Abstract
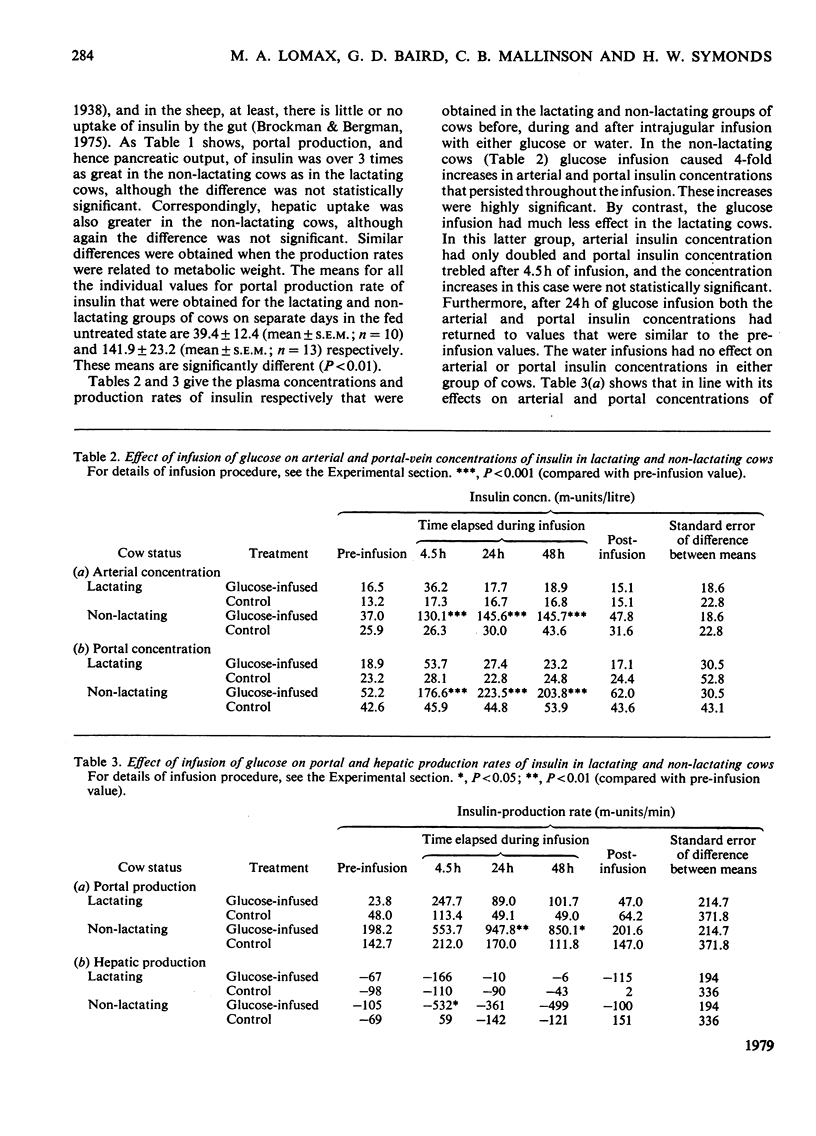
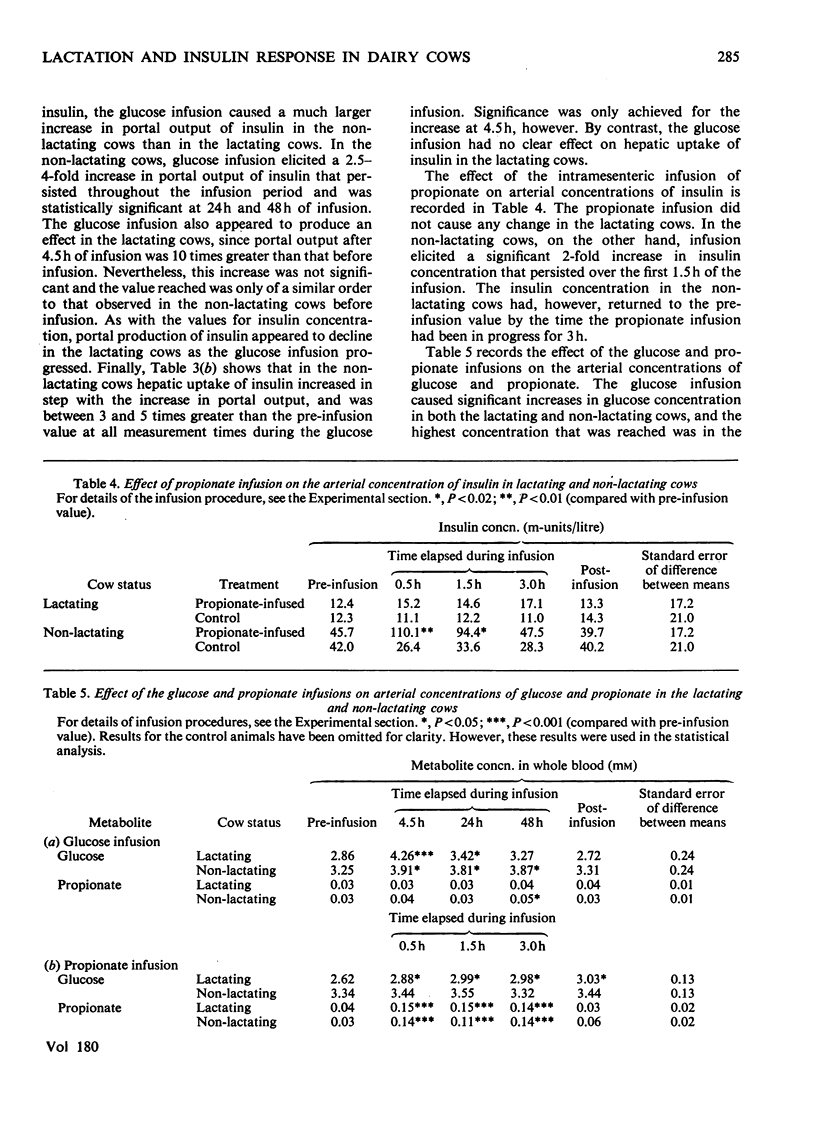
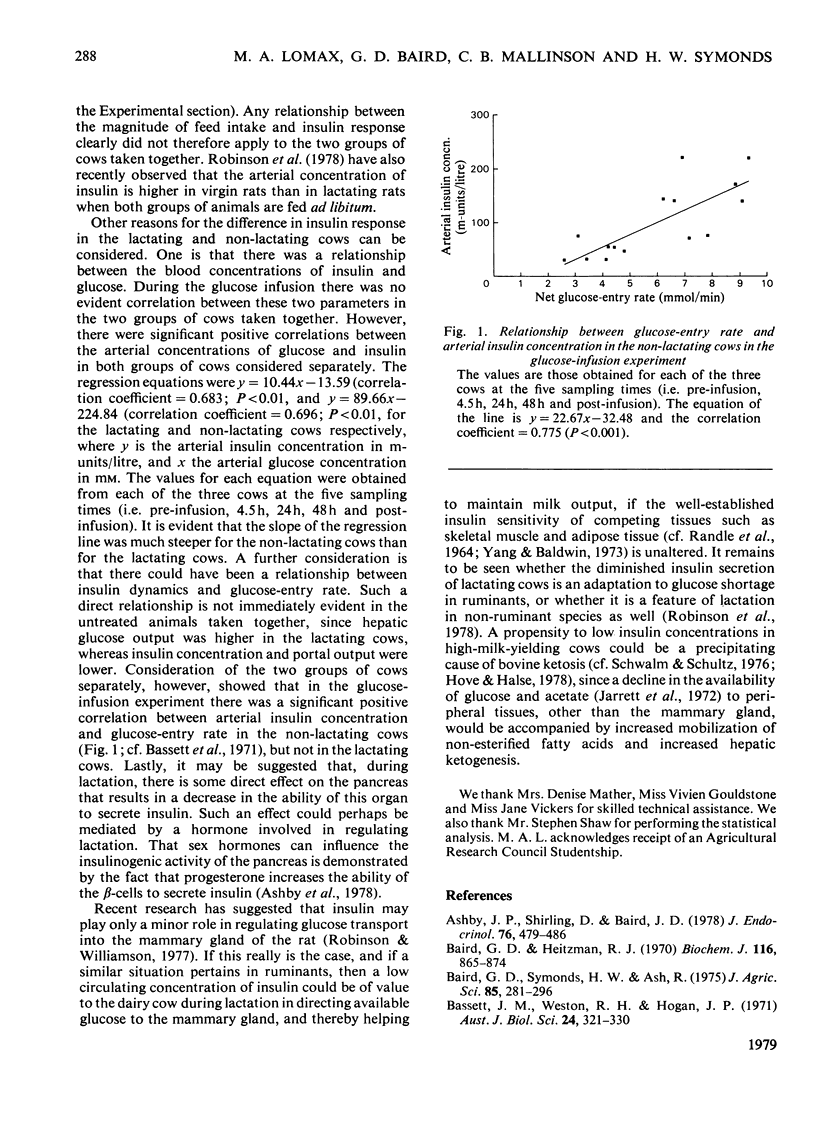
1. Four parameters of insulin metabolism were compared in catheterized lactating and non-lactating Friesian x Ayrshire dairy cows. 2. The four parameters, i.e. arterial and portal-venous concentrations of insulin, and pancreatic output and hepatic uptake of insulin, were approx. 2-, 3-, 3- and 5-fold higher respectively in the non-lactating cows than in the lactating cows in the normal fed state. Statistical significance was not achieved for the differences in magnitude in the case of the latter two parameters, however. 3. All four parameters increased significantly about 4-fold when non-lactating cows were infused intravenously with glucose for 48 h at a rate of 4.2 mmol/min. The parameters also increased in the lactating cows during glucose infusion, but the values reached were substantially lower than in the non-lactating cows and the increases were not statistically significant. 4. Arterial insulin concentrations doubled in the non-lactating cows during a 3 h infusion of propionate into a mesenteric vein, but remained unaltered in the lactating cows. 5. Differences in insulin concentration and output between the lactating and non-lactating cows were not consistently related to differences in either glucose concentration or glucose-entry rate. Arterial propionate concentrations were similar in both groups of cows at all times. 6. It is concluded that in the dairy cow, insulin secretion in response to an insulinotropic agent is diminished during lactation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby J. P., Shirling D., Baird J. D. Effect of progesterone on insulin secretion in the rat. J Endocrinol. 1978 Mar;76(3):479–486. doi: 10.1677/joe.0.0760479. [DOI] [PubMed] [Google Scholar]
- Baird G. D., Heitzman R. J. Gluconeogenesis in the cow. The effects of a glucocorticoid on hepatic intermediary metabolism. Biochem J. 1970 Mar;116(5):865–874. doi: 10.1042/bj1160865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett J. M., Weston R. H., Hogan J. P. Dietary regulation of plasma insulin and growth hormone concentrations in sheep. Aust J Biol Sci. 1971 Apr;24(2):321–330. doi: 10.1071/bi9710321. [DOI] [PubMed] [Google Scholar]
- Brockman R. P., Bergman E. N. Quantitative aspects of insulin secretion and its hepatic and renal removal in sheep. Am J Physiol. 1975 Nov;229(5):1338–1343. doi: 10.1152/ajplegacy.1975.229.5.1338. [DOI] [PubMed] [Google Scholar]
- HARVEY R. B., BROTHERS A. J. Renal extraction of para-aminohippurate and creatinine measured by continuous in vivo sampling of arterial and renal-vein blood. Ann N Y Acad Sci. 1962 Oct 31;102:46–54. doi: 10.1111/j.1749-6632.1962.tb13624.x. [DOI] [PubMed] [Google Scholar]
- Hart I. C., Bines J. A., Balch C. C., Cowie A. T. Hormone and metabolite differences between lactating beef and dairy cattle. Life Sci. 1975 Apr 15;16(8):1285–1291. doi: 10.1016/0024-3205(75)90313-6. [DOI] [PubMed] [Google Scholar]
- Hart I. C., Bines J. A., Morant S. V., Ridley J. L. Endocrine control of energy metabolism in the cow: comparison of the levels of hormones (prolactin, growth hormone, insulin and thyroxine) and metabolites in the plasma of high- and low-yielding cattle at various stages of lactation. J Endocrinol. 1978 Jun;77(3):333–345. doi: 10.1677/joe.0.0770333. [DOI] [PubMed] [Google Scholar]
- Hedeskov C. J., Capito K. The effect of starvation on insulin secretion and glucose metabolism in mouse pancreatic islets. Biochem J. 1974 Jun;140(3):423–433. doi: 10.1042/bj1400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove K., Halse K. Absence of feeding-induced variations in plasma insulin in hypoglycaemic-ketonaemic cows. Acta Vet Scand. 1978;19(2):215–228. doi: 10.1186/BF03547627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett I. G., Filsell O. H., Ballard F. J. Metabolic and endocrine interrelationships in normal and diabetic sheep. Horm Metab Res. 1974;Suppl 4:111–116. [PubMed] [Google Scholar]
- Jenny B. F., Polan C. E., Thye F. W. Effects of high grain feeding and stage of lactation on serum insulin, glucose and milk fat percentage in lactating cows. J Nutr. 1974 Apr;104(4):379–385. doi: 10.1093/jn/104.4.379. [DOI] [PubMed] [Google Scholar]
- Katz M. L., Bergman E. N. Simultaneous measurements of hepatic and portal venous blood flow in the sheep and dog. Am J Physiol. 1969 Apr;216(4):946–952. doi: 10.1152/ajplegacy.1969.216.4.946. [DOI] [PubMed] [Google Scholar]
- Koprowski J. A., Tucker H. A. Bovine serum growth hormone, corticoids and insulin during lactation. Endocrinology. 1973 Sep;93(3):645–651. doi: 10.1210/endo-93-3-645. [DOI] [PubMed] [Google Scholar]
- Leng R. A. Glucose synthesis in ruminants. Adv Vet Sci Comp Med. 1970;14:209–260. [PubMed] [Google Scholar]
- Lohmann D., Liebold F., Heilmann W., Senger H., Pohl A. Diminished insulin response in highly trained athletes. Metabolism. 1978 May;27(5):521–524. doi: 10.1016/0026-0495(78)90017-3. [DOI] [PubMed] [Google Scholar]
- MORTIMORE G. E., TIETZE F., STETTEN D., Jr Metabolism of insulin-I 131; studies in isolated, perfused rat liver and hindlimb preparations. Diabetes. 1959 Jul-Aug;8(4):307–314. doi: 10.2337/diab.8.4.307. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Wright P. H. Effect of fasting upon insulin secretion in the rat. Am J Physiol. 1967 Oct;213(4):843–848. doi: 10.1152/ajplegacy.1967.213.4.843. [DOI] [PubMed] [Google Scholar]
- Manns J. G., Boda J. M., Willes R. F. Probable role of propionate and butyrate in control of insulin secretion in sheep. Am J Physiol. 1967 Apr;212(4):756–764. doi: 10.1152/ajplegacy.1967.212.4.756. [DOI] [PubMed] [Google Scholar]
- McAtee J. W., Trenkle A. Metabolic regulation of plasma insulin levels in cattle. J Anim Sci. 1971 Aug;33(2):438–442. doi: 10.2527/jas1971.332438x. [DOI] [PubMed] [Google Scholar]
- Paterson J. Y., Linzell J. L. Cortisol secretion rate, glucose entry rate and the mammary uptake of cortisol and glucose during pregnancy and lactation in dairy cows. J Endocrinol. 1974 Aug;62(2):371–383. doi: 10.1677/joe.0.0620371. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. M., Girard J. R., Williamson D. H. Evidence for a role of insulin in the regulation of lipogenesis in lactating rat mammary gland. Measurements of lipogenesis in vivo and plasma hormone concentrations in response to starvation and refeeding. Biochem J. 1978 Oct 15;176(1):343–346. doi: 10.1042/bj1760343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Control of glucose metabolism in isolated acini of the lactating mammary gland of the rat. The ability of glycerol to mimic some of the effects of insulin. Biochem J. 1977 Dec 15;168(3):465–474. doi: 10.1042/bj1680465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalm J. W., Schultz L. H. Relationship of insulin concentration to blood metabolites in the dairy cow. J Dairy Sci. 1976 Feb;59(2):255–261. doi: 10.3168/jds.S0022-0302(76)84192-6. [DOI] [PubMed] [Google Scholar]
- Shenkin A., Steele L. W. Clinical and laboratory assessment of nutritional status. Proc Nutr Soc. 1978 May;37(1):95–103. doi: 10.1079/pns19780013. [DOI] [PubMed] [Google Scholar]
- Shikama H., Ui M. Glucose load diverts hepatic gluconeogenic product from glucose to glycogen in vivo. Am J Physiol. 1978 Oct;235(4):E354–E360. doi: 10.1152/ajpendo.1978.235.4.E354. [DOI] [PubMed] [Google Scholar]
- Smith R. D., Hansel W., Coppock C. E. Plasma growth hormone and insulin during early lactation in cows fed silage based diets. J Dairy Sci. 1976 Feb;59(2):248–254. doi: 10.3168/jds.S0022-0302(76)84191-4. [DOI] [PubMed] [Google Scholar]
- Snoswell A. M., Costa N. D., McLean J. G. Interrelationships between acetylation and the disposal of acetyl groups in the livers of dairy cows. J Dairy Res. 1978 Oct;45(3):331–338. doi: 10.1017/s002202990001654x. [DOI] [PubMed] [Google Scholar]
- Symonds H. W., Baird G. D. Cannulation of an hepatic vein, the portal vein and a mesenteric vein in the cow, and its use in the measurement of blood flow rates. Res Vet Sci. 1973 Mar;14(2):267–269. [PubMed] [Google Scholar]
- Thompson J. R., Weiser G., Seto K., Black A. L. Effect of glucose load on synthesis of plasma glucose in lactating cows. J Dairy Sci. 1975 Mar;58(3):362–370. doi: 10.3168/jds.S0022-0302(75)84573-5. [DOI] [PubMed] [Google Scholar]
- Treacher R. J., Baird G. D., Young J. L. Anti-ketogenic effect of glucose in the lactating cow deprived of food. Biochem J. 1976 Jul 15;158(1):127–134. doi: 10.1042/bj1580127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenkle A. Effects of short-chain fatty acids, feeding, fasting and type of diet on plasma insulin levels in sheep. J Nutr. 1970 Nov;100(11):1323–1330. doi: 10.1093/jn/100.11.1323. [DOI] [PubMed] [Google Scholar]
- Walker C. K., Elliot J. M. Effect of roughage restriction on serum insulin in the dairy cow. J Dairy Sci. 1973 Mar;56(3):375–377. doi: 10.3168/jds.S0022-0302(73)85180-X. [DOI] [PubMed] [Google Scholar]
- Yang Y. T., Baldwin R. L. Preparation and metabolism of isolated cells from bovine adipose tissue. J Dairy Sci. 1973 Mar;56(3):350–365. doi: 10.3168/jds.S0022-0302(73)85178-1. [DOI] [PubMed] [Google Scholar]


