Abstract
Microspheres have long been used in drug delivery applications because of their controlled release capabilities. They have increasingly served as the fundamental building block for fabricating scaffolds for regenerative engineering because of their ability to provide a porous network, offer high-resolution control over spatial organization, and deliver growth factors/drugs and/or nanophase materials. Because they provide physicochemical gradients via spatiotemporal release of bioactive factors and nanophase ceramics, microspheres are a desirable tool for engineering complex tissues and biological interfaces. In this review we describe various methods for microsphere fabrication and sintering, and elucidate how these methods influence both micro- and macroscopic scaffold properties, with a special focus on the nature of sintering. Furthermore, we review key applications of microsphere-based scaffolds in regenerating various tissues. We hope to inspire researchers to join a growing community of investigators using microspheres as tissue engineering scaffolds so that their full potential in regenerative engineering may be realized.
Keywords: microspheres, microsphere incorporating scaffolds, microsphere-based scaffolds, microsphere fabrication, microsphere sintering
1. INTRODUCTION
Many scaffold fabrication techniques such as solvent casting, particulate leaching, phase separation, electrospinning, fiber mesh generation, fiber bonding, and additive manufacturing are utilized for tissue engineering applications (1, 2). Microsphere-based scaffold fabrication techniques have attracted attention because these scaffolds may provide excellent initial mechanical properties and allow for controlled release of bioactive molecules to promote tissue regeneration (3). Microspheres are organic or inorganic, spherical, free-flowing particles ranging from 1 to 1,000 μm in diameter that may encapsulate drugs or bioactive molecules. They have been extensively used in drug delivery/targeting applications largely because of their ability to enhance the efficacy of the encapsulated drug by providing both a large surface area–to–volume ratio for drug release and spatial and temporal control over release. In addition to their ability to serve as excellent controlled release vehicles, microspheres are rigid in shape and can be packed together, alone, or in combination with other materials to yield porous three-dimensional (3D) structures that can serve as tissue engineering scaffolds. Scaffolds with microspheres can broadly be divided into two categories: (a) microsphere-incorporating scaffolds and (b) microsphere-based scaffolds. In the former, the microspheres merely serve as one component of the scaffold, whereas in the latter, they are the building blocks of the scaffold framework. Microsphere-based scaffolds are subclassified as either injectable or sintered.
Microsphere-incorporating scaffolds comprise microspheres dispersed into a continuous phase, such as solid polymers or hydrogels. For additional information about hydrogels and hydrogel microspheres, see Reference 4. The incorporated microspheres (a) provide control over the release of proteins/peptides (5–7), nucleotides (8, 9), and antimicrobials (10, 11); (b) deliver bioactive molecules in response to environmental stimuli such as temperature and pH (12, 13); (c) act as miniature bioreactors embedded in a surrounding matrix to create a sectionalized environment for intrinsically complex tissue regeneration (13–15); (d) serve as cell transporters (16); (e) generate a network of pores in the interior of a scaffold to facilitate cellular ingrowth and to accelerate scaffold resorption (17–19); and (f) impart mechanical support to an otherwise weak scaffold matrix (20, 21). For further information about the use of microspheres in microsphere-incorporating scaffolds, see Reference 13. Despite their several advantages over conventional bulk scaffolds, including but not limited to spatiotemporal control over release of bioactive factors and enhanced structural/mechanical properties, microsphere-incorporating scaffolds possess some limitations. Microsphere-incorporating scaffolds are generally fabricated in a multistep process via a top-down approach involving separately creating bulk scaffold matrix and microspheres, and then loading the premade matrix with microspheres to create a final construct. This approach presents challenges with regard to control over biomolecule delivery, cell infiltration and viability within the scaffold matrix, and clinical handling (13, 22). For these reasons, there is a motivation for considering scaffolds composed exclusively of microspheres—that is, microsphere-based scaffolds—instead of merely including the microspheres as one ingredient in another scaffold.
To overcome the drawbacks associated with the top-down approach to the fabrication of microsphere-incorporating scaffolds, a bottom-up approach in which microspheres themselves are the building blocks has become increasingly popular. In 1998, the Laurencin group (20) became the first to report the use of microsphere-based scaffolds for a bone tissue engineering application. Since then, a few dozen research groups have employed microsphere-based scaffolds to regenerate a variety of tissues, and over the past 5 to 6 years, the progress in the microsphere-based scaffold field has been especially rapid, with more than 15 publications coming out per year on the use of these scaffolds (according to a Web of Science database search using the keywords “microsphere based scaffolds”). Several reviews (13, 23–27) have highlighted the use of microspheres as drug delivery agents and cell carriers in microsphere-incorporating scaffolds; however, the field lacks a review that is focused entirely on microsphere-based scaffolds. Wang et al. (13) described the use of microsphere-based scaffolds for bone tissue engineering and outlined the fabrication strategies utilized to synthesize these scaffolds. In a 2011 review, Shi et al. (3) discussed the use of “sintered” microsphere-based scaffolds fabricated via heat and solvent sintering in drug delivery and tissue engineering. Huang et al. (28) examined the use of “sintered” microsphere-based scaffolds for bone tissue engineering applications, discussing different material approaches to the fabrication of such scaffolds. These reviews have underscored the merits of microsphere-based scaffolds in bone regeneration and demonstrated a strong foundation for heat/solvent sintering and for material selection. However, there remains a need for a review that summarizes the numerous available methods for microsphere fabrication/sintering and how these methods affect microsphere and scaffold properties, thereby serving as a valuable guide for the design of microsphere-based scaffolds for diverse clinical needs.
The motivation for this review is that in the quest for scaffolds with high functionality and versatility, microsphere-based scaffolds demonstrate many benefits and have been underutilized as a valuable tool in regenerative engineering. Moreover, the variety of methods available to fabricate microspheres, in addition to the advantages and disadvantages of different sintering methods, can be difficult for an investigator new to the field to navigate. However, microspheres can be made from a plethora of materials via several different methods, injected through most clinical needles, and assembled into a variety of geometries by use of various sintering approaches. Moreover, the methods for microsphere fabrication and sintering are reasonably simple, inexpensive, and in many instances scalable for mass production. Therefore, we encourage scientists in both industry and academia to make use of these microsphere-based scaffolds in repairing a multitude of tissues so that their full potential may be achieved.
2. MICROSPHERE-BASED SCAFFOLDS
Microspheres can be assembled into microsphere-based scaffolds in one of three main packing strategies: random packing, directed assembly, and rapid prototyping (RP) (13). Random packing, as the name suggests, involves the assembly of microspheres in a nonspecific manner, thus allowing for customization of scaffold properties to the microsphere level. Directed assembly of microspheres involves establishing cohesive forces such as electrostatic forces (29), magnetic forces (30), or hydrophobic interactions (31). RP permits layer-by-layer assembly of microsphere-based scaffolds via computer-aided design (CAD) to create scaffolds with precisely tailored architecture (32). Regardless of the packing strategy used to fabricate microsphere-based scaffolds, all microsphere-based scaffolds, at least initially, possess a pore network with 100% interconnectivity due to the nature of sphere packing (33).
Microsphere-based scaffolds can be classified as either (a) injectable or (b) sintered scaffolds. The injectable microsphere-based scaffold exists as a liquid suspension, which acquires the shape of the defect upon implantation inside the body. By contrast, microspheres in a sintered microsphere-based scaffold are fused together to form an integrated “premade” macroscopic scaffold. The spherical nature of microspheres allows injectable microsphere-based scaffolds to be developed as moldable formulations such as suspensions, colloids, and gels that can be administered through most clinical needles in minimally invasive surgery (34–39). A variety of polymers, both natural and synthetic, such as collagen (34), chitosan (36), alginate (38), and poly(lactic-co-glycolic acid) (PLGA) (35, 37, 39), have been used to fabricate injectable microsphere-based scaffolds for engineering different tissue types such as bone (34, 36–38), cartilage (39), and liver (35). Microsphere-based injectable scaffolds may possess superior controlled release and structural properties over other injectable scaffolds such as hydrogels or pastes; however, similar to other injectable scaffolds, they may also be susceptible to migration from defect sites upon implantation as a result of weak interparticle interactions (13). Therefore, glues (40) or cross-linking agents (41) have been investigated to prevent microspheres from flowing out of the defect site, but these agents may be cytotoxic. Directed packing of microspheres by introducing attractive forces among the microspheres provides an appealing alternative; however, directed packing usually requires functionalization of the microspheres that can sometimes be challenging.
By contrast, sintered microsphere-based scaffolds are premade into a specific shape by coalescing the individual microspheres; therefore, these scaffolds do not suffer from the limitation of leaking out from the defect upon implantation. Moreover, sintered microsphere-based scaffolds may be implanted inside the body arthroscopically via a specialized delivery device (42). Numerous studies involving sintered microsphere-based scaffolds have validated their capabilities for controlled release of bioactive factors (43, 44), biocompatibility (45, 46), and potential for tissue regeneration (47, 48).
3. MICROSPHERE FABRICATION METHODS
In this section, we describe three main methods—namely the emulsion-solvent extraction method, precision particle fabrication (PPF), and thermally induced phase separation (TIPS)—used to fabricate microspheres for microsphere-based scaffolds. We also discuss various fabrication process parameters along with the advantages and disadvantages of each method.
3.1. Emulsion-Solvent Extraction Method
The emulsion-solvent extraction method is one of the oldest and most widely used methods for fabricating microspheres. Figure 1a depicts the various steps in the emulsion-solvent extraction method, along with its several variations. There are four major steps in any emulsion-solvent extraction method (49). The first step is dissolution of the microsphere-forming material, typically a polymer, in an organic solvent to create an “oil” phase. The oil phase may contain a bioactive molecule codissolved with the polymer to be encapsulated within the microspheres. In the second step, the oil phase is emulsified in a continuous “water” phase containing an emulsifier (or a surface-active stabilizer) using physical methods such as homogenization and sonication. The third step involves solvent extraction into the water phase, followed by solvent evaporation. The solvent can further be extracted out of the polymer droplets by use of a second solvent that is miscible with an organic solvent but does not dissolve the polymer (50). Owing to the loss of the solvent in the extraction step, the oil phase is enriched in the polymer until the droplets “harden” to become microspheres. In the last step, the “hardened” microspheres are filtered, washed, and lyophilized. On the basis of the number of emulsions used in the microsphere fabrication, the emulsion-solvent extraction method can be classified as either a single emulsion-solvent extraction method (33) or a double emulsion-solvent extraction method (51). On one hand, the single emulsion method involves the creation of only one emulsion by emulsifying the oil phase (possibly with a bioactive molecule) in the “water” phase. On the other hand, the double emulsion method involves first creating a primary emulsion by emulsifying an aqueous solution, which generally contains a bioactive molecule to be encapsulated, in the oil phase, followed by emulsification of the primary emulsion in the water phase to create a second emulsion.
Figure 1.
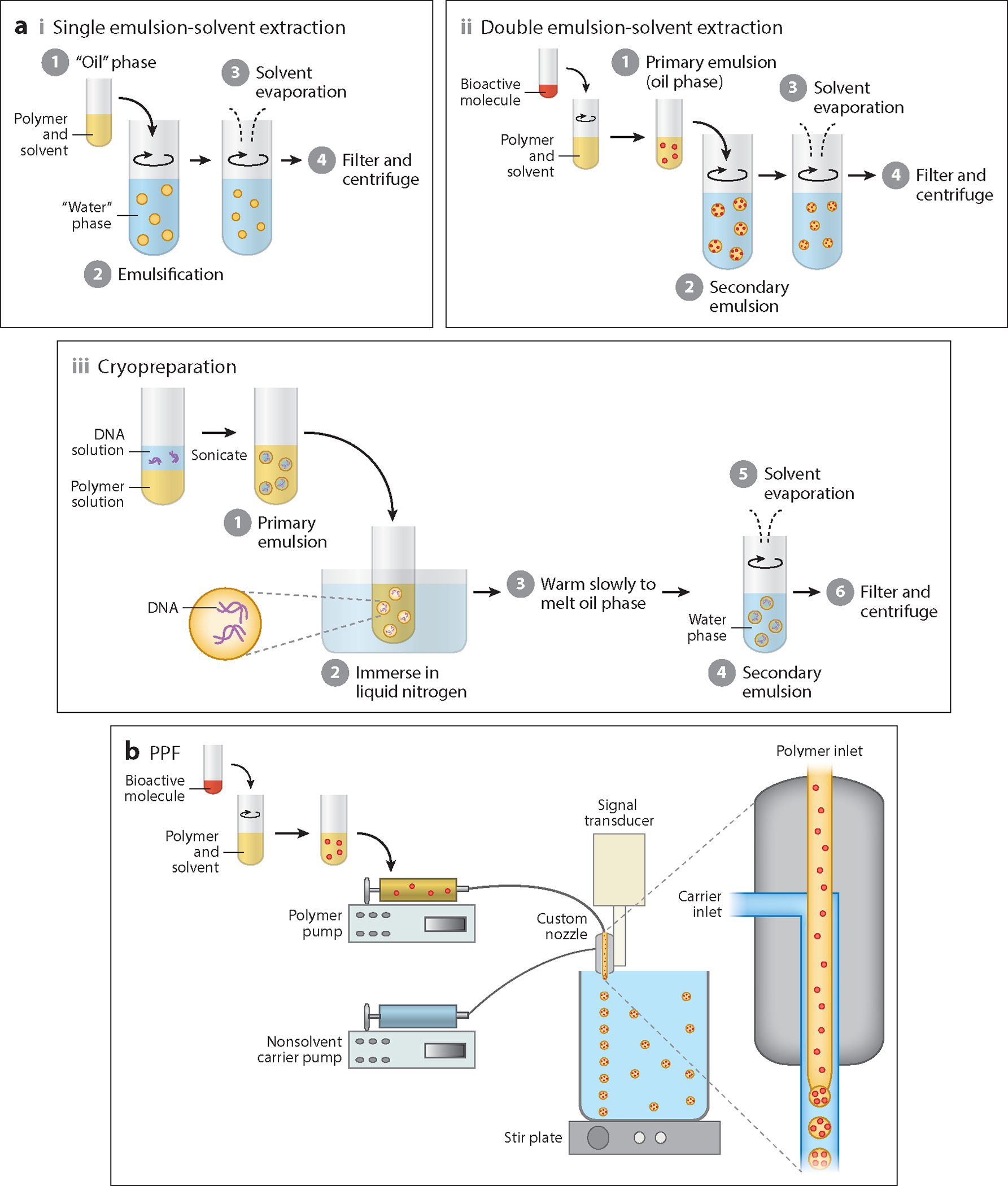
Microsphere fabrication via (a) emulsion-solvent extraction and (b) precision particle fabrication (PPF) methods. (a) The major steps in an emulsion-solvent extraction method along with its several variations: (i) single emulsion, (ii) double emulsion, and (iii) cryopreparation. (b) Schematic of a PPF setup consisting of a custom-designed dual nozzle (expanded view shown on the right) and an acoustic excitation device.
Microspheres with smooth, rough, and porous surface morphologies can be fabricated via the emulsion-solvent extraction method. A smooth morphology is obtained when a homogeneous oil phase is emulsified into the water phase during microsphere fabrication. By contrast, Jiang et al. (52) fabricated rough PLGA microspheres encapsulating chitosan that imparted an uneven surface to the otherwise-smooth PLGA microspheres. Chitosan has a tendency to disperse in water; thus, during the solvent extraction and microsphere hardening steps, only a small amount of chitosan was incorporated into the interior of the microspheres while the rest partitioned onto the microsphere surface, giving it a rough appearance. The incorporation of an inorganic mineral such as hydroxyapatite (HAp) in other emulsion-solvent extraction fabrication methods (53) also led to rough microsphere surfaces. Hong et al. (54) prepared porous polycaprolactone (PCL) microspheres by introducing camphene as a porogen in the oil phase. During the microsphere hardening step, the camphene sublimed, leaving a porous structure within the PCL microspheres. To fabricate PLGA microspheres with a porous morphology, Chou et al. (35) used ammonium bicarbonate as an effervescent agent in a modified double emulsion-solvent extraction method. Ammonium bicarbonate was added to the internal aqueous phase of the primary emulsion. When the double emulsion was created, the ammonium bicarbonate decomposed to form water, carbon dioxide (CO2), and ammonia upon contact between the primary emulsion droplets and the continuous water phase, thus creating an open porous morphology within the microspheres.
The conventional emulsion-solvent extraction method of fabricating DNA-encapsulated microspheres suffers from poor encapsulation efficiency and low loading because of the difficulty of encapsulating such large hydrophilic molecules in a hydrophobic polymer (55). Moreover, shear forces during homogenization can cause the DNA to lose its native supercoiled state, causing loss of bioactivity. Therefore, cryopreparation was developed as an improvement over the traditional double emulsion-solvent extraction method to encapsulate DNA into microspheres (8, 56). Cryopreparation involves lowering the temperature of the DNA-containing primary emulsion below the freezing point of the aqueous inner phase, resulting in a solid particulate suspension. The primary emulsion is then allowed to warm slowly until it reaches a temperature at which the oil phase melts while the aqueous inner phase remains frozen. Afterward, the primary emulsion is homogenized in the continuous water phase to form microspheres. Because the DNA is still entrapped in the frozen aqueous droplets within the primary emulsion, it does not experience shear forces during homogenization (55). Jang & Shea (8) fabricated PLGA microspheres encapsulating plasmid DNA using cryopreparation and reported that the integrity of the encapsulated plasmid was similar to that of the unincorporated DNA.
For the microspheres fabricated through the emulsion-solvent extraction method, microsphere size distribution is determined by the droplet-formation step in the fabrication process (49). The mixing speed during homogenization is the main parameter governing the droplet size and consequently the microsphere size in the continuous phase (49). Increasing the mixing speed results in stronger shear forces and increased turbulence, causing the emulsion to break into smaller droplets and thereby decreasing microsphere size. Additionally, more vigorous mixing results in lower microsphere polydispersity. Other factors such as the viscosity of the dispersed oil and continuous water phases, the interfacial tension between the two phases, and their volume ratio further influence the extent of reduction in size during microsphere fabrication (49). Increasing the viscosity of the dispersed oil phase by using higher concentrations of polymer increases the diameter of the microspheres, as higher shear forces are needed to break the dispersed phase into droplets. Increasing the stabilizer concentration, added during the emulsification step to prevent coalescence of oil-phase droplets, decreases the microsphere size as more stabilizer is available to adsorb on the surface of newly formed polymer droplets, thus preventing their coalescence. There is some disagreement among investigators regarding the effect of the volume ratio of dispersed phase and continuous phase on microsphere size, as some groups have observed a decrease in microsphere size with decrease in volume of the continuous phase, whereas others have observed no such effect (49). Multiple factors, such as miscibility of the dispersed and continuous phases and the osmotic effect due to encapsulation of proteins, may counterbalance the reduction in size caused by solvent extraction, thus leading to a discrepancy in the observed outcomes of volume ratio of the two phases on microsphere size.
A major advantage of fabricating microspheres using the emulsion-solvent extraction method is that it does not require any specialized equipment; a simple beaker and a stirrer are sufficient. This technique can be tailored to produce microspheres over a wide size range, and choosing a suitable solvent (or solvents) allows a broad spectrum of bioactive molecules to be encapsulated into a vast number of microsphere materials (57). However, the emulsion-solvent extraction method suffers from some limitations. It is a batch operation, making scale-up difficult, which further increases the cost of large-scale production of microspheres (55). Additionally, microsphere size distributions are often poorly controllable with the emulsion-solvent extraction method; typical standard deviations of mean diameter are 25–50% of the target size (58). Such high variations in microsphere size can have a profound effect on the release of encapsulated molecules and the injectability of microspheres. Another major disadvantage of the emulsion-solvent extraction method is the relatively poor encapsulation efficiency of water-soluble bioactive molecules (such as proteins), primarily due to the need to expose the hardening microspheres to an aqueous continuous phase for several hours to extract the solvent. The prolonged hardening step causes proteins to agglomerate at the oil–water interface and to be more prone to diffusion into the aqueous continuous phase (58). Moreover, the use of organic solvents may have adverse effects on the encapsulated factors, and there is a concern of toxicity of the residual organic solvents.
3.2. Precision Particle Fabrication
PPF was first introduced by Berkland et al. (59) in 2001, and is an improvement over the emulsion-solvent extraction method in that it allows for precise control over the size of the microspheres while maintaining narrow size distributions (50, 59). Figure 1b shows a schematic of a PPF setup consisting of a custom-designed dual nozzle and an acoustic excitation device (59–61). The solution containing the microsphere material, likely with a bioactive molecule, is pumped through a small inner nozzle to form a smooth cylindrical jet. The jet is acoustically excited with a piezoelectric transducer driven by a frequency generator to controllably break it into uniform droplets. To further control the microsphere size, a “carrier” nonsolvent stream is flowed concentrically around the inner jet through an outer nozzle. The annular carrier stream is pumped at a rate greater than that of the inner polymer jet; thus, frictional contact between the two streams generates an additional downward force that enables greater control over the size of the fabricated microspheres (59). The emanated polymer/carrier streams are flowed into a beaker containing the nonsolvent carrier, and the solvent is allowed to evaporate from the incipient polymer droplets to form microspheres, which are then filtered, washed, and lyophilized.
The size of the microspheres can be varied by controlling process parameters such as flow rates of the polymer and carrier streams and the frequency and amplitude of vibration (59). Increasing the polymer flow rate increases the diameter of the fabricated microspheres, and increasing either the carrier flow rate or the vibration frequency decreases the diameter of the microspheres. Due to the similarity between PPF and the emulsion-solvent extraction method, factors such as polymer concentration and stabilizer concentration can also influence the size and morphology of the fabricated microspheres (49, 59).
Although used primarily with synthetic polymers (59–61), PPF can be employed to fabricate microspheres from natural polymers (62) with some modifications. The greatest advantage of PPF over the conventional emulsion-solvent extraction method is that it offers unprecedented control over microsphere size and distribution. Microspheres fabricated via PPF have a standard deviation that is less than 10% of the target mean diameter of microspheres in the size range 75–300 μm (59). Furthermore, microspheres fabricated in independent batches under the same conditions are indistinguishable from one another (59). However, PPF suffers from some limitations, such as increased polydispersity with microspheres of small size (<5 μm) and the need for more complex apparatus and control systems in comparison to the traditional emulsion-solvent extraction method.
3.3. Thermally Induced Phase Separation
TIPS is a technique that has been widely used for fabricating monolithic tissue engineering scaffolds (63). Blaker et al. (58) were the first to use TIPS for microsphere fabrication. In TIPS, the microsphere-forming material—typically a polymer—is first dissolved in a solvent, possibly alongside a bioactive molecule that is either dispersed in the particulate form or emulsified via an aqueous solution. The polymer and bioactive molecule solution/emulsion is then added dropwise into liquid nitrogen via either a syringe/needle (58) or a piezoelectric nozzle (63). The lowering of temperature of the polymer solution induces phase separation to a stage in which the polymer solution separates into a polymer-rich phase and a polymer-lean phase. Further lowering of the temperature to below the freezing point of the solvent crystallizes the solvent, expelling the polymer and forming a continuous polymeric phase around the solvent crystals (63). The solvent is then removed by sublimation or leaching to yield microspheres with a characteristic porous morphology.
Parameters such as polymer concentration, solvent composition, cooling rate, and incorporation of bioactive factors or nanophase materials affect the size and morphology of the fabricated microspheres (64). Decreasing the polymer concentration reduces the viscosity of the polymer solution, which further decreases the size of the microspheres. Moreover, decreasing the polymer concentration is expected to increase the porosity of the microspheres due to the formation of a thinner polymer-rich phase during phase separation, which has been reported for polymeric foams fabricated through TIPS (65). The solvent composition also influences the microstructure of the pores within the microspheres. Addition of a nonsolvent to the solvent reduces the solubility of the polymer, with liquid–liquid phase separation occurring prior to solvent crystallization, resulting in a more disrupted pore structure (58). The cooling temperature and the cooling rate also influence the morphology of the fabricated microspheres. Lower cooling temperatures (e.g., −196°C) reduce the pore size within the microspheres by providing a faster cooling rate and a shorter time for solvent nucleation, crystal growth, and phase separation (64, 66). Blaker et al. (58) observed that with the incorporation of bioactive glass in PLGA microspheres, the pore structure became progressively less well ordered with increasing bioglass content, due to perturbation of the crystallizing solvent by the bioactive glass particles. Furthermore, other processing parameters, such as the size of the needle orifice or the vibration frequency of the piezoelectric nozzle, control the size of the microspheres, with narrower needles and higher frequencies resulting in smaller microspheres (58, 63).
TIPS is a versatile and scalable technique that can be used to fabricate microspheres in commercial quantities from both synthetic (58, 63) and natural (63, 66, 67) polymers. Microspheres fabricated via TIPS have a characteristic porous morphology that can be tailored by altering various process conditions or variables. Encapsulation of bioactive molecule using the TIPS method is rapid; the amount of time the molecule is exposed to (nonfrozen) solvent is minutes rather than hours, minimizing the adverse effects of prolonged exposure of solvent to the bioactive molecule (58). TIPS allows more control over the microsphere diameter compared with the emulsion-solvent extraction method in terms of droplet-generation parameters such as needle size and vibration frequency of the piezoelectric nozzle. Although TIPS possesses several advantages, it suffers from certain limitations. The method is time-consuming because it entails multiple processing stages. Additionally, the microspheres often coalesce with one another during fabrication, resulting in the formation of undesirable large microspheres that can skew the size distribution of the microspheres thus fabricated. Although a porous structure is likely to facilitate cellular infiltration into the microspheres, it also reduces the mechanical strength of the microspheres (67).
3.4. Summary of Microsphere Fabrication Methods
Table 1 summarizes the merits and drawbacks associated with each microsphere fabrication method, along with each method’s various process parameters that influence microsphere characteristics. Emulsion-based methods are relatively simple and can be used to fabricate microspheres from a variety of materials; however, they yield microspheres with nonuniform size and broad size distribution. By contrast, techniques such as PPF and TIPS can be used to fabricate microspheres with relatively uniform size and narrow size distribution, but they are complex and require special apparatuses with associated capital cost. Moreover, the processing parameters significantly affect the characteristics of the fabricated microspheres. All of these considerations must be taken into account when deciding on a microsphere fabrication method for creating microsphere-based scaffolds.
Table 1.
Merits and drawbacks associated with microsphere fabrication methods along with their various process parameters that influence microsphere characteristics
| Microsphere fabrication method | Process parameters that influence microsphere properties | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Emulsion-solvent extraction | Homogenization speed Viscosity/concentration and composition of the “oil” phase Stabilizer concentration Volume ratio of “oil” and “water” phases |
Simple method that can be easily set up Can be easily tailored to intended application Can be used to encapsulate large hydrophilic molecules such as DNA |
Batch operation increases the cost of commercial production of microspheres Poor control over microsphere size Yields microspheres with high polydispersity Poor encapsulation efficiency of hydrophilic bioactive factors Residual solvent toxicity |
20, 33, 35, 51, 56 |
| Precision particle fabrication (PPF) | Viscosity/concentration and composition of the oil phase Stabilizer concentration Polymer flow rate Carrier flow rate Vibration frequency |
Fabricated microspheres have low polydispersity Reproducibility among batches Allows precise and instantaneous control over microsphere size May be used for natural materials (with some modifications) |
Requires complex apparatus and control systems Increase in polydispersity with small microsphere size |
59–61 |
| Thermally induced phase separation | Viscosity/concentration and composition of the oil phase Solvent composition Cooling temperature/rate Needle size (syringe/needle droplet formation) Nozzle size (piezoelectric nozzle droplet formation) Vibration frequency (piezoelectric nozzle droplet formation) |
Both synthetic and natural materials can be used Fabricated microspheres are inherently porous Rapid encapsulation More control over microsphere size compared with solvent extraction |
Laborious as it entails multiple steps Coalescence of microspheres is an issue Poor control over microsphere size compared with PPF |
58, 67 |
4. MICROSPHERE SINTERING METHODS
The greatest technical achievement in making the leap from microsphere-incorporating scaffolds to microsphere-based scaffolds, which is also one of the greatest challenges, has been the identification of methods to fuse microspheres together to create a single macroscopic unit, as well as to achieve and quantify a desired degree of sintering. Here, we review the available methods for fusing (or sintering) microspheres and their respective merits and drawbacks.
4.1. Heat Sintering
First described by Laurencin et al. (20, 33), the heat sintering method for fabricating sintered microsphere-based scaffolds has gained widespread attention (3, 28). The heat sintering method entails packing fabricated microspheres into a mold and then heating them to a specific temperature above the glass transition temperature (Tg) of the microsphere material for several hours. The heating melts the surface layer of the microspheres and induces them to bond with their proximate neighbors, thus forming a 3D porous scaffold (33).
The sintering temperature and sintering time are the two crucial factors influencing the mechanical properties and porosities of the heat sintered microsphere-based scaffolds (3, 52). A higher sintering temperature and a longer sintering time have equivalent effects on the properties of the scaffolds; an increase in either factor results in an elevated compressive modulus and compressive strength, a smaller pore size, and a decreased pore volume. This is because an elevated sintering temperature leads to greater fusion between the microspheres, contributing to an increase in the compressive properties of the scaffolds. At the same time, greater fusion of microspheres results in possible closure of the pores among them, decreasing the overall pore volume of the scaffolds.
The heat sintering method provides certain benefits for the fabrication of microsphere-based methods. The method is simple and efficient, as it requires moderate temperatures. Moreover, heat sintering offers flexible time constraints (on the order of several minutes). Lastly, a large number of scaffolds can be fabricated via heat sintering (in an oven) at one particular time (68). A major drawback of using heat sintering is that it makes the encapsulation of bioactive molecules difficult. The sintering temperatures and durations of heat exposure used in some previous studies were 75°C for 24 h (33) and 100°C for 4 h (52). Such high temperatures for extended durations may compromise the bioactivity of the encapsulated proteins and bioactive molecules.
4.2. Solvent-Based Methods
Solvent-based sintering is another widely used approach. Such methods are classified as (a) solvent vapor sintering (69, 70), (b) weak solvent sintering (43, 45, 48, 60, 61, 71–79), or (c) solvent/nonsolvent sintering (44, 80, 81).
4.2.1. Solvent vapor sintering.
The solvent vapor sintering scaffold fabrication process relies on diffusion of solvent vapors into the microspheres, which lowers the polymer Tg, thus softening the microspheres and allowing them to fuse (69, 70, 82). Various parameters such as fusion time, microsphere composition, and size affect the resulting scaffold structure and morphology. The rate-limiting step in solvent vapor sintering is the saturation of microspheres with solvent vapors. Once the microspheres are saturated, the subsequent reaction is fast; thus, overexposure to solvent vapors may lead to further dissolution of the polymer, causing closure of pores within the scaffold. Increases in scaffold mass result in longer sintering times as the process is governed by vapor diffusion into the microspheres, and added mass leads to longer diffusion times (70).
Solvent vapor sintering for microsphere-based scaffolds enables inclusion of proteins and bioactive molecules within the scaffolds (69). However, the method suffers from the limitation of strict time constraints (on the order of a few seconds). If a scaffold is left for too long during solvent sintering, it could result in significantly reduced porosity of the structure, thus compromising the mechanical and biological performance of the scaffold (68, 70). Moreover, the presence of residual solvent vapors could have deleterious effects on the encapsulated proteins and bioactive molecules if not adequately removed in subsequent processing.
4.2.2. Weak solvent sintering.
The Detamore and Berkland groups (43, 45, 48, 60, 61, 71–79) have pioneered the weak solvent sintering technique for microsphere sintering by utilizing a weak solvent of the microsphere material as the sintering agent. The process involves stacking of microspheres in a mold followed by treatment with the weak solvent. The solvent treatment causes the Tg of the microsphere material to drop (82), thereby softening the microspheres near the surface and generating a skin layer around them. These skin layers then adjoin, resulting in sintering of the adjacent microspheres (61).
The duration of the solvent soak is an important process parameter, as a longer duration leads to an increase in the degree of sintering, namely in the extent of interconnections between the microspheres, with longer exposure leading to increased deviation of the microspheres from a spherical morphology (61). Polymer properties such as molecular weight and crystallinity affect the required sintering time, as microspheres fabricated from a polymer with a higher molecular weight or higher crystallinity require more time to achieve comparable degrees of sintering (61). The compressive moduli of scaffolds in general increase with longer sintering durations due to an increased degree of sintering between the microspheres.
The weak solvent sintering method is also advantageous in that it allows for incorporation of bioactive molecules and proteins within the scaffolds without significantly altering their bioactivity (43). However, this method requires more time for scaffold fabrication compared with solvent vapor sintering, yet the time required for scaffold fabrication is still comparable to (often less than) the time required for heat sintering.
4.2.3. Solvent/nonsolvent sintering.
Solvent/nonsolvent microsphere sintering, also known as dynamic solvent sintering, involves the use of a solvent and a nonsolvent for the microsphere materials. The solvent and nonsolvent are selected such that the solvent is more volatile than the nonsolvent, both are miscible, and there exist no azeotropes across the range of solvent/nonsolvent ratios (80, 81). Dynamic solvent sintering is based on the concept of fractional solubility defined by Flory–Huggins solution theory, in which polymer dissolution and precipitation are well controlled by the solvent/nonsolvent composition (80). In this sintering method, the dynamic solvent wets the microsphere surface, causing the polymer chains to swell, loosen, and subsequently interact with polymer chains on the adjacent microsphere. The faster evaporation of the solvent compared with the nonsolvent results in precipitation of the polymer chains, causing chain interactions such as locking, entanglement, and intertwining to become permanent with precipitation, and eventually leading to microsphere sintering (81).
Parameters such as solvent/nonsolvent solution composition and sintering duration affect the scaffold characteristics (80). An increase in solvent concentration in the sintering solution reduces scaffold porosity and the pore diameter due to occlusion of pores caused by greater dissolution of the polymer. Moreover, an increase in solvent concentration up to a certain extent increases the scaffold elastic modulus due to an increase in bonding between the microspheres. Further increasing the solvent concentration results in a decrease in the scaffold modulus due to the loss of integrity of individual microspheres caused by extensive dissolution of the polymer (80).
An advantage of the solvent/nonsolvent sintering method is that it can be used to sinter microspheres from a wide variety of polymers, as it is less dependent on the physiochemical properties of a polymer. The method can be tailored to produce the desired degree of sintering by varying the concentration of the solvent/nonsolvent sintering solution. As with other solvent-based sintering methods, the solvent/nonsolvent sintering technique allows for preloading of bioactive factors within the scaffold for sustained release. The major limitation of the method is that it involves considerably large amounts of organic solvents, which if not completely removed can have undesirable effects on the seeded cells and can alter the biological activity of encapsulated proteins and bioactive factors.
4.3. Subcritical CO2 Sintering
The subcritical, dense-phase CO2 sintering method is a modification of the conventional gas-foaming process used for making porous tissue engineering scaffolds. Gas foaming involves saturating a polymer with CO2 at supercritical pressures (>73.8 bar) for several hours, followed by controlled depressurizing to cause nucleation of the gas and formation of pores in the polymer (83). In contrast, with dense-phase CO2 microsphere sintering, the equilibration of CO2 is restricted to lower pressures (15–25 bar) for short durations (around 1 h), leading to a comparatively reduced plasticized state restricted to the surface of the microspheres (83). This leads to retention of the microspheres’ spherical shape during the process, and the slight swelling of the microspheres’ surfaces and subsequent adhesion lead to sintering of the adjoining microspheres (83–86).
The CO2 pressure and exposure time are the primary factors controlling the properties of the scaffolds fabricated through subcritical CO2 sintering. Additionally, the rate of depressurization is an important factor that governs the basic morphology of the scaffolds. Low CO2 pressures (e.g., ≲15 bar) may lead to scaffolds with weak mechanical integrity, as these pressures do not sufficiently increase the mobility of the polymer chains, causing them to fuse with each other. By contrast, high pressures may dissolve the microspheres almost completely, resulting in severe deviations from their spherical morphology and thus leading to a scaffold with closed pores (85). Singh et al. (86) reported that instantaneous depressurization (in less than 5 s) or depressurization at very slow rates (<0.07 bar/s) led to foaming of the formed scaffolds. Depending on the material, pressure alone may not be sufficient for sintering. For example, sintering of PCL required an increase in both pressure (~40 bar) and temperature (~40°C) (84).
Subcritical CO2 sintering is a straightforward method that can be used to fabricate cell-seeded, shape-specific microsphere-based scaffolds in a single step under relatively mild conditions (86). A key advance in microsphere-based scaffolds was made with CO2 sintering through the introduction of a new approach to create shape-specific scaffolds, whereby a negative mold was created from a desired geometry and then filled with microspheres, which were then sintered with CO2 (86). Compared with the other microsphere sintering methods, the CO2 sintering method is a more benign process that allows for incorporation of bioactive molecules into the scaffold. Another advantage of using CO2 as a sintering agent is that it is easily removed from the polymers, so additional washing or lyophilization steps may not be required. Furthermore, CO2 is regarded as a promising green solvent because it has low toxicity and low environmental impact when taken from nonsequestered sources (83). The greatest advantage of CO2 sintering may be its ability to sinter microspheres in the presence of cells, which for certain applications may be highly desirable. However, at high pressures or for long durations, the CO2 sintering technique may not be cytocompatible due to the known sterilization efficacy of CO2 caused by lowering the cytoplasmic pH (87). Moreover, shear forces exerted during gas nucleation upon depressurization, if not done carefully, may have deleterious effects on the cells if present during sintering.
4.4. Selective Laser Sintering
In the last decade, conventional microsphere sintering methods have greatly improved, yet these methods can only produce scaffolds with a simple architecture in a manual and inconsistent manner. To circumvent the limitations associated with the conventional methods, investigators have employed an RP microsphere packing strategy to produce 3D scaffolds with complex shapes and architectures in a layer-by-layer manner using data generated by CAD systems. For instance, microspheres consisting of polymers [such as poly(hydroxybutyrate-cohydroxyvalerate) (PHBV)] or composite [such as calcium phosphate (CaP)/PHBV] were fabricated into scaffolds with an interconnected pore network and high porosity by use of selective laser sintering (SLS) (32, 88). In the SLS process, 3D computer images are first processed into two-dimensional (2D) slices, and then the scaffolds are built layer by layer to the required size, shape, and internal structure by laser-induced fusion of microspheres. The interaction between the laser beam and the microspheres elevates the polymer temperature to Tg, causing the microsphere surfaces in contact to deform and fuse together.
The SLS process parameters that can affect the properties of sintered microsphere-based scaffolds include laser power, scan spacing, layer thickness, part bed temperature, scan speed, and roller speed. Each of these parameters may have different levels of influence, and in addition, these parameters can have combined effects on the final scaffold (32). Duan et al. (32) applied a three-factor, three-level, complete factorial design to investigate the effects of laser power, scan spacing, and layer thickness on scaffold quality. These authors observed that all three of the factors had significant effects on the integrated response of the fabricated scaffolds, which was concerned with their structure and handling stability, dimensional accuracy, and compressive properties.
The potential advantages of SLS include (a) customization (patient-specific) of the scaffolds, (b) fast manufacture speed with reproducibility, (c) the potential to fabricate functionally graded materials for regeneration of complex tissues, (d) freedom from toxic solvents, and (e) controllability over scaffold architecture by creating a macroscopic pore structure that is not possible with random stacking. The primary downside of SLS is that it is not economical to use because commercial SLS machines require large quantities of biomaterial(s) for processing, thus making the process very expensive. Moreover, rolling microspheres over respective layers (e.g., microspheres sticking together or not being spread evenly in successive layers) can present significant logistical challenges.
4.5. Summary of Microsphere Sintering Methods
Table 2 summarizes the merits and limitations associated with each microsphere sintering method, along with each method’s various process parameters that influence the overall properties of the microsphere-based scaffold. Several microsphere sintering techniques are available to fabricate microsphere-based scaffolds ranging from simple shapes to complex geometries. Each sintering method along with its process parameters can have a tremendous influence on the properties of the overall scaffolds. Therefore, the selection of the microsphere sintering method to be employed depends on the desired characteristics of the scaffold and its intended application.
Table 2.
Advantages and disadvantages of microsphere sintering methods along with their various process parameters that influence the overall properties of the microsphere-based scaffold
| Microsphere sintering method | Process parameters that influence scaffold properties | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Heat | Temperature Duration | Simple method; does not require complex apparatus Generally utilizes moderate temperatures Offers flexible time constraints Multiple scaffolds can be fabricated at once |
May require high temperatures or longer durations May result in loss of bioactivity of encapsulated factors |
20, 33, 52, 90 |
| Solvent vapor | Duration Solvent composition Scaffold mass |
Can sinter microspheres quickly Allows for inclusion of bioactive factors |
Strict time constraints Residual solvent toxicity |
69, 70 |
| Weak solvent | Duration Solvent composition |
Allows for inclusion of bioactive factors Moderate time constraints Less concern of residual solvent toxicity as a mild solvent is employed |
Longer sintering durations compared with solvent vapor method | 48, 61, 72, 74 |
| Solvent/nonsolvent | Solution composition Duration | Can be used for a wide range of materials Allows for preloading of bioactive molecules |
Requires large amounts of solvent Residual solvent toxicity |
44, 80, 81 |
| Subcritical CO2 | CO2 pressure Duration Rate of depressurization Temperature (only if necessary) |
Straightforward one-step method May be used to create shape-specific scaffolds Allows for simultaneous cell seeding Benign process Does not require extra washing steps Low environmental impact |
May not be cytocompatible at high CO2 pressures Shear forces may harm concurrently seeded cells |
84–86 |
| Selective laser | Laser power Scan spacing Layer thickness |
Fabrication of patient-specific grafts Reproducible method with fast manufacture speed May be utilized for regeneration of complex tissues Free of toxic solvents Excellent controllability over scaffold architecture Macroporous architecture (not limited to stacking) |
Large quantities of raw materials are required Logistically challenging Expensive |
32, 88 |
5. APPLICATIONS OF MICROSPHERE-BASED SCAFFOLDS
Heretofore, microsphere-based scaffolds have been employed for the regeneration of bone (19, 33, 37, 47, 53, 72, 74, 89–98), cartilage (45, 48, 60, 61, 71, 73, 75–79, 99–101), skin (102), heart (103), liver (35, 104, 105), and nerve (106). In this section, we discuss key applications of microsphere-based scaffolds in terms of their merits for engineering a myriad of functional tissues.
5.1. Bone Regeneration
During the last decade, the use of microsphere-based scaffolds as practical components for bone regeneration has drawn widespread attention. Borden et al. (33) established that microspheres can be used as building blocks in an integrated scaffold for potential use as a substrate for bone regeneration. These authors developed a synthetic microsphere-based scaffold using biodegradable PLGA microspheres fabricated via emulsion-solvent extraction and fused through heat sintering (33). The fabricated scaffold possessed 30–40% porosity with a pore interconnectivity of 100% due to the fundamental quality of sphere packing (33). Thus, the microsphere-based scaffold could serve as a negative template for cancellous bone regeneration, which has a bone volume of around 30%. Additionally, the PLGA microsphere–based scaffolds had a dry compressive modulus ranging between 130 and 300 MPa, underscoring the applicability of these scaffolds for bone regeneration (89). Furthermore, cell-seeding experiments revealed that the cells formed extensive cytoplasmic connections between adjacent microspheres, in concentric rings around the pores of the microsphere-sphere based scaffold (33, 89). Due to the nature of sphere packing, the microsphere-based scaffold organized the regenerating tissue in a manner reminiscent of trabecular bone, which consists of mineralized sheets of collagen organized in concentric rings around a central Haversian canal.
Microspheres can act as both microencapsulation devices and immobilization substrates for bioactive molecule delivery (107). Jiang et al. (47, 94) utilized this property first to create chitosan-encapsulating, PLGA microsphere–based sintered scaffolds, and then to immobilize a negatively charged molecule, heparin, by using the ionic interaction between heparin and protonated chitosan present on the surface of the scaffolds. Additionally, heparin-modified, chitosan-encapsulated, PLGA microsphere–based scaffolds induced bone formation via intramembranous ossification when combined with an osteogenic growth factor, bone morphogenetic protein 2 (BMP-2), and implanted in vivo into critical-sized defects in rabbits (94).
Bone regenerating strategies are evolving to include ceramics (particularly calcium phosphates) in an effort to mimic the natural composition of the tissue, which consists of 70% mineral. Dormer et al. (72) and Gupta et al. (74) demonstrated that calcium phosphates such as HAp and tricalcium phosphate (TCP) can be incorporated into microsphere-based sintered scaffolds. The incorporated ceramics enhanced the endpoint secretion of extracellular matrix (ECM) components relevant to bone tissue and stimulated the differentiation of cells toward an osteoblastic phenotype (72, 74). Other groups have demonstrated the versatility of microsphere-based scaffolds to include other inorganic materials, such as titanium dioxide (TiO2) (97) and hexagonal mesoporous silica (HMS) (98). Relying on the ability of microspheres to act as microscopic bioreactors, Cushnie et al. (90) fabricated microsphere-based scaffolds containing composite microspheres of PLGA and in situ synthesized amorphous HAp to more closely approximate the poorly crystalline structure of bony mineral.
The success of any bone regeneration strategy critically depends on the extent of blood vessel infiltration into the scaffolds (108). In an effort to further validate the potential of microsphere-based scaffolds for bone regeneration, Jabbarzadeh et al. (91, 93) demonstrated that endothelial cells exhibited normal morphological, structural, and functional phenotypes on the microsphere-based scaffolds. These findings suggested that microsphere-based scaffolds for engineering bone might support blood vessel formation within the scaffold and vessel infiltration from the surrounding tissue when implanted in vivo.
To more closely mimic the natural architecture of bone, in which there exists a central cavity where the bone marrow resides, a tubular microsphere-sintered scaffold can be created by either reshaping a preformed scaffold (53) or assembling microspheres via a layer-by-layer approach (32, 88). Furthermore, microsphere-based scaffolds can be used as injectable scaffolds to fill irregularly shaped bone defects (19, 37).
Banking tissue-engineered constructs would allow for immediate procurement upon a surgeon’s request. The limitations of banking of engineered constructs are that significant cell death occurs in the constructs post thawing and that their ECM architecture is altered. Kofron et al. (95) demonstrated that PLGA microsphere–based sintered tissue-engineered constructs could shield cells from the stresses associated with low-temperature tissue banking and retain the ECM architecture post thawing, thus providing further encouragement for these scaffolds to be translated to the clinic.
5.2. Cartilage Regeneration
Microsphere-based scaffolds in cartilage regeneration offer the versatility of serving either as a cell delivery vehicle or as an acellular osteochondral implant, with macroporous architecture conducive to infiltration of endogenous bone marrow cells, via arthroscopic delivery. In 2004, Mercier et al. (99) became the first to demonstrate the potential of PLGA microsphere–based scaffolds as an injectable matrix for delivering chondrocytes in vitro and in vivo. One concern with developing PLGA microsphere–based injectable scaffolds is that cells such as chondrocytes cultured on PLGA microspheres may undergo a loss of phenotype and become fibroblastic (100). To overcome this problem, Park et al. (100) fabricated nanostructured PLGA microsphere–based injectable scaffolds by physically attaching transforming growth factor β3 (TGF-β3)-loaded nanoparticles onto their surfaces.
A prevalent strategy for regenerating articular cartilage leverages the underlying subchondral bone as a reservoir for marrow-residing stem cells and as an anchoring site for an implanted engineered construct (109). Toward this end, the Detamore group (60, 61, 71, 73) demonstrated that microsphere-based sintered scaffolds containing opposing gradients of growth factors can be employed to regenerate cartilage via an acellular, osteochondral route, most notably in a long-term (1-year) study of cartilage regeneration in weight-bearing medial and lateral femoral condyle osteochondral defects in sheep (48). These microsphere-based scaffolds, which had varying microsphere formulations and were fabricated via different sintering approaches, can provide controlled release of encapsulated factors (43). Moreover, microsphere-based scaffolds with spatial control over molecular composition can provide the neighboring progenitor cells with raw materials (bioactive signals and building blocks) for their simultaneous differentiation along chondro- and osteogenic lineages in different regions of the scaffold (48, 76–78). Different encapsulated factors in the chondro- and osteogenic regions of the microsphere-based scaffolds can lead to dissimilar degradation in distinct regions of the scaffold (110), thus having profound implications for osteochondral regeneration, in which scaffolds with different degradation rates may be required to match the properties of the native tissue. Furthermore, ECM materials such as decellularized cartilage and demineralized bone matrix (DBM) can be incorporated into microsphere-based scaffolds, providing the progenitor cells with a “cocktail” of factors (rather than one or two factors) to stimulate their differentiation (45, 75, 79). These ECM-encapsulating microsphere-based scaffolds have tremendous clinical significance, as they may be strategically positioned for more streamlined regulatory approval. Moreover, avoiding the high cost associated with the inclusion of growth factors will translate into higher profit margins for investors, improving the prospects of ECM-encapsulating microsphere–based scaffolds for translation to the clinic.
5.3. Skin Regeneration
Microspheres made of biocompatible polymers can serve as cell microcarriers by either cell encapsulation or cell attachment at the exposed surface. For example, Kim et al. (102) demonstrated the feasibility of using PLGA microsphere–based scaffolds as both a cell culture substrate and a transplantation vehicle for skin cells. Three weeks following implantation in vivo, these microsphere-based scaffolds showed signs of dermal regeneration and differentiated epithelium, demonstrating a significant advantage over current skin substitutes, such as epidermal sheet grafts, that fail to restore fully functional skin (111).
5.4. Heart Regeneration
A major challenge in cardiac tissue engineering is that the engineered myocardium must contain a dense population of properly aligned and electrically connected cardiomyocytes. Additionally, the scaffolds need to be highly porous to enable the passage of nutrients needed for cells to survive at the center of a large construct. Due to their spherical nature, microspheres can be densely packed in a regular arrangement to yield porous microsphere-based scaffolds. Depending on the packing arrangement, the porosity of the scaffolds can be tailored to meet the specific requirements of the tissue of interest. Smith et al. (103) assembled poly(ethylene glycol) (PEG) microspheres around HL-1 cardiomyocytes to produce microsphere-based scaffolds. The HL-1 cardiomyocytes exhibited high cell viability, expressed cardiac functional markers, and were spontaneously depolarizing even 38 days after scaffold formation. These findings suggest that microsphere-based scaffolds have the potential to be utilized in cardiovascular tissue engineering applications.
5.5. Liver Regeneration
Microspheres allow for easy and controllable surface modification for enhanced cell–material interaction, thus guiding cellular growth. Zhu et al. (104) modified and covalently conjugated PHBV microspheres with three ECM proteins to enhance the proliferation of Hep3B cells. Additionally, the same authors showed that delivery of hepatocyte growth factor (HGF) to the primary hepatocytes from PHBV microspheres maintained cell viability and phenotype better than the delivery of protein via the cell culture medium (105). Microsphere-based scaffolds fabricated from porous microspheres may permit cells to infiltrate the interior of the microspheres as well as the interstitial space among the microspheres, thus facilitating cell–cell interactions within and between the microspheres. Chou et al. (35) demonstrated that hepatocytes along with nonparenchymal cells cultured on porous PLGA microspheres can be developed as an injectable 3D scaffold that can be used for functional restoration of hepatic tissue.
5.6. Nerve Regeneration
Severe nerve injuries result in large gaps between portions of a nerve and require a scaffold with excellent mechanical properties and a large surface area to promote proliferation of support cells and axonal regeneration (112). Microspheres possess a large surface area due to their inherently small size, and they easily allow for surface modifications that can further enhance their specific area. Valmikinathan et al. (106) developed a novel spiral-shaped microsphere-based scaffold with a nanofibrous surface to increase scaffold surface area. To demonstrate the potential of these scaffolds for peripheral nerve regeneration in vitro, these authors showed that Schwann cells had higher rates of cell attachment and proliferation on microsphere-based scaffolds in comparison to other contemporary tubular scaffolds (106).
5.7. Summary of Microsphere-Based Scaffolds in Regenerative Engineering
Microsphere-based scaffolds have innate qualities that make them well suited for functional regeneration of a variety of tissues. Densely packed microsphere-based porous scaffolds can both serve as a template for cell proliferation and act as a guide for establishing intricate cell–cell/cell–ECM connections. Microsphere-based loosely packed scaffolds can function as either cell culture substrates or transplantable devices. Microsphere-based scaffolds can either immobilize bioactive molecules or synthesize them in situ to enhance the biological activity of the scaffolds. Moreover, microsphere-based scaffolds can enable spatial and temporal control over the release of bioactive molecules that can trigger the same population of progenitor cells to differentiate along discrete pathways in distinct regions of the scaffold.
6. DESIGN STRATEGIES FOR MICROSPHERE-BASED SCAFFOLDS
As discussed in the preceding section, microsphere-based scaffolds hold immense promise for regenerating a multitude of tissues, yet their use has been limited primarily to the repair of bone and/or cartilage. In this section, we present the process of designing microsphere-based scaffolds in a series of steps with the goal of stimulating the interest of researchers across the tissue engineering field. Figure 2 depicts the various design considerations for fabricating microsphere-based scaffolds.
Figure 2.
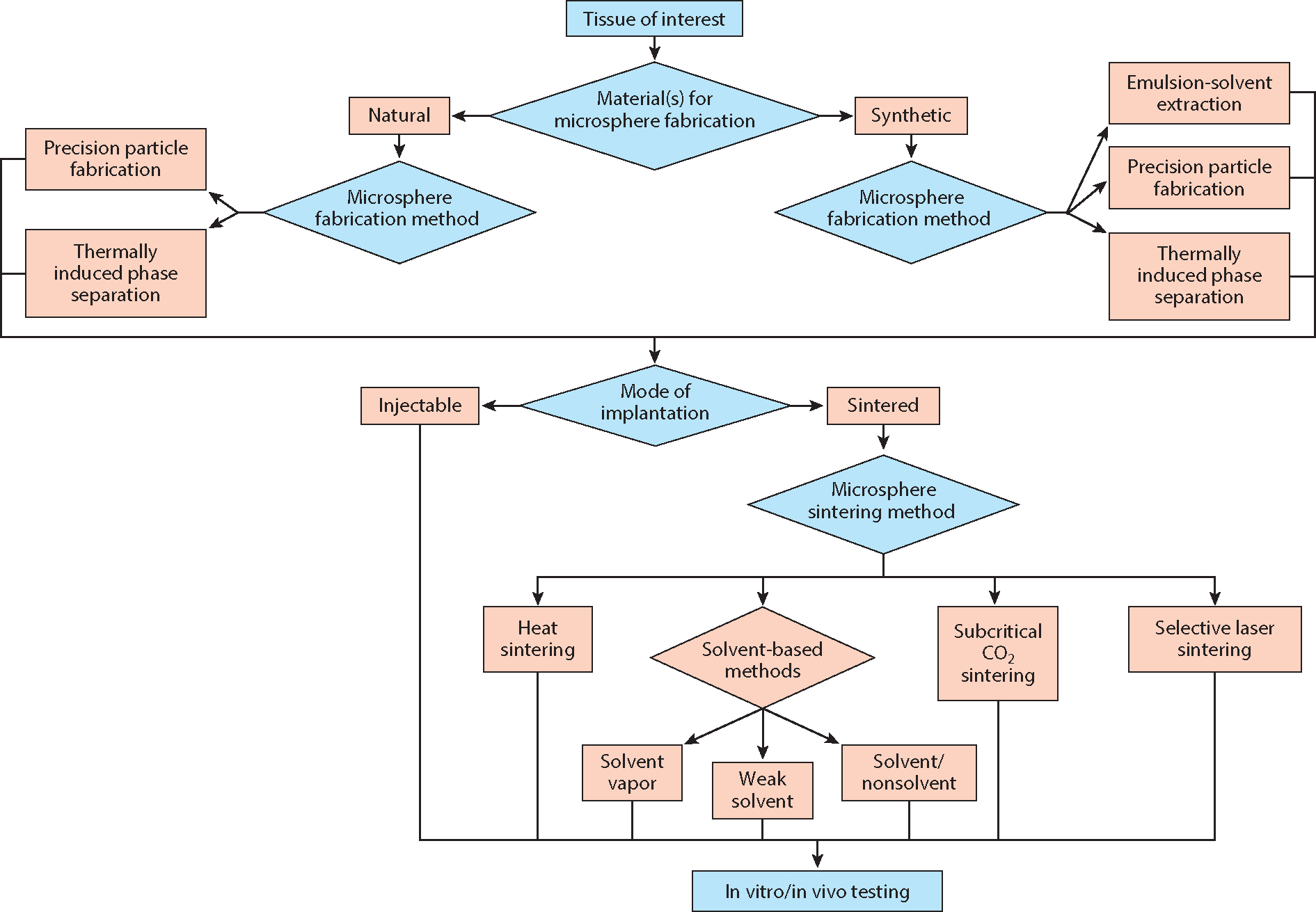
Design strategies for fabricating microsphere-based scaffolds.
6.1. Tissue of Interest
The first and most crucial step in designing a microsphere-based scaffold is to determine a specific application in need of spatiotemporal control over biomaterial composition and/or controlled release. The intended application of the microsphere-based scaffolds will determine parameters such as microsphere composition and scaffold morphology that can have great implications for regenerating a particular type of tissue.
6.2. Material(s) for Microsphere Fabrication
The second step involves selecting a combination of materials to be used for microsphere fabrication. The tissue of interest and the desired characteristics of the microsphere-based scaffold dictate the choice of materials. For instance, collagen along with calcium phosphates may be a suitable choice for fabricating microspheres for bone regeneration.
6.3. Microsphere Fabrication Method
The next step after material selection is to select the method for microsphere fabrication. In this review, we have divided the materials into two categories: natural and synthetic. As discussed above, the emulsion-solvent extraction method may not be suitable for fabricating microspheres from natural polymers such as alginate and gelatin. But other available methods that are not discussed in this review, such as interfacial polymerization (50) and ionotropic gelation (113), can be used to fabricate microspheres from these natural polymers.
6.4. Microsphere Diameter
Smaller microsphere diameters allow for greater spatial resolution, although if they are too small they may lead to undesirable restrictions on cell infiltration (114) and may have implications for reproducible degrees of sintering. Generally, smaller diameters are avoided, although there may be advantages pertaining to capitalizing on the capillary method (115, 116) associated with either cell seeding or endogenous body fluid (e.g., bone marrow with associated cells) infiltration in vivo. As a general guideline, we recommend microspheres with diameters of at least 70 μm (71), but ideally in the 200–300-μm range.
6.5. Mode of Implantation
The next step in the microsphere-based scaffold design process is to establish the mode of implantation, which can be categorized as either injectable or sintered. Injectable scaffolds comprising microsphere cell carriers and arthroscopically implantable scaffolds can then be examined for their intended application.
6.6. Microsphere Sintering Method
The selection of sintering method is governed primarily by the intended application of the scaffold and the composition of the microsphere. The microsphere fabrication method may not have a significant effect on the choice of sintering method. The major focus of the sintering step is to achieve a desired degree of sintering. There is a delicate balance involved with the degree of sintering, as undersintering results in poor mechanical and structural integrity and oversintering results in pore closure, both of which defeat the purpose of a microsphere-based approach. In order to find that “sweet spot” where mechanical integrity and pore interconnectivity coexist in harmony, all microsphere-based scaffold studies should implement the following three measures of the degree of sintering.
Mechanical testing to failure (quantitative). Insufficient sintering will be revealed immediately if scaffolds easily crumble at low strains and/or low stresses.
Porosity measurement (quantitative). Porosity may be measured directly, for example, by mercury porosimetry, or indirectly, by simple mass and volume calculation, which can provide a reasonably accurate approximation of direct porosity measurements (61). Computed tomography (CT) is another option, but it can be cost and time prohibitive, and resolution can be a major limitation. We advise mass and volume calculation, verified in house by initial mercury porosimetry and/or nano-CT measurements. Porosity based on sphere stacking will have an upper limit of ~45%, so a target range may be 30–45% porosity, and more advanced manufacturing methods (e.g., 3D printing) can be employed if there is a need to surpass the porosity associated with the geometric stacking limit.
Visual measurement. Arguably the best and simplest yet least quantitative method of determining the degree of sintering involves the use of scanning electron microscopy (SEM) to directly visualize the pore network, sintering junctions, and (if applicable) cell infiltration.
6.7. In Vitro or In Vivo Testing
The last step in designing microsphere-based scaffolds is to employ the scaffolds in their intended application. The results obtained from scaffold testing can inform the design of the microsphere-based scaffolds, thus allowing researchers to further improve on their designs.
The microsphere-based scaffold design process is iterative when microspheres or even material combinations may be tested for their intended application before being fabricated into microspheres or scaffolds. This kind of approach is encouraged in order to identify and rectify problems when the process is still in the early stages.
7. DISCUSSION AND CONCLUSIONS
The potential of microspheres in drug delivery has been investigated since the 1970s, whereas their use in regenerative engineering scaffolds has been increasingly endorsed only over the last two decades. Typically, microspheres can be used either as one component of a scaffold or as building blocks to create unified high-utility microsphere-based scaffolds. Microsphere-based scaffolds can be assembled via several combinations of microsphere fabrication and sintering techniques, each of which can have a wide-ranging effect on microsphere and scaffold properties. Microsphere-based scaffolds are highly desirable for delivering payloads of growth factors or “raw materials” (79, 117) such as bioactive glass or natural ECM components, either by encapsulation or by surface adsorption (79), in applications requiring spatiotemporal control over the distribution and release of these components. Moreover, microsphere-based scaffolds offer a straightforward alternative to traditional additive manufacturing methods for creating macroporous, 3D shape–specific constructs. Compared with conventional tissue engineering scaffolds, microsphere-based scaffolds exhibit numerous advantages that are related to (a) an enhanced porous network, (b) high-resolution control over spatial organization, (c) the creation of physicochemical gradients via spatiotemporal release of bioactive factors and nanophase ceramics, (d) the practicality of producing patient-specific biological grafts, and (e) the feasibility of being implanted arthroscopically in a minimally invasive procedure.
ACKNOWLEDGMENTS
M.S.D. and C.J.B. acknowledge support from the National Institute for Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH) (R01 AR056347). C.T.L. acknowledges support from the NIH (AR060480, AR062711, AR066320, AR063698, MD009476, and NIH Pioneer Award DP1OD019349-01). C.T.L. also acknowledges support from the National Science Foundation (NSF) (EFRI-1332329, IIP-1311907, and EFRI-0710321). C.T.L. is a recipient of the Presidential Faculty Fellow Award (NSF); the Presidential Award for Excellence in Science, Math and Engineering Mentoring (NSF); and the National Medal of Technology and Innovation.
Footnotes
DISCLOSURE STATEMENT
C.J.B. is a shareholder and cofounder of Orbis Biosciences. The other authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Ravichandran R, Sundarrajan S, Venugopal JR, Mukherjee S, Ramakrishna S. 2012. Advances in polymeric systems for tissue engineering and biomedical applications. Macromol. Biosci. 12:286–311 [DOI] [PubMed] [Google Scholar]
- 2.Subia B, Kundu J, Kundu S. 2010. Biomaterial Scaffold Fabrication Techniques for Potential Tissue Engineering Applications. www.intechopen.com: INTECH [Google Scholar]
- 3.Shi X, Su K, Varshney RR, Wang Y, Wang D-A. 2011. Sintered microsphere scaffolds for controlled release and tissue engineering. Pharm. Res. 28:1224–28 [DOI] [PubMed] [Google Scholar]
- 4.Elbert DL. 2011. Liquid–liquid two phase systems for the production of porous hydrogels and hydrogel microspheres for biomedical applications: a tutorial review. Acta Biomater. 7:31–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roam JL, Yan Y, Nguyen PK, Kinstlinger IS, Leuchter MK, et al. 2015. A modular, plasmin-sensitive, clickable poly(ethylene glycol)–heparin–laminin microsphere system for establishing growth factor gradients in nerve guidance conduits. Biomaterials 72:112–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nath SD, Linh NT, Sadiasa A, Lee BT. 2014. Encapsulation of simvastatin in PLGA microspheres loaded into hydrogel loaded BCP porous spongy scaffold as a controlled drug delivery system for bone tissue regeneration. J. Biomater. Appl. 28:1151–63 [DOI] [PubMed] [Google Scholar]
- 7.Niu X, Feng Q, Wang M, Guo X, Zheng Q. 2009. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J. Control. Release 134:111–17 [DOI] [PubMed] [Google Scholar]
- 8.Jang J-H, Shea LD. 2003. Controllable delivery of non-viral DNA from porous scaffolds. J. Control. Release 86:157–68 [DOI] [PubMed] [Google Scholar]
- 9.Rooney GE, Knight AM, Madigan NN, Gross L, Chen B, et al. 2011. Sustained delivery of dibutyryl cyclic adenosine monophosphate to the transected spinal cord via oligo[(polyethylene glycol) fumarate] hydrogels. Tissue Eng. A 17:1287–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Zhang L, Shi P, Zou Q, Zuo Y, Li Y. 2010. Hydroxyapatite/polyurethane scaffold incorporated with drug-loaded ethyl cellulose microspheres for bone regeneration. J. Biomed. Mater. Res. B 95:36–46 [DOI] [PubMed] [Google Scholar]
- 11.Meng D, Francis L, Thompson ID, Mierke C, Huebner H, et al. 2013. Tetracycline-encapsulated P(3HB) microsphere-coated 45S5 Bioglass®-based scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 24:2809–17 [DOI] [PubMed] [Google Scholar]
- 12.Sanborn TJ, Messersmith PB, Barron AE. 2002. In situ crosslinking of a biomimetic peptide–PEG hydrogel via thermally triggered activation of factor XIII. Biomaterials 23:2703–10 [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Leeuwenburgh SC, Li Y, Jansen JA. 2012. The use of micro- and nanospheres as functional components for bone tissue regeneration. Tissue Eng. B 18:24–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson HC, Garimella R, Tague SE. 2005. The role of matrix vesicles in growth plate development and biomineralization. Front. Biosci. 10:822–37 [DOI] [PubMed] [Google Scholar]
- 15.Pederson AW, Ruberti JW, Messersmith PB. 2003. Thermal assembly of a biomimetic mineral/collagen composite. Biomaterials 24:4881–90 [DOI] [PubMed] [Google Scholar]
- 16.Leong W, Lau TT, Wang DA. 2013. A temperature-cured dissolvable gelatin microsphere-based cell carrier for chondrocyte delivery in a hydrogel scaffolding system. Acta Biomater. 9:6459–67 [DOI] [PubMed] [Google Scholar]
- 17.De Nardo L, Bertoldi S, Cigada A, Tanzi MC, Haugen HJ, Farè S. 2012. Preparation and characterization of shape memory polymer scaffolds via solvent casting/particulate leaching. J. Appl. Biomater. Funct. Mater. 10:119–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dellinger JG, Wojtowicz AM, Jamison RD. 2006. Effects of degradation and porosity on the load bearing properties of model hydroxyapatite bone scaffolds. J. Biomed. Mater. Res. A 77:563–71 [DOI] [PubMed] [Google Scholar]
- 19.Habraken WJEM Wolke JGC, Mikos AG Jansen JA. 2006. Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J. Biomater. Sci. Polym. Ed. 17:1057–74 [DOI] [PubMed] [Google Scholar]
- 20.Laurencin CT, Ko FK, Attawia MA, Borden MD. 1998. Studies on the development of a tissue engineered matrix for bone regeneration. Cells Mater. 8:175–81 [Google Scholar]
- 21.Matsuno T, Hashimoto Y, Adachi S, Omata K, Yoshitaka Y, et al. 2008. Preparation of injectable 3D-formed β-tricalcium phosphate bead/alginate composite for bone tissue engineering. Dent. Mater. J. 27:827–34 [DOI] [PubMed] [Google Scholar]
- 22.Nichol JW, Khademhosseini A. 2009. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter 5:1312–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Tabata Y, Tong YW. 2010. Fabricating tissue engineering scaffolds for simultaneous cell growth and drug delivery. Curr. Pharm. Des. 16:2388–94 [DOI] [PubMed] [Google Scholar]
- 24.Davis HE, Leach JK. 2011. Designing bioactive delivery systems for tissue regeneration. Ann. Biomed. Eng. 39:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin Y, Eldardiri M, Lawrence-Watt DJ, Sharpe JR. 2011. Microcarriers and their potential in tissue regeneration. Tissue Eng. B 17:71–80 [DOI] [PubMed] [Google Scholar]
- 26.Park JH, Perez RA, Jin GZ, Choi SJ, Kim HW, Wall IB. 2013. Microcarriers designed for cell culture and tissue engineering of bone. Tissue Eng. B 19:172–90 [DOI] [PubMed] [Google Scholar]
- 27.Perez RA, Kim HW. 2015. Core–shell designed scaffolds for drug delivery and tissue engineering. Acta Biomater. 21:2–19 [DOI] [PubMed] [Google Scholar]
- 28.Huang W, Li X, Shi X, Lai C. 2014. Microsphere based scaffolds for bone regenerative applications. Biomater. Sci. 2:1145–53 [DOI] [PubMed] [Google Scholar]
- 29.Van Tomme SR, van Steenbergen MJ, De Smedt SC, van Nostrum CF, Hennink WE. 2005. Self-gelling hydrogels based on oppositely charged dextran microspheres. Biomaterials 26:2129–35 [DOI] [PubMed] [Google Scholar]
- 30.Alsberg E, Feinstein E, Joy MP, Prentiss M, Ingber DE. 2006. Magnetically-guided self-assembly of fibrin matrices with ordered nano-scale structure for tissue engineering. Tissue Eng. 12:3247–56 [DOI] [PubMed] [Google Scholar]
- 31.Van Tomme SR, Mens A, van Nostrum CF, Hennink WE. 2008. Macroscopic hydrogels by self-assembly of oligolactate-grafted dextran microspheres. Biomacromolecules 9:158–65 [DOI] [PubMed] [Google Scholar]
- 32.Duan B, Wang M, Zhou WY, Cheung WL, Li ZY, Lu WW. 2010. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 6:4495–505 [DOI] [PubMed] [Google Scholar]
- 33.Borden M, Attawia M, Laurencin CT. 2002. The sintered microsphere matrix for bone tissue engineering: in vitro osteoconductivity studies. J. Biomed. Mater. Res. 61:421–29 [DOI] [PubMed] [Google Scholar]
- 34.Chan BP, Hui TY, Wong MY, Yip KH, Chan GC. 2010. Mesenchymal stem cell–encapsulated collagen microspheres for bone tissue engineering. Tissue Eng. C 16:225–35 [DOI] [PubMed] [Google Scholar]
- 35.Chou M-J, Hsieh C-H, Yeh P-L, Chen P-C, Wang C-H, Huang Y-Y. 2013. Application of open porous poly(d,l-lactide-co-glycolide) microspheres and the strategy of hydrophobic seeding in hepatic tissue cultivation. J. Biomed. Mater. Res. A 101:2862–69 [DOI] [PubMed] [Google Scholar]
- 36.Jayasuriya AC, Bhat A. 2010. Mesenchymal stem cell function on hybrid organic/inorganic microparticles in vitro. J. Tissue Eng. Regen. Med. 4:340–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang S-W, Yang HS, Seo S-W, Han DK, Kim B-S. 2008. Apatite-coated poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for bone tissue engineering. J. Biomed. Mater. Res. A 85:747–56 [DOI] [PubMed] [Google Scholar]
- 38.Lee CS, Moyer HR, Gittens RA, Williams JK, Boskey AL, et al. 2010. Regulating in vivo calcification of alginate microbeads. Biomaterials 31:4926–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercier NR, Costantino HR, Tracy MA, Bonassar LJ. 2005. Poly(lactide-co-glycolide) microspheres as a moldable scaffold for cartilage tissue engineering. Biomaterials 26:1945–52 [DOI] [PubMed] [Google Scholar]
- 40.Lemperle G, Morhenn VB, Pestonjamasp V, Gallo RL. 2004. Migration studies and histology of injectable microspheres of different sizes in mice. Plast. Reconstr. Surg. 113:1380–90 [DOI] [PubMed] [Google Scholar]
- 41.Arimura H, Ouchi T, Kishida A, Ohya Y. 2005. Preparation of a hyaluronic acid hydrogel through polyion complex formation using cationic polylactide–based microspheres as a biodegradable cross-linking agent. J. Biomater. Sci. Polym. Ed. 16:1347–58 [DOI] [PubMed] [Google Scholar]
- 42.Lozier AJ, Murphy DP. 2013. Osteochondral graft delivery device and uses thereof. US Patent 8435305B2 [Google Scholar]
- 43.Dormer NH, Gupta V, Scurto AM, Berkland CJ, Detamore MS. 2013. Effect of different sintering methods on bioactivity and release of proteins from PLGA microspheres. Mater. Sci. Eng. C 33:4343–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oredein-McCoy O, Krogman NR, Weikel AL, Hindenlang MD, Allcock HR, Laurencin CT. 2009. Novel factor–loaded polyphosphazene matrices: potential for driving angiogenesis. J. Microencapsul. 26:544–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta V, Tenny KM, Barragan M, Berkland CJ, Detamore MS. 2016. Microsphere-based scaffolds encapsulating chondroitin sulfate or decellularized cartilage. J. Biomater. Appl. 31:328–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson C, Khan Y, Laurencin CT. 2014. Nanofiber–microsphere (nano-micro) matrices for bone regenerative engineering: a convergence approach toward matrix design. Regen. Biomater. 1:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang T, Khan Y, Nair LS, Abdel-Fattah WI, Laurencin CT. 2010. Functionalization of chitosan/poly(lactic acid–glycolic acid) sintered microsphere scaffolds via surface heparinization for bone tissue engineering. J. Biomed. Mater. Res. A 93:1193–208 [DOI] [PubMed] [Google Scholar]
- 48.Mohan N, Gupta V, Sridharan BP, Mellott AJ, Easley JT, et al. 2015. Microsphere-based gradient implants for osteochondral regeneration: a long-term study in sheep. Regen. Med. 10:709–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freitas S, Merkle HP, Gander B. 2005. Microencapsulation by solvent extraction/evaporation: reviewing the state of the art of microsphere preparation process technology. J. Control. Release 102:313–32 [DOI] [PubMed] [Google Scholar]
- 50.Mao S, Guo C, Shi Y, Li LC. 2012. Recent advances in polymeric microspheres for parenteral drug delivery—part 2. Expert Opin. Drug Deliv. 9:1209–23 [DOI] [PubMed] [Google Scholar]
- 51.Shi X, Wang Y, Varshney RR, Ren L, Gong Y, Wang D-A. 2010. Microsphere-based drug releasing scaffolds for inducing osteogenesis of human mesenchymal stem cells in vitro. Eur. J. Pharm. Sci. 39:59–67 [DOI] [PubMed] [Google Scholar]
- 52.Jiang T, Abdel-Fattah WI, Laurencin CT. 2006. In vitro evaluation of chitosan/poly(lactic acid–glycolic acid) sintered microsphere scaffolds for bone tissue engineering. Biomaterials 27:4894–903 [DOI] [PubMed] [Google Scholar]
- 53.Kofron MD, Cooper JA, Kumbar SG, Laurencin CT. 2007. Novel tubular composite matrix for bone repair. J. Biomed. Mater. Res. A 82:415–25 [DOI] [PubMed] [Google Scholar]
- 54.Hong S-J, Yu H-S, Kim H-W. 2009. Tissue engineering polymeric microcarriers with macroporous morphology and bone-bioactive surface. Macromol. Biosci. 9:639–45 [DOI] [PubMed] [Google Scholar]
- 55.Varde NK, Pack DW. 2004. Microspheres for controlled release drug delivery. Expert Opin. Biol. Ther. 4:35–51 [DOI] [PubMed] [Google Scholar]
- 56.Ando S, Putnam D, Pack DW, Langer R. 1999. PLGA microspheres containing plasmid DNA: preservation of supercoiled DNA via cryopreparation and carbohydrate stabilization. J. Pharm. Sci. 88:126–30 [DOI] [PubMed] [Google Scholar]
- 57.Watts PJ, Davies MC, Melia CD. 1990. Microencapsulation using emulsification/solvent evaporation: an overview of techniques and applications. Crit. Rev. Ther. Drug Carr. Syst. 7:235–59 [PubMed] [Google Scholar]
- 58.Blaker JJ, Knowles JC, Day RM. 2008. Novel fabrication techniques to produce microspheres by thermally induced phase separation for tissue engineering and drug delivery. Acta Biomater. 4:264–72 [DOI] [PubMed] [Google Scholar]
- 59.Berkland C, Kim K, Pack DW. 2001. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J. Control. Release 73:59–74 [DOI] [PubMed] [Google Scholar]
- 60.Dormer NH, Singh M, Wang L, Berkland CJ, Detamore MS. 2010. Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Ann. Biomed. Eng. 38:2167–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh M, Morris CP, Ellis RJ, Detamore MS, Berkland C. 2008. Microsphere-based seamless scaffolds containing macroscopic gradients of encapsulated factors for tissue engineering. Tissue Eng. C 14:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choy YB, Choi H, Kim KK. 2007. Uniform biodegradable hydrogel microspheres fabricated by a surfactant-free electric-field-assisted method. Macromol. Biosci. 7:423–28 [DOI] [PubMed] [Google Scholar]
- 63.Go DP, Harvie DJE, Tirtaatmadja N, Gras SL, O’Connor AJ. 2014. A simple, scalable process for the production of porous polymer microspheres by ink-jetting combined with thermally induced phase separation. Part. Part. Syst. Charact. 31:685–98 [Google Scholar]
- 64.Akbarzadeh R, Yousefi AM. 2014. Effects of processing parameters in thermally induced phase separation technique on porous architecture of scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B 102:1304–15 [DOI] [PubMed] [Google Scholar]
- 65.Zhang R, Ma PX. 1999. Poly(α-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J. Biomed. Mater. Res. 44:446–55 [DOI] [PubMed] [Google Scholar]
- 66.Ro IJ, Kwon IC. 2002. Fabrication of a pure porous chitosan bead matrix: influences of phase separation on the microstructure. J. Biomater. Sci. Polym. Ed. 13:769–82 [DOI] [PubMed] [Google Scholar]
- 67.Keshaw H, Thapar N, Burns AJ, Mordan N, Knowles JC, et al. 2010. Microporous collagen spheres produced via thermally induced phase separation for tissue regeneration. Acta Biomater. 6:1158–66 [DOI] [PubMed] [Google Scholar]
- 68.Wright LD, Young RT, Andric T, Freeman JW. 2010. Fabrication and mechanical characterization of 3D electrospun scaffolds for tissue engineering. Biomed. Mater. 5:055006. [DOI] [PubMed] [Google Scholar]
- 69.Jaklenec A, Hinckfuss A, Bilgen B, Ciombor DM, Aaron R, Mathiowitz E. 2008. Sequential release of bioactive IGF-I and TGF-β1 from PLGA microsphere–based scaffolds. Biomaterials 29:1518–25 [DOI] [PubMed] [Google Scholar]
- 70.Jaklenec A, Wan E, Murray ME, Mathiowitz E. 2008. Novel scaffolds fabricated from protein-loaded microspheres for tissue engineering. Biomaterials 29:185–92 [DOI] [PubMed] [Google Scholar]
- 71.Dormer NH, Busaidy K, Berkland CJ, Detamore MS. 2011. Osteochondral interface regeneration of rabbit mandibular condyle with bioactive signal gradients. J. Oral Maxillof. Surg. 69:e50–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dormer NH, Qiu Y, Lydick AM, Allen ND, Mohan N, et al. 2012. Osteogenic differentiation of human bone marrow stromal cells in hydroxyapatite-loaded microsphere–based scaffolds. Tissue Eng. A 18:757–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dormer NH, Singh M, Zhao L, Mohan N, Berkland CJ, Detamore MS. 2012. Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J. Biomed. Mater. Res. A 100:162–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gupta V, Lyne DV, Barragan M, Berkland CJ, Detamore MS. 2016. Microsphere-based scaffolds encapsulating tricalcium phosphate and hydroxyapatite for bone regeneration. J. Mater. Sci. Mater. Med. 27:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta V, Lyne DV, Laflin AD, Zabel TA, Barragan M, et al. 2016. Microsphere-based osteochondral scaffolds carrying opposing gradients of decellularized cartilage and demineralized bone matrix. ACS Biomater. Sci. Eng. In press. 10.1021/acsbiomaterials.6b00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gupta V, Mohan N, Berkland CJ, Detamore MS. 2015. Microsphere-based scaffolds carrying opposing gradients of chondroitin sulfate and tricalcium phosphate. Front. Bioeng. Biotechnol. 3:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohan N, Dormer NH, Caldwell KL, Key VH, Berkland CJ, Detamore MS. 2011. Continuous gradients of material composition and growth factors for effective regeneration of the osteochondral interface. Tissue Eng. A 17:2845–55 [DOI] [PubMed] [Google Scholar]
- 78.Mohan N, Gupta V, Sridharan B, Sutherland A, Detamore MS. 2014. The potential of encapsulating “raw materials” in 3D osteochondral gradient scaffolds. Biotechnol. Bioeng. 111:829–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sutherland AJ, Detamore MS. 2015. Bioactive microsphere-based scaffolds containing decellularized cartilage. Macromol. Biosci. 15:979–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown JL, Nair LS, Laurencin CT. 2008. Solvent/non-solvent sintering: a novel route to create porous microsphere scaffolds for tissue regeneration. J. Biomed. Mater. Res. B 86:396–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nukavarapu SP, Kumbar SG, Brown JL, Krogman NR, Weikel AL, et al. 2008. Polyphosphazene/nano-hydroxyapatite composite microsphere scaffolds for bone tissue engineering. Biomacromolecules 9:1818–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qutachi O, Vetsch JR, Gill D, Cox H, Scurr DJ, et al. 2014. Injectable and porous PLGA microspheres that form highly porous scaffolds at body temperature. Acta Biomater. 10:5090–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhamidipati M, Scurto AM, Detamore MS. 2013. The future of carbon dioxide for polymer processing in tissue engineering. Tissue Eng. B 19:221–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhamidipati M M, Sridharan B, Scurto AM, Detamore MS. 2013. Subcritical CO2 sintering of microspheres of different polymeric materials to fabricate scaffolds for tissue engineering. Mater. Sci. Eng. C 33:4892–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jeon JH, Bhamidipati M, Sridharan B, Scurto AM, Berkland CJ, Detamore MS. 2013. Tailoring of processing parameters for sintering microsphere-based scaffolds with dense-phase carbon dioxide. J. Biomed. Mater. Res. B 101:330–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh M, Sandhu B, Scurto A, Berkland C, Detamore MS. 2010. Microsphere-based scaffolds for cartilage tissue engineering: using subcritical CO2 as a sintering agent. Acta Biomater. 6:137–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang J, Davis TA, Matthews MA, Drews MJ, LaBerge M, An YH. 2006. Sterilization using high-pressure carbon dioxide. J. Supercrit. Fluids 38:354–72 [Google Scholar]
- 88.Zhou WY, Lee SH, Wang M, Cheung WL, Ip WY. 2008. Selective laser sintering of porous tissue engineering scaffolds from poly(L-lactide)/carbonated hydroxyapatite nanocomposite microspheres. J. Mater. Sci. Mater. Med. 19:2535–40 [DOI] [PubMed] [Google Scholar]
- 89.Borden M, El-Amin SF, Attawia M, Laurencin CT. 2003. Structural and human cellular assessment of a novel microsphere-based tissue engineered scaffold for bone repair. Biomaterials 24:597–609 [DOI] [PubMed] [Google Scholar]
- 90.Cushnie EK, Khan YM, Laurencin CT. 2008. Amorphous hydroxyapatite-sintered polymeric scaffolds for bone tissue regeneration: physical characterization studies. J. Biomed. Mater. Res. A 84:54–62 [DOI] [PubMed] [Google Scholar]
- 91.Jabbarzadeh E, Jiang T, Deng M, Nair LS, Khan YM, Laurencin CT. 2007. Human endothelial cell growth and phenotypic expression on three dimensional poly(lactide-co-glycolide) sintered microsphere scaffolds for bone tissue engineering. Biotechnol. Bioeng. 98:1094–102 [DOI] [PubMed] [Google Scholar]
- 92.Jabbarzadeh E, Nair LS, Khan YM, Deng M, Laurencin CT. 2007. Apatite nano-crystalline surface modification of poly(lactide-co-glycolide) sintered microsphere scaffolds for bone tissue engineering: implications for protein adsorption. J. Biomater. Sci. Polym. Ed. 18:1141–52 [DOI] [PubMed] [Google Scholar]
- 93.Jabbarzadeh E, Starnes T, Khan YM, Jiang T, Wirtel AJ, et al. 2008. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: a combined gene therapy–cell transplantation approach. PNAS 105:11099–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang T, Nukavarapu SP, Deng M, Jabbarzadeh E, Kofron MD, et al. 2010. Chitosan–poly(lactide-co-glycolide) microsphere–based scaffolds for bone tissue engineering: in vitro degradation and in vivo bone regeneration studies. Acta Biomater. 6:3457–70 [DOI] [PubMed] [Google Scholar]
- 95.Kofron MD, Opsitnick NC, Attawia MA, Laurencin CT. 2003. Cryopreservation of tissue engineered constructs for bone. J. Orthop. Res. 21:1005–10 [DOI] [PubMed] [Google Scholar]
- 96.Wang J, Yu X. 2010. Preparation, characterization and in vitro analysis of novel structured nanofibrous scaffolds for bone tissue engineering. Acta Biomater. 6:3004–12 [DOI] [PubMed] [Google Scholar]
- 97.Wang Y, Shi X, Ren L, Yao Y, Zhang F, Wang D-A. 2010. Poly(lactide-co-glycolide)/titania composite microsphere–sintered scaffolds for bone tissue engineering applications. J. Biomed. Mater. Res. B 93:84–92 [DOI] [PubMed] [Google Scholar]
- 98.Xu W, Wang L, Ling Y, Wei K, Zhong S. 2014. Enhancement of compressive strength and cytocompatibility using apatite coated hexagonal mesoporous silica/poly(lactic acid–glycolic acid) microsphere scaffolds for bone tissue engineering. RSC Adv. 4:13495 [Google Scholar]
- 99.Mercier NR, Costantino HR, Tracy MA, Bonassar LJ. 2004. A novel injectable approach for cartilage formation in vivo using PLG microspheres. Ann. Biomed. Eng. 32:418–29 [DOI] [PubMed] [Google Scholar]
- 100.Park JS, Park K, Woo DG, Yang HN, Chung H-M, Park K-H. 2008. PLGA microsphere construct coated with TGF-β3 loaded nanoparticles for neocartilage formation. Biomacromolecules 9:2162–69 [DOI] [PubMed] [Google Scholar]
- 101.Wang Y, Kim U-J, Blasioli DJ, Kim H-J, Kaplan DL. 2005. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials 26:7082–94 [DOI] [PubMed] [Google Scholar]
- 102.Kim S-S, Gwak S-J, Choi CY, Kim B-S. 2005. Skin regeneration using keratinocytes and dermal fibroblasts cultured on biodegradable microspherical polymer scaffolds. J. Biomed. Mater. Res. B 75:369–77 [DOI] [PubMed] [Google Scholar]
- 103.Smith AW, Segar CE, Nguyen PK, MacEwan MR, Efimov IR, Elbert DL. 2012. Long-term culture of HL-1 cardiomyocytes in modular poly(ethylene glycol) microsphere–based scaffolds crosslinked in the phase-separated state. Acta Biomater. 8:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu XH, Gan SK, Wang C-H, Tong YW. 2007. Proteins combination on PHBV microsphere scaffold to regulate Hep3B cells activity and functionality: a model of liver tissue engineering system. J. Biomed. Mater. Res. A 83:606–16 [DOI] [PubMed] [Google Scholar]
- 105.Zhu XH, Wang C-H, Tong YW. 2009. In vitro characterization of hepatocyte growth factor release from PHBV/PLGA microsphere scaffold. J. Biomed. Mater. Res. A 89:411–23 [DOI] [PubMed] [Google Scholar]
- 106.Valmikinathan CM, Tian J, Wang J, Yu X. 2008. Novel nanofibrous spiral scaffolds for neural tissue engineering. J. Neural Eng. 5:422–32 [DOI] [PubMed] [Google Scholar]
- 107.Brady D, Jordaan J. 2009. Advances in enzyme immobilisation. Biotechnol. Lett. 31:1639–50 [DOI] [PubMed] [Google Scholar]
- 108.Patel ZS, Mikos AG. 2004. Angiogenesis with biomaterial-based drug- and cell-delivery systems. J. Biomater. Sci. Polym. Ed. 15:701–26 [DOI] [PubMed] [Google Scholar]
- 109.Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. 2015. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 11:21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sridharan B, Mohan N, Berkland CJ, Detamore MS. 2016. Material characterization of microsphere-based scaffolds with encapsulated raw materials. Mater. Sci. Eng. C 63:422–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wong DJ, Chang HY. 2008. Skin Tissue Engineering. Cambridge, MA: Harvard Stem Cell Inst. [PubMed] [Google Scholar]
- 112.Belkas JS, Shoichet MS, Midha R. 2004. Peripheral nerve regeneration through guidance tubes. Neurol. Res. 26:151–60 [DOI] [PubMed] [Google Scholar]
- 113.Ribeiro CC, Barrias CC, Barbosa MA. 2006. Preparation and characterisation of calcium–phosphate porous microspheres with a uniform size for biomedical applications. J. Mater. Sci. Mater. Med. 17:455–63 [DOI] [PubMed] [Google Scholar]
- 114.Poellmann MJ, Harrell PA, King WP, Wagoner Johnson AJ. 2010. Geometric microenvironment directs cell morphology on topographically patterned hydrogel substrates. Acta Biomater. 6:3514–23 [DOI] [PubMed] [Google Scholar]
- 115.Bai H, Wang D, Delattre B, Gao W, De Coninck J, et al. 2015. Biomimetic gradient scaffold from ice-templating for self-seeding of cells with capillary effect. Acta Biomater. 20:113–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Polak SJ, Rustom LE, Genin GM, Talcott M, Wagoner Johnson AJ. 2013. A mechanism for effective cell-seeding in rigid, microporous substrates. Acta Biomater. 9:7977–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Renth AN, Detamore MS. 2012. Leveraging “raw materials:” building blocks and bioactive signals in regenerative medicine. Tissue Eng. B 18:341–62 [DOI] [PMC free article] [PubMed] [Google Scholar]


