brains and tissue cultures (24). Later on, this protein was described as an N-glycosylated viral protein with a molecular mass of 17 to 18 kDa, which exists in a tetrameric form (15, 30, 31, 32). Glycosylation of p16 has been shown by lectin staining, endoglycosidase treatment of BDV-infected rat brain material, in vitro transcription and translation experiments in the presence and absence of canine microsomal membranes, and mass spectrometry (15, 31). Antibodies to native or recombinant protein of ORF III have been shown to possess neutralizing activities (14, 30). In addition, antisera raised against BDV-specific synthesized glycoconjugates showed neutralizing capacity, which is thought to be partially attributable to gp18 (30, 32). These data suggested that the protein of the ORF III may resemble an integral membrane protein rather than an ordinary viral matrix protein, which lines the inside of the viral envelope. However, comparison of ORF III with homologous ORFs in other members of Mononegavirales reveals that they all encode typical nonglycosylated matrix proteins. Because of this discrepancy, we studied the unusual properties of the BDV-M protein. We carefully analyzed the carbohydrate content, studied putative N-glycosylation attachment sites of p16 by mutational analysis, and determined its topology by flotation of p16-containing membranes on density gradients under various conditions. Our results strongly indicate that p16 is a normal, nonglycosylated matrix protein.
FIG. 1.
Schematic presentation of the BDV matrix protein p16. The gene product of ORF III of BDV is a polypeptide containing 142 amino acids. Homologous peptides used for raising the polyclonal antibodies αM1 and αM128 in rabbits are indicated. Putative carbohydrate attachment sites N74IY and LN88SLS (15) are marked by rhombs. Hydrophobic regions are shown in boxes.
(Part of this work was performed by I. Kraus in partial fulfillment of the requirements for a Ph.D. degree from the Philipps University, Marburg, Germany.)
MATERIALS AND METHODS
Cells and virus material.
Persistently BDV-infected Vero (strain No/98), MDCK (strain He/8o), and C6 (strain He/80) cells, uninfected Vero, MDCK, and C6 cells as a control, and HeLa and U373 cells (human glia blastoma cells) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 50 units of penicillin/ml, and 50 μg of streptomycin/ml. The cell lines were kept at 37°C with 5% CO2. In order to increase the BDV particle production, BDV-infected MDCK cells were treated with 8 mM sodium n-butyrate (Sigma, Deisenhofen, Germany) for 48 h (21). Virus from cell supernatants was pelleted through a 20% sucrose cushion, resuspended in 200 μl of phosphate-buffered saline (PBS), and purified by velocity gradient centrifugation in an iodixanol gradient (OptiPrep; Sigma) which was formed by 11 steps with 1.2% increments of iodixanol ranging from 6 to 18% diluted in PBS. Resuspended virus pellet was layered onto the top of the gradient and centrifuged for 1.5 h at 250,000 × g in a Beckmann-SW41 rotor. Fractions were collected from the top of the gradient and analyzed for protein content (10).
Modified vaccinia virus Ankara-T7 (MVA-T7) expressing the T7 RNA polymerase was grown in chicken embryo fibroblasts (3). Virus was released 48 to 72 h postinfection by freezing-thawing and sonication (40 W, 30 s).
Extracts of BDV-infected rat brain.
Extracts of BDV-infected and uninfected rat brains were prepared as described previously, but in a slightly modified manner (13). Briefly, 4- to 6-week-old Lewis rats were intracerebrally infected with a rat-adapted BDV strain (derived from strain He/80) and sacrificed 3 weeks later. Rat brains were homogenized in a 10-fold volume of 200 mM Tris-HCl, pH 7.2, containing 100 mM NaCl, 1% Triton X-100, and 0.5% sodium deoxycholate and stirred for 1 h at room temperature. Particulate matter was removed by centrifugation in a Beckmann-45Ti rotor at 100,000 × g for 2 h. The supernatant was removed and used for immunoblot analysis.
In vitro transcription and translation.
The TNT T7 Quick Coupled Transcription/Translation System (Promega, Madison, Wis.) was used for in vitro transcription and translation in the presence or absence of canine pancreatic microsomal membranes (a generous gift from B. Dobberstein and M. Froeschke). A plasmid DNA template (0.5 μg) (p16 cDNA in pTM1; see “Site-directed mutagenesis and MVA-T7 expression system,” below) was incubated with 20 μl of TNT T7 Quick Master Mix and 10 μCi of [35S]methionine (specific activity, >1,000 Ci/mmol; Amersham-Buchler, Braunschweig, Germany) or 1 μl of 1 mM methionine for 90 min at 30°C using standard protocols. Samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography.
Peptide antisera.
Peptides corresponding to the amino acid positions 2 to 16 and 128 to 142 of p16 were chemically synthesized, conjugated to keyhole limpet hemocyanin (Calbiochem, Frankfurt, Germany) as a carrier protein by using N-(α-maleimido-acetoxy)succinimide ester (Pierce, Bonn, Germany), and used for repeated immunization of rabbits. The resulting antisera were designated αM1 and αM128, respectively. The monospecific antiserum Rb-αgp2 recognizes BDV-gp94, and the cleavage product gp43 was prepared previously (23).
Immunoblot analysis.
Samples were supplemented with sample buffer (100 mM Tris-HCl [pH 6.8], 4% SDS, 10% 2-mercaptoethanol, 20% glycerol. and 0.05% bromophenol blue), heated for 5 min at 96°C, separated on 15% polyacrylamide gels by SDS-PAGE, and electrophoretically transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). The membranes were blocked at 4°C overnight with a solution of 3% bovine serum albumin in PBS containing 0.1% Tween 20 and incubated for 1 h with peptide antisera diluted 1 to 2,000 in PBS-Tween (0.1%), followed by an incubation of a 1-to-2,000-diluted anti-rabbit-immunoglobulin G (swine) complexed with horseradish peroxidase (DAKO, Hamburg, Germany). Protein bands were visualized using the SuperSignal chemoluminescence substrate as described by the supplier (Pierce).
Radioactive labeling, immunoprecipitation, and deglycosylation.
Permanently BDV-infected Vero cells and uninfected cells as controls were radioactively labeled with [35S]methionine (50 μCi/ml; specific activity, >1,000 Ci/mmol; Amersham-Buchler). Cells were harvested in RIPA buffer (1% Triton X-100, 1% deoxycholate, 0.1% SDS, 50-μl/ml aprotinin [Trasylol; Bayer, Wuppertal, Germany], 150 mM NaCl, 20 mM Tris-HCl [pH 7.4], 10 mM EDTA, 1.85-mg/ml iodoacetamide), sonicated (40 Watts, 30 s), and centrifuged at 20,000 × g for 30 min at 4°C. The supernatant was immunoprecipitated with peptide antisera and protein A-Sepharose beads (Sigma). Immunoprecipitates were heated for 10 min at 100°C with denaturation buffer (0.5% SDS, 1% β-mercaptoethanol) and incubated with endoglycosidase H (Endo H) or peptide-N-glycosidase F (PNGase F) (New England BioLabs, Schwalbach, Germany), respectively, in the appropriated reaction buffers (50 mM sodium citrate [pH 5.5] [Endo H], 50 mM sodium phosphate [pH 7.5], 1% NP-40 [PNGase F]) for 1 h at 37°C. The samples were heated in sample buffer for 5 min at 96°C, separated on 18% polyacrylamide gels, and visualized by autoradiography.
Lectin blot analysis.
Lectin binding studies were performed by using the DIG (digoxigenin) Glycan Differentiation Kit (Roche, Mannheim, Germany). Immunoprecipitated proteins from BDV-infected and uninfected Vero cells and glycoproteins, supplied with the kit as positive controls, were separated on 15% polyacrylamide gels by SDS-PAGE and electrophoretically transferred onto nitrocellulose membranes (Schleicher & Scheull). The nitrocellulose membranes were incubated in the blocking solution of the kit (Roche) overnight at 4°C, and the blots were washed in Tris-buffered-saline (TBS) containing 50 mM Tris-HCl[pH 7.5]–150 mM NaCl and then in TBS supplemented with 1 mM MgCl2, 1 mM MnCl2, and 1 mM CaCl2 and incubated with specific digoxigenin-labeled lectins solved in the supplemented TBS for 1 h at room temperature. The lectins on the nitrocellulose membranes were exposed to antidigoxigenin immunoglobulins complexed with alkaline phosphatase for 1 h. Bound lectins were stained with 4-nitroblue tetrazolium–X-phosphate (supplied with the kit).
Site-directed mutagenesis and MVA-T7 expression system.
The p16 cDNA of BDV strain He/80 was cloned into pTM1 for vectorial expression in mammalian cells using the modified vaccinia virus strain Ankara-T7 (MVA-T7) expression system (33). Point mutations were introduced using the QuikChange Site-directed Mutagenesis Kit (Stratagene, Amsterdam, The Netherlands). The PCR was carried out according to the protocol of the manufacturer with the primer pairs 5′-CCC-TGG-TCA-ACA-TAA-CCT-TCC-AGA-TTG-ACG-3′ and 5′-CGT-CAA-TCT-GGA-AGG-TTA-TGT-TGA-CCA-GGG-3′ for the Y76T mutant, 5′-CCT-AAC-ACT-CAA-CTC-AAC-GTC-CGT-GTA-CAA-AGA-CC-3′ and 5′-GGT-CTT-TGT-ACA-CGG-ACG-TTG-AGT-TGA-GTG-TTA-GG-3′ for the L90T mutant. Plasmids carrying the wild type and the mutated cDNA of the p16 gene were used for transfection experiments. HeLa or U373 cells were infected with MVA-T7, and 1 h postinfection the cells were transfected with pTM1-p16 using lipofectin reagent (Life Technologies, Eggenstein, Germany). Cells were radioactively labeled with [35S]methionine (50 μCi/ml; specific activity, >1,000 Ci/mmol; Amersham-Buchler) and lysed 8 h posttransfection. The recombinant p16 was immunoprecipitated, separated by SDS-PAGE, and visualized by autoradiography.
Flotation experiments.
Flotation experiments were carried out as described previously with some modifications (4, 19). About 106 BDV-infected Vero cells and uninfected cells were radioactively labeled with [35S]methionine (50 μCi/ml; specific activity, >1,000 Ci/mmol; Amersham-Buchler) for 2 h at 37°C. Cells were harvested and disrupted in a hypotonic Tris buffer (20 mM Tris-HCl [pH 7.4]) by 20 strokes of a Dounce homogenizer on ice. Nuclei and cell debris were removed from the cell lysate by centrifugation at 700 × g for 5 min at 4°C. OptiPrep (Sigma) was added to the postnuclear supernatant to a final concentration of 35% in a total volume of 500 μl, which was placed at the bottom of a Beckmann-SW60 centrifuge tube. It was overlaid with 3.5 ml of 30% OptiPrep and then with 200 μl of TNE buffer (25 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA). All OptiPrep solutions were prepared in TNE containing the protease inhibitor mixture Complete (Roche). The gradient was centrifuged to equilibrium at 165,000 × g for 4 h at 4°C. The cellular membranes which were moved by flotation into the interface between 30% OptiPrep and TNE were treated with 1 M sodium bicarbonate buffer (pH 10) for 1 h at 4°C and then neutralized with 1 M Tris-HCl, pH 6.8, or were treated with 2 M KCl or with 50 mM EDTA for 1 h at room temperature. The pretreated membranes were subjected to flotation again as described above. Fractions were collected from the top, mixed with 2× RIPA buffer, immunoprecipitated, subjected SDS-PAGE, and visualized by autoradiography.
RESULTS
Immune detection analysis of p16.
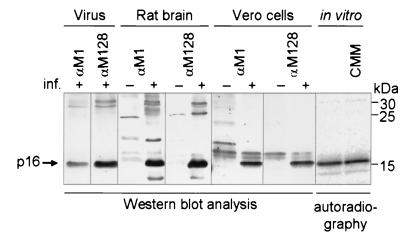
In order to identify the p16 BDV protein, monospecific antibodies were raised against peptides comprising the N and C termini of p16 (Fig. 1). The peptide antisera were designated αM1 and αM128 according to the first amino acid position of the peptides used for immunization. The p16 BDV-protein used for these studies was derived from different sources, e.g., from purified BDV particles, from persistently BDV-infected eukaryotic cells, from BDV-infected rat brain, and from in vitro transcribed and translated p16 cDNA. In all of these preparations, the monospecific antisera αM1 and αM128 recognized a virus-specific protein with an apparent molecular mass of 16 kDa (Fig. 2). Upper bands of about 30 kDa most likely represent dimers of p16. No staining was detected in uninfected control preparations, indicating the viral nature of the detective protein. The p16 BDV protein isolated from persistently BDV-infected MDCK and C6 cells or from rat brain displayed the same size irrespective of whether αM1 or αM128 antiserum was used (data not shown). In vitro-translated p16, which by definition is not glycosylated, shows the same molecular mass in the presence and absence of microsomal membranes, as is found with p16 expressed in mammalian cells. According to previous reports, expression of p16 in mammalian cells would allow N glycosylation of the protein, indicated by a shift in its molecular mass. There are two explanations for our results: (i) p16 is glycosylated but posttranslationally truncated, or (ii) no covalently linked carbohydrate is present on p16 expressed in eukaryotic systems. The fact that both antisera, one directed against the N terminus (amino acids [aa] 1 to 15) and one against the C terminus (aa 128 to 142), recognize p16 at the same position on the gel conclusively indicates that neither an N-terminal signal peptide nor a C-terminal peptide is co- or posttranslationally cleaved off. These results challenge the reinvestigation of the putative glycosylation of p16.
FIG. 2.
Immunodetection of p16 from different BDV sources. Virus isolated from permanent BDV-infected MDCK cells (He/80), brain tissue from uninfected and experimentally BDV-infected rats (He/80), cell lysates from uninfected and permanently BDV-infected Vero cells (strain No98), and in vitro-translated p16 in the absence and presence of microsomal membranes (CMM) are shown. Proteins were separated on 15% polyacrylamide gels by SDS-PAGE. Either the proteins were electrophoretically transferred to a nitrocellulose membrane and p16 was detected by Western blot analysis using the immune sera αM1 and αM128, or p16 was detected by autoradiography. Molecular mass calibration was done with the Rainbow marker proteins (Amersham). inf., infection.
Carbohydrate analyses.
p16 BDV protein, derived from persistently infected Vero cells, was metabolically labeled with [35S]methionine, immunoprecipitated with αM128, treated with two different glycosidases (Endo H and PNGase F), and separated on 18% polyacrylamide gels by SDS-PAGE. Neither the incubation with Endo H nor that with PNGase F resulted in a decrease of molecular mass, indicating that p16 does not contain detectable amounts of carbohydrate (Fig. 3).
FIG. 3.
Examination of the putative N glycosylation of p16 by endoglycosidase digestion. Aliquots of immunoprecipitated p16 of cell lysates of metabolically [35S]methionine-labeled, uninfected (mock) and permanently BDV-infected Vero cells were incubated with Endo H (E) or PNGase F (P) or kept untreated (–). The p16 protein was subjected to SDS-PAGE on an 18% polyacrylamide gel and detected by autoradiography. Molecular masses of the Rainbow marker proteins are indicated.
In addition, various lectins were used to identify carbohydrates of the p16 BDV protein. The lectins selected recognized a broad range of N and O glycans. Immunoprecipitates of p16 from uninfected and persistently BDV-infected Vero cells were subjected to SDS-PAGE, transferred onto nitrocellulose membranes, exposed to several lectins, and analyzed using a detection kit of high sensitivity. None of the lectins gave a positive signal in the expected molecular mass range of 15 to 18 kDa, whereas the control glycoproteins were easily detected (Fig. 4, upper panel). After removal of the lectins from the nitrocellulose membranes, the presence of p16 was demonstrated by immune detection. The lectin-analyzed samples contained enough p16 for immune detection (Fig. 4, lower panel). It should be noted that the nonspecific bands found in Fig. 4 are also present in the uninfected controls. In conclusion, two independent experimental approaches, one using different glycosidases and the other using different lectins, indicate that p16 is not glycosylated.
FIG. 4.
Lectin blot analysis of p16. p16 was immunoprecipitated with αM128 from cell lysates of permanently BDV-infected Vero cells (+), and the control was performed with lysates of uninfected cells (–). The precipitated proteins and control glycoproteins (C), submitted with the Glycan Detection Kit, were separated by SDS-PAGE, transferred onto a nitrocellulose membrane, and incubated with different digoxigenin-labeled lectins: GNA (Galanthus nivalis agglutinin), SNA (Sambucus nigra a.), MAA (Maackia amurensis a.), PNA (Peanut a.), and DSA (Datura stramonium a.). Incubation with anti-digoxigenin-alcaline phosphatase and staining with 4-nitroblue tetrazolium–X-phosphate solution identified the kit glycoproteins but did not stain p16. The arrow marks the position of 16 kDa, where putative glycosylated p16 would appear (upper panel). After removal of the lectins from the blots, the blotted p16 reacted with αM128, as shown by immunodetection (lower panel). Rainbow molecular mass marker proteins are indicated.
Putative carbohydrate attachment sites.
No consensus amino acid N-X-S/T sequences for putative attachment of N-glycosidic carbohydrates exist within p16. However, alternative attachment sites for N glycosylation have been suggested but not verified to date. The supposed N-glycosylation sites were N74-I-Y and L-N88-S-L-S (15). In order to examine whether these amino acid positions within the polypeptide chain of p16 can be used for N glycosylation, we changed these two sites to the consensus N-glycosylation sites N74-I-T76 and L-N88-S-T90-S, respectively, by site-directed mutagenesis of the cloned p16 gene. The resulting mutants, Y76T and L90T, showed no difference in electrophoretic mobility in comparison with the wild-type p16 when they were expressed by the MVA-T7 expression system in HeLa cells (Fig. 5A). Furthermore, additional treatment of immunoprecipitated wild-type and mutated p16 with the glycosidases Endo H and PNGase F revealed no electrophoretic mobility shift (Fig. 5B). A distinct decrease in molecular mass would be expected on gels after electrophoresis if one or two N-linked carbohydrate chains were removed from p16.
FIG. 5.
Examination of N glycosylation by an interchange of alternative glycosylation sites to general attachment sites. The putative N-glycosylation attachment sites N74-I-Y and L-N88-S-L-S were mutated to consensus N-glycosylation motives by site-directed mutagenesis (Y76T and L90T). HeLa cells (HeLa) were infected with MVA-T7 (MVA inf.) and transfected with p16 recombinant plasmids pTM1-M-WT (WT), pTM1-M-L90T (L90T), and pTM1-M-Y76T (Y76T). After radioactive labeling with [35S]methionine, p16 was immunoprecipitated from lysed cells and the proteins were separated on 15% polyacrylamide gels and visualized by autoradiography (A). The immunoprecipitated samples were treated with Endo H (E) and PNGase F (P) or kept untreated (–) before they were subjected to SDS-PAGE (B). The positions of Rainbow molecular mass markers are indicated.
These data indicate that the newly introduced carbohydrate attachment sites within p16 were not used. This raised the question of whether the introduced glycosylation sites on p16 are generally accessible for glycosylation in the lumen of the endoplasmic reticulum and in subsequent compartments of the exocytic pathway. Therefore, it was necessary to study the topology of p16 in cellular membranes.
Topology of p16 in cellular membranes.
A viral surface protein requires at least one hydrophobic transmembrane domain in the polypeptide chain for its transition into the lumen of the endoplasmic reticulum, where N glycosylation takes place. Such integrated glycoproteins can be released only by destroying the membrane with detergents. In contrast to viral integral glycoproteins, membrane-associated nonglycosylated viral matrix proteins can be dissociated from cellular membranes without destroying the lipid bilayer. With this knowledge, we analyzed the membrane topology of p16 to find out whether it is a peripheral membrane-associated protein or an integral membrane protein. Cellular membranes were prepared from metabolically labeled persistently BDV-infected cells and subjected to flotation analysis by ultracentrifugation through an OptiPrep gradient. In Fig. 6A, it is shown that membranes containing the BDV-specific proteins p16 and gp94 move from a layer of high density (fraction 5) to a less dense layer of the gradient (fraction 1). In order to discriminate membrane-associated proteins from integral membrane proteins, the membrane samples were treated either with sodium bicarbonate buffer at pH 10, with 2 M KCl, or with 50 mM EDTA, respectively. Aliquots of the fractions were immunoprecipitated, and the proteins were separated by SDS-PAGE and analyzed by autoradiography (Fig. 6A to D). Treatment of membranes at pH 10 transforms vesicles into membrane sheets, which consequently releases soluble or peripheral proteins trapped in vesicles (4). Treatment of membranes with 2 M KCl shields charges and weakens ionic interactions, which bind peripheral proteins to membranes either directly or indirectly through other membrane proteins. The 50 mM EDTA treatment disrupts membrane association of proteins, which is mediated by divalent cation bridge formation (4). In our experiments, the treatment of membranes from BDV-infected cells either with pH 10 (Fig. 6B, upper panel) or with 2 M KCl (Fig. 6C, upper panel) detached substantial amounts of p16 from the membranes, since after centrifugation it is predominantly found in fractions of gradients near the bottom of the centrifuge tube (fraction 5). This indicates that the p16 BDV protein can easily be dissociated from membrane layers. In the case of EDTA treatment, p16 could not be separated from the membranes (Fig. 6D, upper panel). This indicates that the binding is not predominantly mediated by divalent cation bridge formation. The same fractionated gradients were analyzed using an immune serum against the membrane-anchored glycoprotein gp94. They comigrate in the gradient with the membrane fraction (Fig. 6A to D, lower panels, fraction 1). The flotation experiments clearly demonstrated that p16 is released from membranes at high salt concentrations or at high pH, whereas BDV-gp94 cannot dissociate from the cell membranes without disintegration of the lipid layer of the membranes. BDV p16 and glycoprotein gp94 were released from the cellular membranes after treatment with Triton X-100 at a final concentration of 2% (data not shown). We therefore conclude that p16 is a typical membrane-associated matrix protein of BDV and not an integral membrane protein.
FIG. 6.
Dissociation of p16 from cellular membranes by OptiPrep flotation gradient centrifugation. Cellular membranes of radioactively labeled permanent BDV-infected Vero cells and control cells (not shown here) were prepared and purified by flotation through an OptiPrep step gradient. Aliquots of these membranes were treated with carbonate buffer pH 10 (B), 2 M KCl (C), or 50 mM EDTA (D) or kept untreated (A) and ultracentrifuged again. The flotation gradient was fractionated from top to bottom (fractions 1 to 5), and aliquots of each fraction were immunoprecipitated by a p16-specific antiserum, αM128 (upper panel), and by a gp94-specific antiserum, αgp2 (lower panel). The immunoprecipitated proteins were separated on 15% or 12% polyacrylamide gels by SDS-PAGE and visualized by autoradiography. The positions of the molecular mass markers are indicated.
DISCUSSION
BDV p16 is assumed to be synthesized as a soluble cytoplasmic protein, which converts to a membrane-associated protein during virus maturation. BDV-infected cells contain soluble and membrane-bound fractions with the same electrophoretical mobilities on polyacrylamide gels, indicating the same molecular mass (data not shown). The membrane-bound fraction of p16 has been of interest in this study, because it is the only relevant p16 form that would allow N glycosylation. We demonstrate in this report that the gene product of the ORF III of the BDV encodes a nonglycosylated, not proteolytically processed typical viral matrix protein which lines the inner side of a lipid-containing viral envelope and therefore should be termed p16 or matrix protein M but no longer glycoprotein gp18. The evidence for the lack of carbohydrate on p16 is given by several independent findings: first, p16 has the same electrophoretic mobility on gels independently of whether it was in vitro translated in the absence of a glycosylation system or biosynthesized in eukaryotic cells in the presence of glycosylation facilities. Second, examinations for deglycosylation of p16 expressed in eukaryotic cells by Endo H and PNGase F do not change the electrophoretic mobility of p16. Third, binding of several different lectins to p16 failed although p16-specific antibodies easily recognized the protein in immunoblots. Fourth, the proposed alternative glycosylation sites, N-I-Y or L-N-S-L-S, were not used by the eukaryotic glycosylation machinery even when they were mutated into typical consensus sequences for N glycosylation (N-X-T). Our results differ from previously published data (14, 15, 31, 32). In addition to lectin blots, endoglycosidase treatment, and in vitro transcription-translation assays, we mutagenized the putative N-glycosylation sites and solved the glycosylation problem definitely.
The question of why previous experiments indicated an N-glycosylated p16 remains. A plausible explanation is that BDV p16 from brain material or different cell cultures has been basically subjected to the same procedure described by Schädler and colleagues (24), which does not achieve pure p16. Therefore, these p16 preparations may contain impurities from cell material, such as carbohydrates or proteoglycans, which comigrated with p16 on gels during electrophoresis and, thus, caused carbohydrate staining (15). The coinciding of p16 and carbohydrate does not imply that the carbohydrate is covalently linked to p16. When monospecific antibodies are raised against p16 together with carbohydrates, they will recognize not only p16 but also carbohydrates. This may be one reason why p16 was accounted a glycoprotein. This false conclusion has serious consequences: the misinterpretation of p16 as an N-glycosylated matrix protein, the occurrence of p16 on the virus surface, and the misleading interpretation that antibodies to p16 have neutralizing activity (14, 15, 30–32). A recent publication (11) shows that neutralizing activity for BDV infection was found only with monoclonal antibodies raised against gp94, not with those against p16, which were raised against recombinant BDV proteins. Moreover, previous immunocytochemical investigations which used antibodies directed to carbohydrate-contaminated p16 need to be reexamined.
A putative topological analysis of p16 was performed by computer-aided programs, which dissected p16 in hydrophobic, predominantly uncharged domains and hydrophilic domains (EMBL-HUSAR, Heidelberg, Germany). The amino acid sequences between positions 14 and 32 and between positions 71 and 93 present hydrophobic regions with a certain probability for transmembrane helices (Fig. 1). Transmembrane-anchored p16 would implicate the exposition of at least one hydrophobic domain, resulting in the exposure of a peptide containing at least several amino acids on the surface of BDV-infected cells or virus particles. Biotinylation analysis failed to reveal surface-expressed domains of p16, although several ɛ-amino groups of lysine residues, which are the target of biotinylation, are present in p16 (data not shown here). Thus, these findings do not support the concept that a peptide domain of p16 is accessible on the virus surface. Moreover, the flotation density gradients of membranes from BDV-infected cells exclude a hydrophobic transmembrane domain as an anchor for p16 in a lipid bilayer. The data indicate, however, that the 16-kDa protein is associated with the internal side of the viral envelope.
The homologous matrix proteins of influenza virus and vesicular stomatitis virus which have significantly higher molecular masses than BDV p16 are synthesized as soluble proteins, which are later on in part tightly associated with membranes; they cannot be detached from these membranes either by addition of KCl or EDTA or by high-pH treatment (4, 17). In contrast, the BDV matrix protein p16 can be removed from membranes by treatments with KCl or at high pH, but with variable efficiencies. Whether hydrophobic peptide domains within p16 interact with membranes is therefore not clear yet. Additional studies using methods which can identify weak protein-membrane interactions are needed. Interestingly, specific hydrophobic peptide domains are apparently not a prerequisite for membrane binding of viral matrix proteins, as shown for the influenza matrix protein (17).
In conclusion, the protein of the ORF III of BDV is in structural and most likely functional analogy with the matrix proteins of all other members of Mononegavirales. It represents a nonglycosylated membrane-associated viral protein of 16 kDa in size, and thus, it is the smallest matrix protein among the mammalian negative-stranded RNA viruses. The architecture of the BDV envelope will be better understood when the final atomic structure of BDV p16 is available.
ACKNOWLEDGMENTS
We are very grateful to R. Rott (Giessen) and H.-D. Klenk (Marburg) for their interest, helpful discussions, and critical reading of the manuscript. Microsomal membranes were kindly provided by B. Dobberstein and M. Froeschke (Zentrum Molekulare Biologie, Heidelberg). Peptides were synthesized and kindly provided by M. Krause (Institut für Molekularbiologie und Tumorforschung).
This work was supported by the Deutsche Forschungsgemeinschaft, SFB 286 (W.G.), SFB 535 (J.A.R.), and Ga282/3-1 (W.G.).
REFERENCES
- 1.Briese T, de la Torre J C, Lewis A, Ludwig H, Lipkin W I. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci USA. 1992;89:11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briese T, Schneemann A, Lewis A J, Park Y S, Kim S, Ludwig H, Lipkin W I. Genomic organization of Borna disease virus. Proc Natl Acad Sci USA. 1994;91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroll M W, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology. 1997;238:198–211. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- 4.Chong L D, Rose J K. Membrane association of functional vesicular stomatitis virus matrix protein in vivo. J Virol. 1993;67:407–414. doi: 10.1128/jvi.67.1.407-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cubitt B, Oldstone C, de la Torre J C. Sequence and genome organization of Borna disease virus. J Virol. 1994;68:1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cubitt B, de la Torre J C. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J Virol. 1994;68:1371–1381. doi: 10.1128/jvi.68.3.1371-1381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cubitt B, Oldstone C, Valcarcel J, de la Torre J C. RNA splicing contributes to the generation of mature mRNAs of Borna disease virus, a non-segmented negative strand RNA virus. Virus Res. 1994;34:69–79. doi: 10.1016/0168-1702(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 8.Cubitt B, Ly C, de la Torre J C. Identification and characterization of a new intron in Borna disease virus. J Gen Virol. 2001;82:641–646. doi: 10.1099/0022-1317-82-3-641. [DOI] [PubMed] [Google Scholar]
- 9.De la Torre J C. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dettenhofer M, Yu X F. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J Virol. 1999;73:1460–1467. doi: 10.1128/jvi.73.2.1460-1467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furrer E, Bilzer T, Stitz L, Planz O. Neutralizing antibodies in persistent Borna disease virus infection: prophylactic effect of gp94-specific monoclonal antibodies in preventing encephalitis. J Virol. 2001;75:943–951. doi: 10.1128/JVI.75.2.943-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Dunia D, Cubitt B, Grasser F A, de la Torre J C. Characterization of Borna disease virus p56 protein, a surface glycoprotein involved in virus entry. J Virol. 1997;71:3208–3218. doi: 10.1128/jvi.71.4.3208-3218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas B, Becht H, Rott R. Purification and properties of an intranuclear virus-specific antigen from tissue infected with Borna disease virus. J Gen Virol. 1986;67:235–241. doi: 10.1099/0022-1317-67-2-235. [DOI] [PubMed] [Google Scholar]
- 14.Hatalski C G, Kliche S, Stitz L, Lipkin W I. Neutralizing antibodies in Borna disease virus-infected rats. J Virol. 1995;69:741–747. doi: 10.1128/jvi.69.2.741-747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kliche S, Briese T, Henschen A H, Stitz L, Lipkin W I. Characterization of a Borna disease virus glycoprotein, gp18. J Virol. 1994;68:6918–6923. doi: 10.1128/jvi.68.11.6918-6923.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohno T, Goto T, Takasaki T, Morita C, Nakaya T, Ikuta K, Kurane I, Sano K, Nakai M. Fine structure and morphogenesis of Borna disease virus. J Virol. 1999;73:760–766. doi: 10.1128/jvi.73.1.760-766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kretzschmar E, Bui M, Rose J K. Membrane association of influenza virus matrix protein does not require specific hydrophobic domains or the viral glycoproteins. Virology. 1996;220:37–45. doi: 10.1006/viro.1996.0283. [DOI] [PubMed] [Google Scholar]
- 18.Malik T H, Kishi M, Lai P K. Characterization of the P protein-binding domain on the 10-kilodalton protein of Borna disease virus. J Virol. 2000;74:3413–3417. doi: 10.1128/jvi.74.7.3413-3417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mañes S, Mira E, Gómez-Moutón C, Lacalle R A, Keller P, Labrador J P, Martínez A C. Membrane raft microdomains mediated front-rear polarity in migrating cells. EMBO J. 1999;18:6211–6220. doi: 10.1093/emboj/18.22.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowotny N, Kolodziejek J, Jehle C O, Suchy A, Staeheli P, Schwemmle M. Isolation and characterization of a new subtype of Borna disease virus. J Virol. 2000;74:5655–5658. doi: 10.1128/jvi.74.12.5655-5658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauli G, Ludwig H. Increase of virus yields and releases of Borna disease virus from persistently infected cells. Virus Res. 1985;2:29–33. doi: 10.1016/0168-1702(85)90057-7. [DOI] [PubMed] [Google Scholar]
- 22.Richt J A, Clements J E, Herzog S, Pyper J, Wahn K, Becht H. Analysis of virus-specific RNA species and proteins in Freon-113 preparations of the Borna disease virus. Med Microbiol Immunol. 1993;182:271–280. doi: 10.1007/BF00579625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richt J A, Fürbringer T, Koch A, Pfeiffer I, Herden C, Bause-Niedrig I, Garten W. Processing of the Borna disease virus glycoprotein gp94 by the subtilisin-like endoprotease furin. J Virol. 1998;72:4528–4533. doi: 10.1128/jvi.72.5.4528-4533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schädler R, Diringer H, Ludwig H. Isolation and characterization of a 14500 molecular weight protein from brains and tissue cultures persistently infected with Borna disease virus. J Gen Virol. 1985;66:2479–2484. doi: 10.1099/0022-1317-66-11-2479. [DOI] [PubMed] [Google Scholar]
- 25.Schneider P A, Hatalski C G, Lewis A J, Lipkin W I. Biochemical and functional analysis of the Borna disease virus G protein. J Virol. 1997;71:331–336. doi: 10.1128/jvi.71.1.331-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider P A, Kim R, Lipkin W I. Evidence for translation of the Borna disease virus G protein by leaky ribosomal scanning and ribosomal reinitiation. J Virol. 1997;71:5614–5619. doi: 10.1128/jvi.71.7.5614-5619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwemmle M, Salvatore M, Shi L, Richt J, Lee C H, Lipkin W I. Interactions of the Borna disease virus P, N, and X proteins and their functional implications. J Biol Chem. 1998;273:9007–9012. doi: 10.1074/jbc.273.15.9007. [DOI] [PubMed] [Google Scholar]
- 28.Schwemmle M, Jehle C, Formella S, Staeheli P. Sequence similarities between human bornavirus isolates and laboratory strains question human origin. Lancet. 1999;354:1973–1974. doi: 10.1016/S0140-6736(99)04703-0. [DOI] [PubMed] [Google Scholar]
- 29.Staeheli P, Sauder C, Hausmann J, Ehrensperger F, Schwemmle M. Epidemiology of Borna disease virus. J Gen Virol. 2000;81:2123–2135. doi: 10.1099/0022-1317-81-9-2123. [DOI] [PubMed] [Google Scholar]
- 30.Stoyloff R, Briese T, Borchers K, Zimmermann W, Ludwig H. N-glycosylated protein(s) are important for the infectivity of Borna disease virus (BDV) Arch Virol. 1994;137:405–409. doi: 10.1007/BF01309486. [DOI] [PubMed] [Google Scholar]
- 31.Stoyloff R, Strecker A, Bode L, Franke P, Ludwig H, Hucho F. The glycosylated matrix protein of Borna disease virus is a tetrameric membrane-bound viral component essential for infection. Eur J Biochem. 1997;246:252–257. doi: 10.1111/j.1432-1033.1997.t01-2-00252.x. [DOI] [PubMed] [Google Scholar]
- 32.Stoyloff R, Bode L, Borchers K, Ludwig H. Neutralization of Borna disease virus depends upon terminal carbohydrate residues alpha-d-man, beta-d-GlcNAc of glycoproteins gp17 and gp94. Intervirology. 1998;41:135–140. doi: 10.1159/000024926. [DOI] [PubMed] [Google Scholar]
- 33.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker M P, Jordan I, Briese T, Fischer N, Lipkin W I. Expression and characterization of the Borna disease virus polymerase. J Virol. 2000;74:4425–4428. doi: 10.1128/jvi.74.9.4425-4428.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wehner T, Ruppert A, Herden C, Frese K, Becht H, Richt J A. Detection of a novel Borna disease virus-encoded 10 kDa protein in infected cells and tissues. J Gen Virol. 1997;78:2459–2466. doi: 10.1099/0022-1317-78-10-2459. [DOI] [PubMed] [Google Scholar]
- 36.Wolff T, Pfleger R, Wehner T, Reinhardt J, Richt J A. A short leucine-rich sequence in the Borna disease virus p10 protein mediates association with the viral phospho- and nucleoproteins. J Gen Virol. 2000;81:939–947. doi: 10.1099/0022-1317-81-4-939. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann W, Breter H, Rudolph M, Ludwig H. Borna disease virus: immunoelectron microscopic characterization of cell-free virus and further about the genome. J Virol. 1994;68:6755–6758. doi: 10.1128/jvi.68.10.6755-6758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]