Abstract
Photoresponsive soft materials are everywhere in the nature, from human’s retina tissues to plants, and have been the inspiration for engineers in the development of modern biomedical materials. Light as an external stimulus is particularly attractive because it is relatively cheap, noninvasive to superficial biological tissues, can be delivered contactless and offers high spatiotemporal control. In the biomedical field, soft materials that respond to long wavelength or that incorporate a photon upconversion mechanism are desired to overcome the limited UV–visible light penetration into biological tissues. Upon light exposure, photosensitive soft materials respond through mechanisms of isomerization, crosslinking or cleavage, hyperthermia, photoreactions, electrical current generation, among others. In this review, we discuss the most recent applications of photosensitive soft materials in the modulation of cellular behavior, for tissue engineering and regenerative medicine, in drug delivery and for phototherapies.
Keywords: Photoisomerization, Photothermal, Photodynamic, Photocleavage, Photovoltaic/Optoelectronic, Smart Soft Materials, Phototherapies, Regenerative Medicine, Cell Behavior, Drug Delivery
1. Introduction
Soft matter is present everywhere in nature and it has been the inspiration for engineers in the development of modern materials. Nature’s soft materials are composed of lipids, saccharides, peptides, and nucleotides that assemble into multiresponsive dynamic structures such as biological tissues, fat, muscles, etc. The term “soft” comes from their relatively low Young’s modulus, in the range of 104 to 109 Pa, in comparison with other classes of materials [1]. Artificial materials that can recapitulate the mechanical properties of biological materials such as polymers, gels, colloids, or liquid crystals, are of great interest for the design and fabrication of modern tools with applications in biomedicine [2], nanotechnology [3], and robotics [4,5]. However, prevalent applications require of soft materials with multifunctional dynamic properties just as found in nature. Synthetic soft materials undergoing rapid conformational changes in response to internal/external stimulus such as pH, ionic strength, temperature, or irradiation are of high scientific interest [6].
Biomedical soft materials that respond to internal stimulus such as pH, metabolite concentration or temperature may benefit from undesired foreign disturbances, however, naturally occurring physiological and pathological stimuli offer poor control over the dynamics of the material. Electrical currents, magnetic fields, ultrasound, and light are among the most popular external stimuli technologies investigated for biomedical soft materials [7]. For instance, electrical stimulation has been applied to manipulate the behavior of electroconductive soft materials [8-10]. However, this external stimulation approach requires a device/electrode wired to the system to deliver the electrical currents, which is often damaging to biological tissues [11]. The heat dissipation and reactive oxygen species (ROS) production through soft magnetic materials has been widely exploited in the biomedical field utilizing wireless magnetic fields as external stimuli [12,13]. The weak magnetic properties and low electrical conductivity of tissue allow magnetic fields to reach deep into the body with no attenuation of the signal [14-17]. Magnetic field stimulus strategies are limited by the potential off-target heating effects and the challenges in scaling high-frequency magnetic coils, which impede their universal adoption in clinical practices. The use of soft materials for stimulation with ultrasound has open the possibility for a technology with higher spatial resolution, however, even in the form of focused ultrasound, it has a broad radius of action on the order of millimeters to centimeters [18,19], resulting in undesired perturbation of biological tissues. Special attention in the biomedical field has been given to photoresponsive soft materials. Light provides a unique combination of wireless energy delivery with high spatial and temporal resolution [20]. Additionally, low-cost light sources can be tuned for their wavelength/frequency, intensity and chirality offering well-controlled stimulation [21]. A clear disadvantage of photosensitive soft materials in the biomedical field is the poor optical penetration of biological tissues at short wavelengths. To overcome this deficiency soft materials that respond to light in the near infrared wavelengths and nanotechnologies for photon upconversion have been developed. In general, photosensitive soft materials are designed to respond to light stimuli through specific light-triggered mechanisms as are photoisomerization, photocleavage, photocrosslinking, photothermal, photodynamic, photovoltaic, among others [22]. Photoisomerization implies a conformational change upon light illumination about a bond with restricted rotation, in general a double bond. In organic molecules with double bonds, such as azobenzenes, it involves isomerization between the trans and cis orientations upon irradiation with light of different wavelengths [23]. Isomers exhibit geometric structural differences, for instance, planar trans orientation of azobenzenes is more hydrophobic than the nonplanar cis orientation. While the microscopic geometrical change can be translated into macroscopic motion to control material shape or exert mechanical stress, the change in wettability can be used to trigger assembly-disassembly of nanocarriers and tune material interactions with drugs, proteins and cells [23,24]. Photocleavage involves the fission or splitting of chemical bonds upon light illumination. Photocleavage can be used to modulate the degree of gel crosslinking and soften a gel, remove protecting groups to expose targeting ligands or to trigger dissociation for controlled drug release [21,25]. Photocrosslinking is the light-induced formation of bonds linking polymer chains. It can be used to in situ control hydrogel gelation and stiffness, for cell encapsulation in mild conditions and for phototriggered shrinking of polymer multilayer particles followed by drug release [22,26,27]. A photothermal material is able to convert light energy into thermal energy [28]. Polymers and soft materials exhibiting photothermal conversion capacity can be used to, upon light illumination, stimulate temperature sensitive cells or kill specific cell populations such as tumor or bacteria cells. Photothermal materials can be also coupled with thermoresponsive materials, i.e., materials able to experience geometric or physicochemical changes following temperature variations, to control cells or trigger drug release [29,30]. Photodynamic effect refers to the generation of reactive oxygen species or free radicals upon illumination of a photosensitizer [31]. The local increase of concentration of these cytotoxic species is generally used to kill cancer or bacteria cells [32]. Finally, photovoltaic effect is the conversion of light energy into electrical power. Photovoltaic biomaterials are often used in soft electronic tools and interfaces and to stimulate electrogenic cells such as neurons and muscle [33,34]. Here, we will review the most recent advances in photoresponsive soft materials for the modulation of cellular behavior, in tissue engineering and regenerative medicine, for the delivery of therapeutics and for phototherapies (Scheme 1).
Scheme 1.
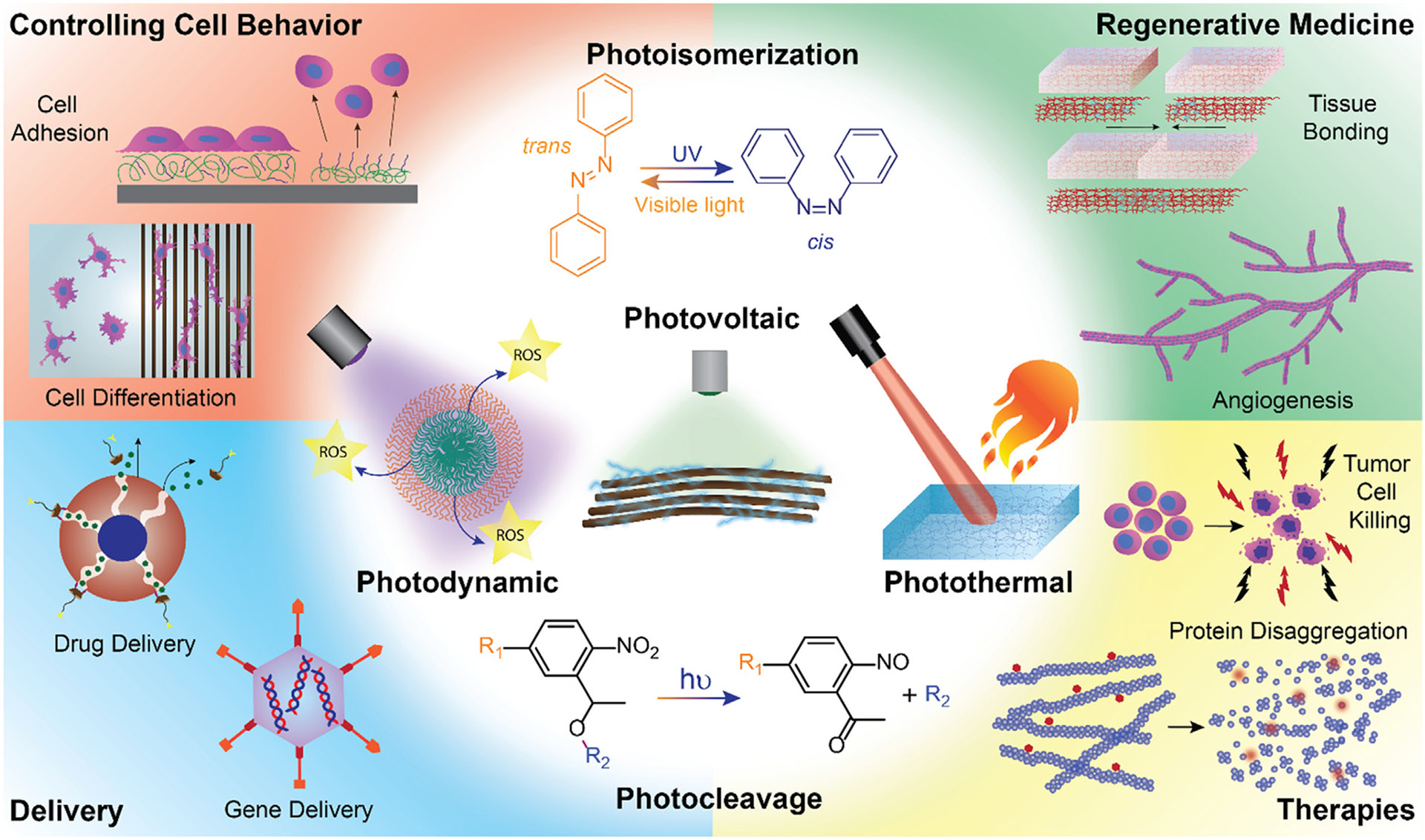
Schematic representation of different photo-triggered effects and the use of photo-responsive materials for controlling cell behavior, for delivery and regenerative medicine strategies and for phototherapies.
2. Photoresponsive soft materials for controlling cell function
The extracellular matrix (ECM) is a crucial, non-cellular component in tissues and organs that surrounds them and acts as the cell’s physical environment, modulating their behavior [35]. As such, ECM serves as nature’s template for the development of new biomaterials and scaffolds that frequently try to emulate its natural properties, sensitivity and dynamic behavior [36,37]. The ECM has intrinsic biochemical and mechanical cues that regulate cell phenotype and function, tissue morphogenesis, homeostasis and response to injury [38]. Furthermore, each tissue has a unique ECM composition and topography that interacts dynamically and reciprocally with the cells [39]. Since static scaffolds often fail to mimic the ECM dynamicity, it is of outmost importance to develop stimuli responsive scaffolds able to change their biochemical and/or physical properties in response to biocompatible stimuli [40]. In this vein, molecular photoswitches, such as azobenzene or spiropyran, and other reversible chemistries have been incorporated into hydrogels to control polymer chain conformation and thereby emulate the dynamic nature of the ECM [41]. The development of new biomaterials to study the influence of isolated materials properties on cell behavior, and to understand the interplay of multiple factors on cellular function is fundamental for basic cell biology studies and for generating new in vitro models [42,43]. Moreover, as the ECM is a crucial mediator for most of the intracellular events, abnormalities in the ECM lead to the development of most mammalian diseases [44]. Thus, gaining knowledge on ECM-cell interactions may lead to the development of new therapeutic options. Cells’ interactions with surfaces depends on material’s properties such as chemistry, surface energy and charge, wettability, roughness, morphology, rigidity and deformability [45]. Different nano and microscale engineering techniques such as layer-by-layer assembly [46], microcontact printing [47], and electrospinning [48] can be used to synthetize soft biomaterials with controlled biochemical and physical properties. Soft photoresponsive nanomaterials and biointerfaces have been also developed for engineering precise and dynamic cell microenvironments, manipulate cell behavior and trigger specific cellular responses [26,49,50]. Different photoresponsive reactions, such as photocrosslinking, photocleavage and photoisomerization, have been employed to elicit specific material responses such as stiffness change, degradation or shape change [26]. In the following subsections, the use of different photo-responsive soft materials for controlling cell behavior will be reviewed.
2.1. Cell adhesion, spreading and morphology
Cell adhesion, i.e., the ability of a cell to attach to other cells or to a surface through specialized protein complexes, is the first of many physiological and pathological processes such as morphogenesis, wound healing, and tumor progression [51,52]. Material characteristics such as topography [53], stiffness [54], surface physicochemical properties (e.g. wettability, polarity and charge) and pro-adhesive protein adsorption [45,55], influence cell adhesion, often in a cell-line specific fashion, and may be controlled to obtain a particular cellular response. Depending on the application, preventing or promoting selective cell adhesion may be desired. Photoresponsive materials may take advantage of photoisomerization, photocleavage or photothermal effects for controlling cell adhesion [56]. In reference [57], Wu et al. presented a photocured thiolated hyaluronic acid (HA) hydrogel and its in-situ remodeling capacities with light stimuli and small molecule diffusion. Synthetic thiolated HA polymers were crosslinked by UV irradiation in the presence of a radical photoinitiator, which lead to the radical-mediated photooxidation of thiols into disulfide. The obtained hydrogels presented both self and photoinduced healing properties. The authors further explored the hydrogel remodeling via photodegradation, being able to obtain patterns of soft gel areas within the stiff gel by employing a photomask. Hydrogel reversible remodeling was possible by free thiol molecules such as glutathione at physiologically relevant concentrations. Photocrosslinked thiolated HA hydrogels with Young’s modulus in the range 260–680 Pa were able to sustain human mesenchymal stem cells (hMSCs) adhesion and spreading, but adhesion was impaired in hydrogels with larger crosslinking densities. In reference [58], Karimipour et al. developed spiropyran (SP)-based photoswitchable acrylic nanofibers for dynamic control of cell adhesion and detachment. The authors used emulsion polymerization to synthetize photoresponsive poly (methyl methacrylate-co-hydroxy ethyl methacrylate-co-spiropyran ethyl acrylate) terpolymer that was further used to create nanofibers and film coatings by electrospinning and drop-casting techniques, respectively. Reversible photoswitching between orthogonal, hydrophobic, colorless SP and hydrophilic, zwitterionic and colored merocyanine (MC) can be achieved upon illumination with UV and visible light. It was found that the higher exposure surface and porosity of the nanofibrillar structure can be occupied by hydrophobic air leading to higher water contact angle (higher hydrophobicity) in the SP nanofibers relative to the SP films. Furthermore, it explains the faster photoswitching rate of MC to SP found in the nanofibers in comparison to the films. Switchable glioma cancer C6 cells attachment and detachment on nanofibrillar samples was achieved upon visible light and UV light illumination. In another approach using SP-functionalized polymer, He et al. developed a photoresponsive interface able to spatiotemporally modulate wettability, cell adhesion and detachment in a reversible manner [59]. SP molecules were introduced into the hydrophobic block of an amphiphilic diblock copolymer composed of poly (methyl methacrylate) (PMMA) and polyethylene glycol (PEG). Then, the copolymer was immobilized on a glass substrate by spin-coating. The surface exhibited reversible changes in wettability with the alternating irradiation of UV and visible light. Interestingly, due to the diblock structure of the copolymers the direction of change was opposite to the polarity change in the photoisomerization of SP. The obtained surface is cell repellent and can be turned into cell-adhesive upon UV-light illumination. Cell detachment can be further induced by visible light irradiation.
Smart surfaces may be further modified with target bioactive ligands such as DNA or peptides to obtain selective cell adhesion. In reference [60] Zhang et al. developed photoswitchable selective protein/cell adhesion surfaces via self-assembled monolayers (SAMs) based on sugar(galactose/mannose)-modified azobenzene derivatives. The inactive cis-state of the monosaccharide-azobenzene modified surface can be switched upon visible light illumination to the trans-state where the hydrophilic saccharide protrudes. This allows selective binding of lectins (proteins that recognize sugars) and cells that highly express sugar receptors, such as HepG2 cells that overexpress the galactose-selective asialoglycoprotein receptor and macrophage cells that express mannose receptor. The smart surface can be switched off by UV-light illumination, which induces photoisomerization towards the cis-state and exposure of the hydrophobic azobenzene moiety, leading to weakened affinity towards biomacromolecules and cells. In another approach for the reversible capture and release of targeted cells, Li et al. developed a photoresponsive SP-coated nanostructured surface with both structural and molecular recognition (Fig. 1a-d) [61]. The first arises from the interaction between the nanofractal silica surface and the protrusions of the cell surface that improves cell capture capability. The latter is regulated by phototriggered wettability changes. The ring-closed SP form can be switched to the ring-opened MC form upon UV illumination, leading to a surface wettability transition from hydrophobic to hydrophobic. The MC form can be switched back again to the SP form upon visible light exposure. However, in general, the light-triggered wettability transition on photoresponsive surfaces is limited, reducing cell release efficiency. Thus, the authors amplified the wettability transition by introducing photo-irresponsive and hydrophilic 2-hydroxyethyl methacrylate (HEMA) to the spiropyran derivatives (SPMA) (Fig. 1b). As HEMA provides more free space for SPMA isomerization, the phototriggered wettability switch is facilitated. Furthermore, HEMA hydrophilicity resists nonspecific cell adhesion. Biotinylated bovine serum albumin (BSA) can be adsorbed onto hydrophobic poly-(HEMA-co-SPMA) through hydrophobic interactions, and detached from the surface upon UV-light illumination (Fig. 1c). Using streptavidin as a linker, biotinylated epithelial cell adhesion molecule antibody can be further connected to biotinylated BSA as a cell capture agent (Fig. 1d). The designed surface presented high specific isolation of epithelial cell adhesion molecule-positive cells and high resistance to non-specific adhesion. Cell specificity can be also achieved by other targeting ligands such as aptamers, i.e. single-stranded short nucleotide sequences [62]. In this vein, Huang et al. employed o-nitrobenzyl phosphate ester photoprotected nucleic acids for photopatterning of nucleic acid tethers on polyacrylamide hydrogel films [63]. Stiffness differences between the patterned regions and the surrounding hydrogel matrix were observed. Mucin 1 protein (MUC-1) is a glycoprotein which is overexpressed in some human cancer cells membranes. By using anti MUC-1 DNA aptamers, HeLa cells were deposited on domains with circular shape.
Fig. 1.
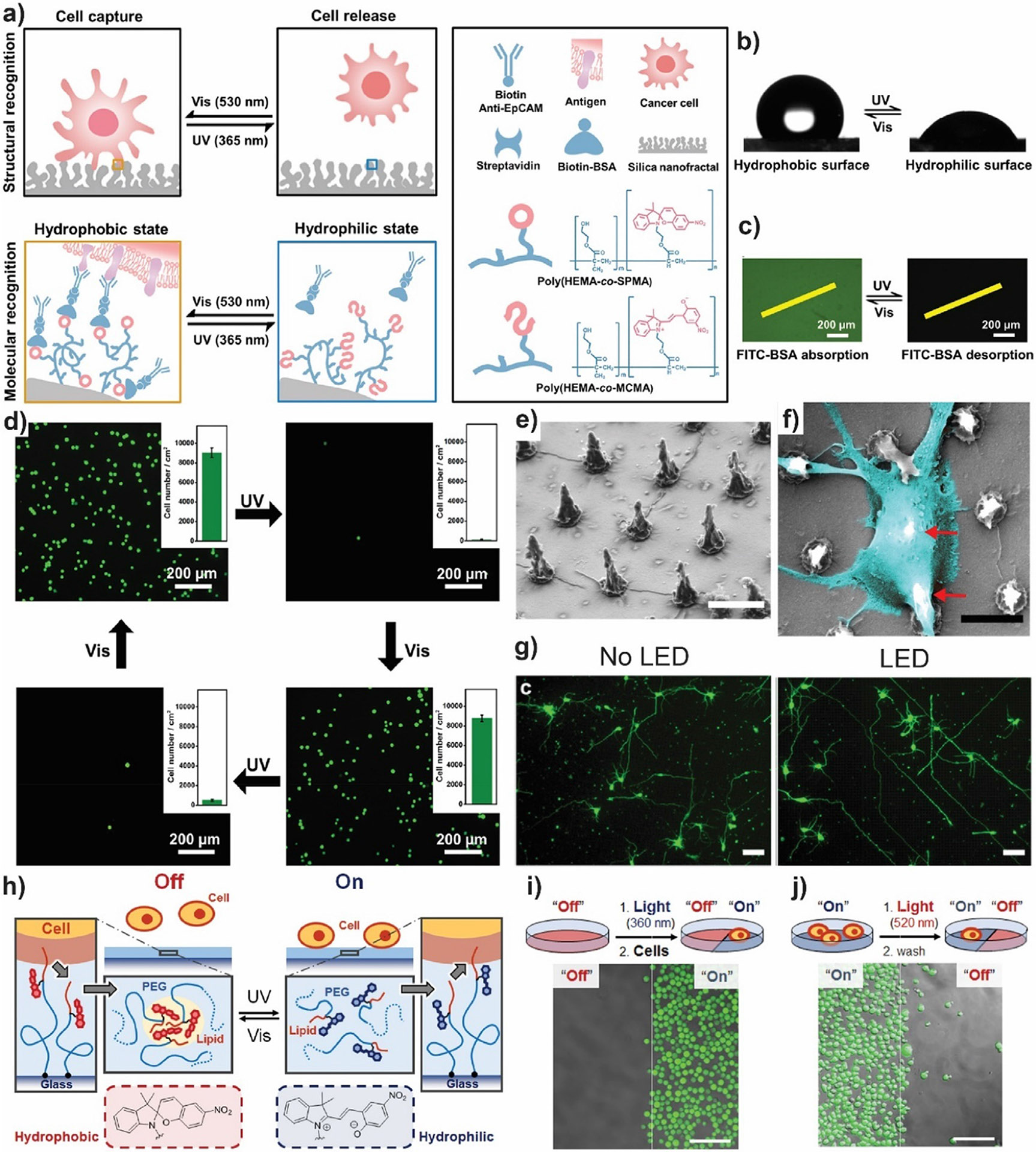
a) Scheme of the photo-irresponsive molecule-enhanced wettability switch on photoresponsive nanostructured surfaces toward targeted cancer cells reversible capture. Yellow and blue color in the nanostructured surfaces represent the hydrophobic and hydrophilic properties of photoresponsive components, respectively. b) Evaluation of the surface wettability of the modified silica nanofractal under UV and visible light irradiation. c) Adsorption/desorption of green fluorescent BSA under UV and visible light irradiation. d) Reversible capture and release of target breast cancer cell line (MCF-7) on the photoresponsive nanostructured surface by alternately changing UV and vis light irradiation and employing epithelial cell adhesion molecule antibody as a cell capture agent. Insets show the number of cells adhered on the surface during the reversible capture and release process. e) Scanning electron microscopy (SEM) images of P3HT micropillar array. Scale bar: 5 μm. f) Focused ion beam/SEM characterization of a rat primary cortical neuron soma suspended over two pillars (red arrows). Scale bar: 5 μm. g) Growth of neuronal cells on P3HT micropillars after 3 days in vitro with and without photostimulation (green: β-III-tubulin). Scale bar: 50 μm. h) Scheme of the working hypothesis of reversible cell immobilization with spiropyran-functionalized PEG-lipids conjugates. In the hydrophobic ‘closed’ form, the lipid moiety may be localized in hydrophobic assemblies inside the PEG layer, thus preventing their interaction with cells. In the hydrophilic ‘open’ form, the PEG-lipid may be dispersed in solution facilitating interaction between lipid moiety and the lipid bilayer of cells. Confocal microscopy images of partial cell immobilization (i) and release (j) of murine pro-B non-adherent cell line expressing green fluorescent protein. Merged fluorescence and phase contrast image is shown. Scale bar: 100 μm. a)-d) Adapted with permission from [61]. Copyright 2019 American Chemical Society. e)-g) Adapted with permission from [64], 2020 American Chemical Society. h)-j) Adapted with permission from [65]. Copyright 2019 American Chemical Society.
Though adherent cells are mainly cultured on two-dimendional (2D) substrates, in vivo they are embedded in a three-dimendional (3D) dynamic environment composed by ECM and cells. Thus, developing dynamic and controllable 3D microenvironments is desired to better mimic in vivo conditions. In this context, Nagata et al. presented a 3D microfiber scaffold with photoresponsive polymer surfaces for regulating cell adhesion by means of light stimulation [66]. The authors employed a core-sheath electrospinning technique to form bilayer fibers composed of an inner core of PMMA with a diameter of ca. 1 μm and a 30 nm thickness outer sheath of copolymers of SPMA and PMMA (poly (SPMA-co-MMA)). Adhesion of bovine aortic endothelial cells on the core-sheath nanostructures could be regulated by the light-induced chemical changes. While hydrophobic SP in the sheath layer prevented cell adhesion, the isomerization to hydrophilic MC upon UV exposure promoted cell adhesion, probable due to an enhanced interaction with amino acids in ECM proteins and formation of stable complexes. In another study, Ma et al. developed a 3D cell culture system with controlled cell adhesion and detachment based on photoresponsive smart hydrogel microspheres [67]. Hydrogel microspheres consisting of PEG and β-cyclodextrin (β-CD) modified poly (methyl vinyl ether-alt-maleic acid) were allowed to assemble with trans-azobenzene modified arginine-glycine-aspartate (RGD) integrin-binding peptide via host–guest interactions. Upon UV irradiation, photoisomerization of trans-azobenzene to cis-azobenzene leads to unbinding of the β-CD and azobenzene-RGD complex and subsequent cell detachment.
It is worth noting that cells are able to sense and respond to spatial variations or gradients in environmental physicochemical properties. In fact, rather than being homogeneous materials, several body tissues exhibit local gradual variations and a directional dependence in their biochemical and physical/structural properties [68]. These inhomogeneities may be reflected in patterning or in the change of concentration of biochemical cues along a direction, and also in patterning and gradients of structural and physical characteristics such as topography, mechanical or optical properties [69]. Thus, anisotropic biomaterials are of interest for tissue engineering applications. Moreover, as they allow to study the effect on cell behavior of a wide range of values of a determined property on the same surface, materials with gradient properties are very appealing for cell biology studies [70]. In reference [71], Ender et al. developed a photochemical strategy to generate gradients of integrin-binding peptide RGD on amyloid-like fibril scaffolds. A self-assembling β-sheet forming peptide was connected to an RGD motif using a photocleavable nitrobenzil linker. Then, the fibrils were spray-coated on glass substrates and the RGD motif was cleaved in a dose-dependent manner by a gradual irradiation with UV-light. A homogeneous distribution of nanofibrils with a cm-length scale gradient of RGD was obtained. A gradient of A459 adenocarcinoma epithelial lung cells adhesion was obtained, with less cells on the sections exposed to longer UV irradiation, i.e., lower RGD concentration. Gradients and patterned mechanical properties with submicrometer resolution have been obtained on photodegradable hydrogels by means of photolithography [72]. Even gradients of distinct parameters have been developed to study their cooperative influence on cell behavior. Rape et al. developed a HA hydrogel with orthogonally pattern gradients of substrate stiffness and fibronectin density using distinct wavelengths of light and a gradient photomask [73].
Multifunctional scaffolds and external stimuli can be combined to synergistically guide cell behavior and enhance desired outcomes. In reference [64], Milos et al. combined optical stimulation with topographical cues of the substrate to regulate neuronal growth (Fig. 1e-g). Thiophene-based polymer poly(3-hexylthiophene-2,5-diyl) (P3HT) was used due to its electrical and photoelectrical properties and biocompatibility [74]. Optically active P3HT micropillars array platform was obtained by push-coating technique (Fig. 1e). Micropillars topography allowed a close contact with cells, as shown in the actin cytoskeleton staining and in the SEM images (Fig. 1f). Neurons seeded on P3HT micropillars presented neurites ~ 40 % larger than on flat P3HT substrates or glass, showing the effect of micro and nano scale topography in promoting neurite growth. Moreover, while neurites of neurons seeded on flat substrates without topographical cues presented a random angle distribution, neurites of neurons seeded on the micropillar array were mostly aligned at 0, 45 and 90° relative to the direction of the pattern. Photostimulation of neurons seeded on optically inert substrates (glass or hybrid organic/ceramic polymer OrmoComp) had no observable effect on axon length. However, cells growing on P3HT substrates and stimulated with a green LED presented significantly larger axons than non-stimulated cells or cells stimulated with a red LED with negligible overlap with P3HT absorption (Fig. 1g). All in all, micro/nano topography of a photoresponsive substrate and wireless, noninvasive light stimulation can be combined to modulate the growth of rat primary cortical neurons in vitro.
Being able to separate or trap individual or a group of cells on substrates at a high density in a simple and accurate manner is essential for high-throughput single cell analysis and organ-on-chip technologies. However, retrieval of adherent cells may restrict target cell recovery. On the other hand, trapping weakly adherent and non-adherent cells may be troublesome. In reference [75] Jarzębska et al. developed a photoresponsive material with strong trapping capability based on photo-cleavable PEG-lipid. The material consisted of a long PEG chain, which is known to inhibit cell adhesion, connected to a photo-cleavable linker moiety and an oleyl group able to interact with lipid bilayers of cell membranes. In order to improve cell trapping, different spacers were placed between the photo-cleavable linker moiety, finding the best results with oligo (ethylene glycol) spacers. The obtained material was used to coat a collagen surface. Then, UV-light and a photomask were used for light-guided micropatterning, removing oleyl groups via photo-induced cleavage. Model immunocytes were selectively trapped in non-irradiated areas as single cells, and were further released from the substrates following UV light illumination. The same group reported the reversible, photoresponsive and spatioselective trapping of nonadherent cells by SP-functionalized PEG-lipids conjugates [65]. SP was attached to different PEG-lipid conjugates to find the optimal for photoswitchable cell trapping. SP-conjugated PEG – lipid were immobilized on collagen-coated substrates. In the ‘closed’ and hydrophobic SP form the PEG-lipid may form hydrophobic assemblies and hamper interaction between cells and lipid moieties (Fig. 1h). Upon UV light illumination, isomerization to the ‘open’ hydrophilic MC form induced the PEG-lipid to be disperse in solution, allowing the lipid moiety to interact with cells. The substrate enabled fast and switchable non-adherent and adherent cells immobilization and release upon UV and visible light irradiation, respectively (Fig. 1i-j). Spatially selective photo-switching was also demonstrated illuminating part of the surface area. Using a different approach, Yamaguchi et al. developed a photocleavable RGD-PEG conjugate surface for light-guided patterning and recovery of adherent cells [76]. The RGD peptide that binds to integrins and leads to cell adhesion and spreading can be released from the surface upon exposure to nontoxic levels of light, allowing for cell recovery. Facile patterning of cell adhesive and non-adhesive regions and further cell release was achieved with light.
Cell shape and cell membrane curvature dynamically change in response to complex intracellular and extracellular cues, affecting several curvature-dependent intracellular processes such as endocytosis, exocytosis and actin dynamics [77]. Though prefabricated nano and micro structured static surfaces are useful for studying curvature-dependent processes, they fail to mimic dynamicity aspects of cell membrane curvature and cell-ECM interactions. In this regard, De Martino et al. developed a light-responsive azobenzene-based polymer structure in which the shape changed from vertical pillar to an elongated vertical bar upon green light illumination [78]. Ordered arrays of vertical micropillars of poly (dispersed Red 1 methacrylate) (pDR1m) were fabricated on glass coverslips by soft lithography. By means of green polarized light, a trans–cis isomerization can be induced and an athermal transition from glassy azopolymer to a fluid state is achieved. This leads to a distinct mass migration along the direction of the light polarization and a pillar to ellipsoidal bar shape transition. The light-triggered reshaping process induced dynamic changes in human bone osteosarcoma epithelial cells (U2OS) membrane curvatures, promoting accumulation of actin fibers and actin nucleation factor in locations with high curvature. Rossano et al. employed pDR1m as a substrate to display dynamic concentric rings patterns to fibroblastic NIH-3T3 cells by means of a focused laser beam of a confocal microscope [79]. Cells were strongly influenced by 10 μm crest-to-crest distance circular patterns but perceived 5 and 15 μm circular patterns as flat surface. As fast as 2 h after pattern inscription, cells reconfigure their shape and align along pattern direction assuming a circular disposition. Fibroblasts cultivated on 10 μm patterned substrates possessed a significantly softer cell body than cells on flat surfaces. Moreover, cells presented a less organized actin network and shorter focal adhesion, probably due to substrate curvature impairment maturation of the cytoskeleton.
A brief summary of strategies for controlling cell adhesion reviewed in this subsection is presented in Table 1.
Table 1.
Summary of strategies for controlling cell adhesion reviewed in this article.
| Phototunable Property |
Material | Phototriggered effect | Observed Properties and Effects | Ref |
|---|---|---|---|---|
| Stiffness | Thiolated HA polymers | Photocrosslinking and photodegradation | Photoinduced healing, patterned stiffness, and control of hMSCs adhesion | [57] |
| Wettability | SP based acrylic nanofibers | Photoisomerization | Dynamic control of cell adhesion and detachment | [58] |
| Wettability | Amphiphilic diblock copolymer with SP moieties in the hydrophobic block | Photoisomerization | Opposite polarity changes to the expected for photoisomerization of SP, switchable cell-repellent cell-adhesive surface | [59] |
| Ligand presentation | Sugar-modified azobenzene derivatives | Photoisomerization | Switchable and selective protein/cell adhesion | [60] |
| Wettability | Copolymer of SP derivatives and photoirresponsive molecule. Molecular recognition system | Photoisomerization | Amplified wettability transition, reversible capture and release of targeted cells | [61] |
| Stiffness and ligand presentation | O-nitrobenzylphosphate ester photoprotected nucleic acid-based polyacrylamide hydrogel | Photocleavage | Patterned and selective HeLa cell adhesion using specific aptamers | [63] |
| Wettability | Core-sheath fiber scaffold, the sheath being a SP containing copolymer | Photoisomerization | 3D scaffold with controllable cell adhesion | [66] |
| Ligand presentation | Hydrogel microspheres with β-CD moieties and RGD-functionalized azobenzene | Photoisomerization | 3D scaffold with controllable cell detachment | [67] |
| Ligand presentation | β-sheet forming peptide connected to RGD motif using a nitrobenzil linker | Photocleavage | Gradient of RGD on the fibrils and gradient of cell adhesion | [71] |
| Electrical stimulation | P3HT micropillars | Photovoltaic | Alignment and length increase of neurons axons | [64] |
| Ligand presentation | PGG connected to an oleyl group by a photocleavable linker | Photocleavage | Patterned nonadherent cell trapping and controllable release | [75] |
| Ligand presentation | SP-conjugated PEG – lipids | Photoisomerization | Spatially, switchable non-adherent and adherent cells immobilization | [65] |
| Ligand presentation | Photocleavable RGD-PEG conjugate surface | Photocleavage | Patterning of cell adhesive and non-adhesive regions and further release | [76] |
| Substrate topography | Vertical micropillars of pDR1m | Photoisomerization and mass migration | Pillar to ellipsoidal bar shape transition and changes in cell actin dynamics | [78] |
| Substrate topography | pDR1m substrate | Photoisomerization and mass migration | Generation of dynamic concentric rings patterns led to changes in fibroblasts’ shape | [79] |
2.2. Cell migration and mechanobiology studies
Both individual and collective cell migrations play crucial roles in several physiological and pathological processes [80]. For instance, single-cell migration towards or between tissue compartments takes place with leukocytes migration during inflammation. Collective cell migration, i.e., the movement of physically and functionally connected cells in a coordinated manner, is essential in embryogenesis, wound healing and is involved in many disease processes such as cancer metastasis [81]. Different external cues such as substrate topography [82] and gradients of substrate stiffness or soluble chemoattractants [83] are known to interplay with cell intrinsic cues in order to regulate cell migration [84]. Smart photoresponsive materials offer the possibility of mimicking the dynamic microenvironment of cells and allow the in -situ control of extrinsic cell migration regulators.
The programmable and adaptive nature of photoresponsive liquid crystal networks (LCN) coatings has been used to study the effect of in situ changes of micro and nanoscale topographical cues in cell adhesion and migration (Fig. 2a-c) [85]. A mixture of methacrylate functionalized azobenzene and liquid crystalline monomers were photopolymerized. Illumination using a photomask leads to local trans to cis transition of azobenzene and local formation of protrusions and increased in nanoroughness. Cell adhesion and migration were analyzed either on surfaces with fixed microscale hexagonally arranged pillars (Fig. 2b-c), or on flat surfaces where nanoscale topography was changed in situ by UV-light illumination. A decrease in mean cell speed was observed after illumination, correlated with the increase in nanoroughness. In another approach using photoresponsive azobenzene-based materials, Isomäki et al. developed a light-responsive cell culture bilayer interface for reversible cell guidance [86]. The platform consisted on a thin layer of azobenzene-based molecular glass coated with a thin polydimethylsiloxane (PDMS) layer (Fig. 2d and 2e). Surface relief gratings (SRGs) were easily photo-inscribed and photo-erased, therefore allowing for reversible photomodulation (Fig. 2f). The inscribed gratings can guide epithelial cell orientation and migration along the topography. For small groups of cells, grating erasure led to rearrangement of phosphorylated focal adhesion kinase, an effect which was not visible for confluent layers of cells. In reference [87], Liu et al. developed a near-infrared (NIR) light-responsive optomechanical actuator for controllable modulation of collective cell migration. A thermoresponsive copolymer hydrogel composed of N-isopropylacrylamide (NIPAM) and acrylamide (AM) was doped with gold nanorods, which can transform NIR light illumination into heat, leading to hydrogel contraction and increase in Young’s modulus. Following surface functionalization with cell adhesive ligands, it was shown that applying contraction forces to cells by means of NIR light illumination, significantly increased random cell motility in comparison to controls. Using a microcontact printing strategy, patterned presentation of adhesive ligands and further cell alignment was achieved. Directed collective cell migration was stimulated by NIR light. Wu et al. designed a photo-responsive hydrogel with reversible tunable mechanical properties for optically controlling collective cell migration (Fig. 2g-j) [88]. The hydrogels were made of four-armed PEG-maleimide molecules crosslinked by the photoswitchable protein Dronpa145N (Fig. 2g and 2 h). Dronpa145N forms tetrameric species under violet light illumination and can be rapidly switched to the monomeric state under cyan light illumination, leading to a gel-sol transition and softening of the hydrogel (Fig. 2g and 2i). Cell adhesive ligand RGD was introduced to Dronpa145N proteins for promoting cell attachment to the hydrogels to perform a wound healing cell migration assay. The authors found a correlation between stiffness of the hydrogels and cell migration and were able to modulate cell migration rate in real time (Fig. 2j).
Fig. 2.
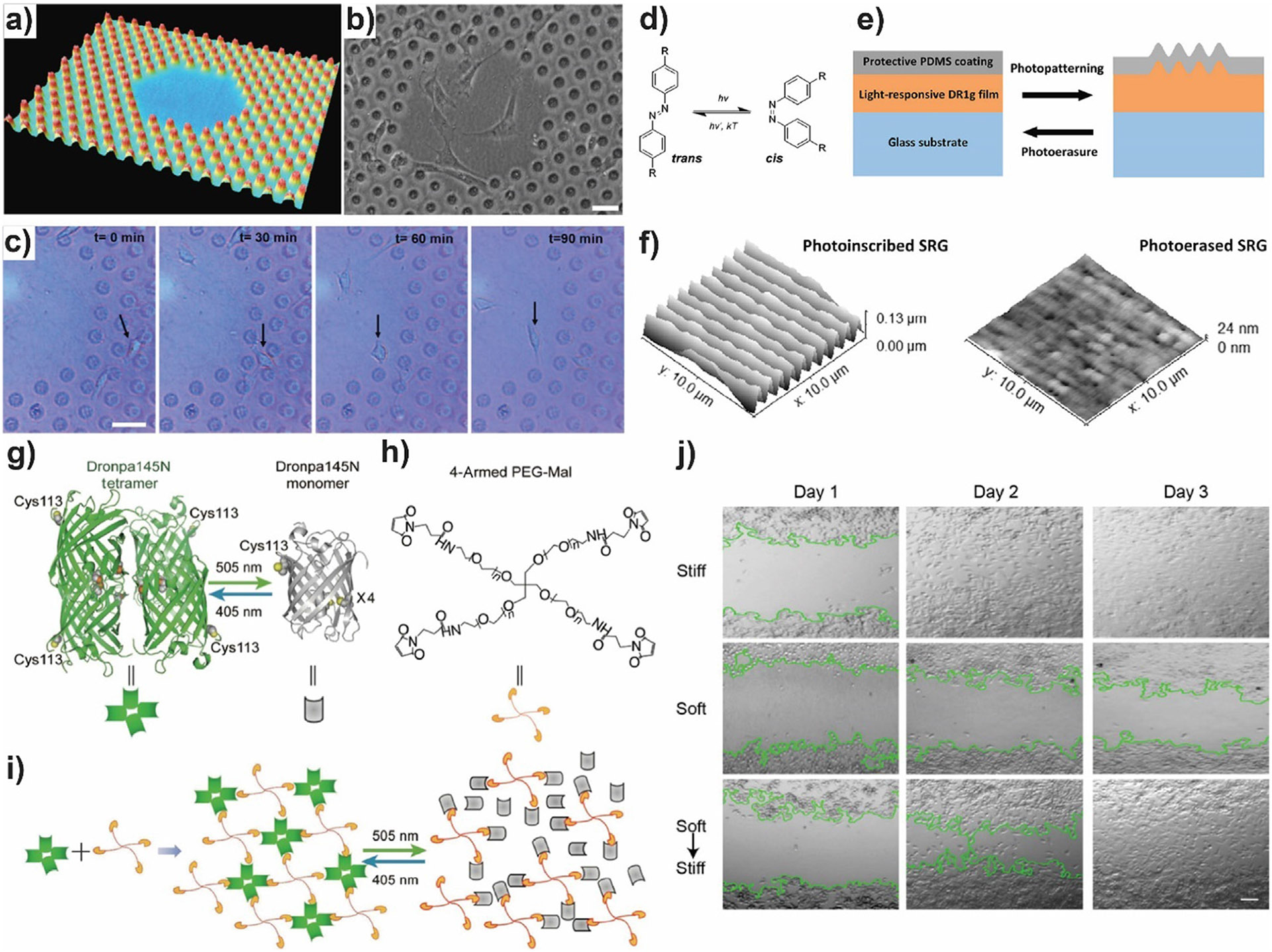
a) Three-dimensional representation of the mixed flat not entirely illuminated surface surrounded by hexagonally arranged pillars with a height of 1.1 μm. b) Phase contrast image of NIH3T3 fibroblasts on liquid crystal polymer network (LCN) surface after 3 days of culture. c) Live cell imaging of a representative cell going from the pillar area to the flat area. Scale bar: 50 μm. d) Photoisomerization of azobenzene between the thermodynamically stable trans isomer and the metastable cis isomer. e) Scheme of the bilayer structure and the azobenzene-driven surface relief gratings (SRG) formation and erasure (DR1g: disperse Red 1-containing molecular glass; PDMS, polydimethylsiloxane). f) AFM images of the surface topography of DR1g-PDMS1 after inscription (left) and erasure (right). g-j) Scheme of the photoresponsive hydrogel made of four-armed PEG crosslinked by Dronpa145N. g) Dronpa145N oligomerization states can be switched by irradiation with cyan and violet light. In both states, only one thiol group is exposed. h) Four-armed PEG- maleimide chemical structure. Maleimide end of each arm can react with thiol exposed from Dronpa145N.i) Illustration of the gel (left), sol (right) and photocontrolled sol–gel transition. j) Controlling cell migration in a wound closure experiment by tuning hydrogel stiffness. Representative pictures taken at different time points. The green line shows the colony interface. Scale bar 50 μm. a)-c) Adapted with permission from [85]. Copyright 2017 Wiley. d)-f) Adapted from [86]. g)-j) Reprinted by permission from [88], Copyright 2018 Springer Nature.
Although extrinsic factors such as chemical and mechanical cues may affect collective cell migration, intrinsic conditions such as cell density and colony shape may also play an important role [89,90]. In order to study and resolve the interplay between mechanical, chemical and geometrical factors, Yamamoto et al. developed a photoactivable hydrogel platform to study cell migration under standardized conditions [91]. The substrate consisted on a poly(acrylamide) hydrogel, which allowed controlling mechanical properties, functionalized with poly-d-lysine, which allowed for controlling ECM proteins adsorption, and photocleavable PEG, which allowed to control colony geometry by photopatterning. Even though 2D cell migration has been studied in detail, elucidating how cells migrate in the complex 3D environments of living tissues is important for understanding several biological processes and for tissue engineering applications [92]. In reference [93], Cao et al. studied how endothelial cells (ECs) migrate into photoresponsive HA hydrogels with tunable stiffness under the presence of proinflammatory macrophages (M1). HA was grafted with methacrylate moieties, which crosslinking is insensitive to environmental stimuli and thus maintain hydrogel integrity, and with coumarin, which dimers formed at 365 nm can be decomposed by irradiation at 254 nm leading to a decrease in hydrogel compressive modulus and increase in mesh size and swelling. Hydrogels irradiated or not at 254 nm were placed in a Boyden chamber and ECs were seeded on top, while pro-inflammatory macrophages were seeded in the well. After 7 days, a significant larger displacement of ECs was found in the softer hydrogel irradiated at 254 nm than in the stiffer one.
The process by which cells are able to sense mechanical cues from their environment and transduce these cues into biochemical signals is called mechanotransduction. Active biomaterials that can apply dynamic mechanical cues to cells and tissues in a controllable manner are very appealing for mechanobiology studies [94]. Different light manipulation strategies have been employed to temporarily modulate mechanical properties in synthetic matrices. Lee et al. incorporated an azobenzene crosslinker to a polyacrylamide-based hydrogel to obtain a scaffold with photo-switchable mechanical properties for minimally invasive mechanotransduction in vitro studies [95]. Softening and stiffening of the hydrogel was induced by near-UV and visible blue light, respectively, leading to changes in primary human MSCs spreading and morphology. Brown et al. synthetized soft hydrogels via strain-promoted azide – alkyne cycloaddition (SPAAC) reaction and exploited the ability of azadibenzocyclooctyne to undergo a photocrosslinking reaction for on-demand stiffening of the 3D scaffold [96]. Taking advantage of the favorable kinetics of the SPAAC reaction at physiological conditions, C2C12 mouse myoblasts were encapsulated in the 3D hydrogels and further subjected to dynamic stiffening of the extracellular environment. Cells sensed and responded changing their spreading and localization of Yes-associated protein 1. In reference [97] Homma et al. designed a hydrogel composed of an azobenzene acrylate and N,N-dimethyl acrylamide copolymer that undergoes stiffness changes by photo-induced phase transition. The combination of hydrophilic N,N-dimethyl acrylamide and hydrophobic azobenzene acrylate allows the copolymer to phase separate in aqueous solution with the increase of temperature. Furthermore, the photoisomerization of azobenzene that causes changes in hydrophilicity shifts the phase separation temperature. The authors tuned the amount of azobenzene so the phase transition solely occurs by light irradiation and azobenzene photoisomerization at around 37 °C. Upon UV and visible light illumination, the hydrogel undergoes reversible swelling-deswelling and softening-stiffening. Human breast cancer MCF-7 cells seeded on the gels showed varied responses in gene expression levels of E-cadherin upon stiffening at different timepoints.
Measuring scaffold stiffness in situ may be time consuming, invasive and difficult for some systems such as microfluidic chips. In this vein, Li et al. developed a photoresponsive and photonic hydrogel for in situ light-manipulation and monitoring of scaffold stiffness via color change [98]. The photonic hydrogel was synthetized by copolymerizing a coumarin-containing acrylate monomer and PEG diacrylate in the presence of mono-dispersed silica nanoparticles (Fig. 3a-c). Coumarin reversible dimerization upon illumination was used to dynamically control hydrogel stiffness. Silica nanoparticles form periodic structures during hydrogel formation leading to a photonic band gap that reflects light at specific wavelengths depending on the center-to-center distance between nearest particles (Fig. 3a). Light-induced changes in hydrogel mechanical properties are associated with swelling-deswelling processes that lead to changes in the distance between silica nanoparticles and thus, to a change in hydrogel color (Fig. 3d-f). The authors demonstrate the feasibility of applying this photonic hydrogel strategy to microfluidic chips and cell culture microarrays. As in many physio and pathological processes cells are exposed to transient, cyclic forces, Chandorkar et al. developed a patterned beating hydrogel system that can be spatiotemporally controlled using NIR light trigger [99]. By mixing N-isopropyl acrylamide and N-ethyl acrylamide a volume phase transition temperature around 37 °C was achieved. Gold nanorods were added for photothermal heating and the surface was coated with cell adhesive proteins for cell attachment. Local nN-scale mechanical actuation (beats) with specific amplitude and variable frequencies was achieved by using a NIR laser (Fig. 3g). L929 fibroblast cells exhibited changes in migration, nuclear translocation of the mechanosensory protein myocardin related transcription factor A and increased secretion and alignment of fibronectin upon actuation (Fig. 3h and 3i). Liu et al. reported a modular semisynthetic approach to create protein-polymer hydrogel biomaterials for cyclic stiffness modulation upon user-specified stimuli [100]. By employing a dual-chemoenzymatic modification strategy, the authors created different fusion protein-based gel crosslinkers with stimuli-dependent intramolecular association. Taking advantage of reversible dissociation between light, oxygen, and voltage sensing domain 2 (LOV2) protein and Jα helix upon blue light illumination, the authors obtained hydrogels with phototunable mechanical properties. Moreover, they showed that cyclic mechanical loading drive fibroblasts to myofibroblasts transdifferentiation.
Fig. 3.
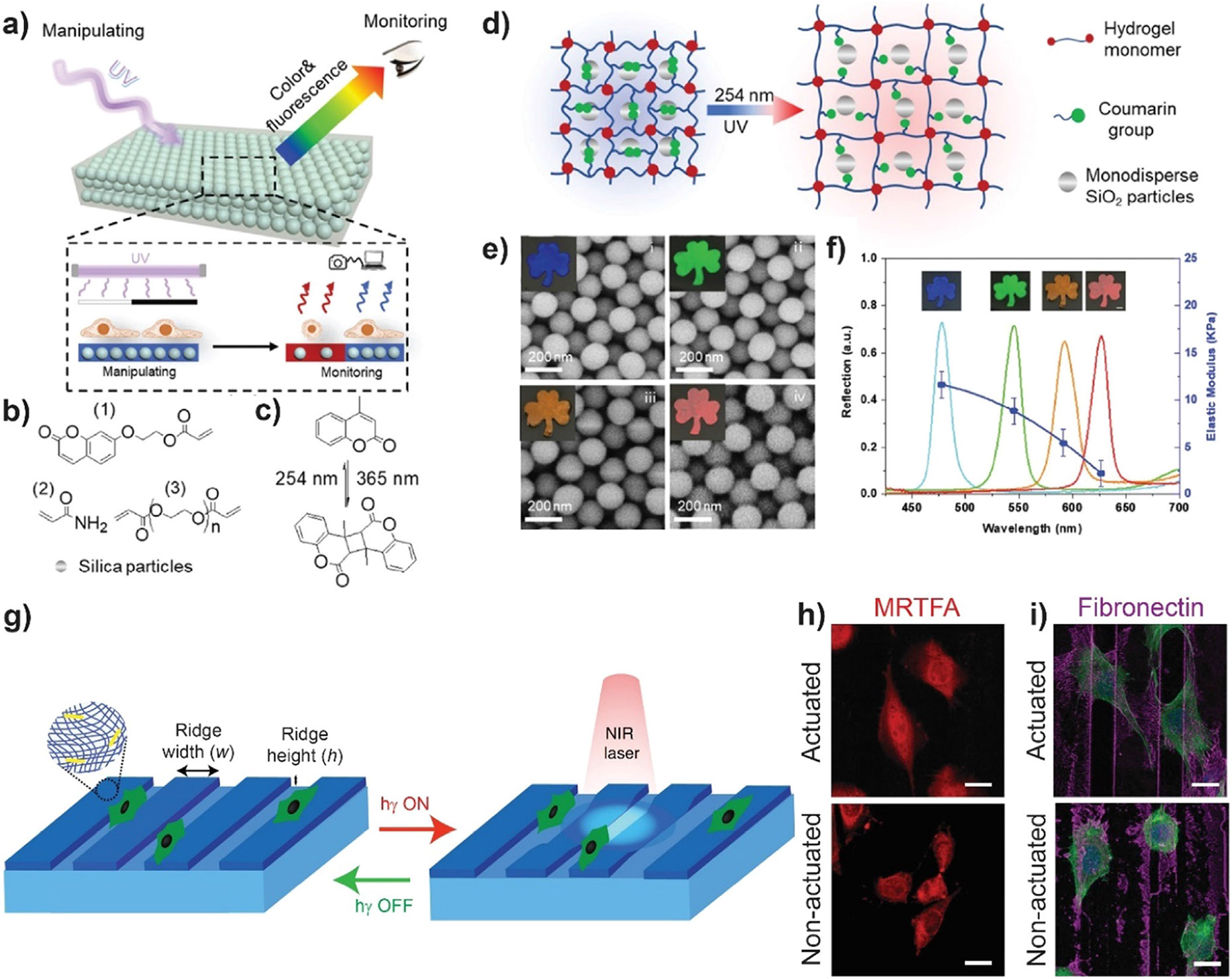
a) Scheme of the principle of in situ manipulation and monitoring of mechanical properties using photonic hydrogel. b) The hydrogel is synthesized by copolymerization of acrylate monomers in the presence of coumarin-containing acrylate and silica nanoparticles. c) Reversible photo-induced dimerization and cleavage of methyl coumarin group. d) Scheme of the reconfiguration and self-reporting of the photonic hydrogel. e) SEM and optical (inset) images of the generated hydrogel before and after reconfiguration. i) original hydrogel and after 2 (ii), 4 (iii) and 5 (iv) min illumination. As the hydrogels were freeze dried before SEM visualization, polymer networks were not visible from a top view. The center-to-center distance between the particles increases and the color of the hydrogel gradually changed from blue to red. f) Reflection spectra and the corresponding stiffness of the reconfigured hydrogels. g) Scheme of the soft and patterned light-triggered hydrogels composed of thermoresponsive polymer and gold nanorods. Gold nanorods convert NIR light-illumination into heat, leading to gel collapse. Pulsing laser results in actuating (beating) hydrogels. h-i) Immunofluorescent images showing the effect of actuation on mechanosensitive protein myocardin related transcription factor A (MRTFA) localization and fibronectin distribution. Cells in the actuating area show nuclear localization of MRTFA, whereas for cells in the non-actuating area MRTFA is in the cytoplasm (h). When cells are actuated, fibronectin secretion is more aligned (i). a)-f) Republished with permission of Royal Society of Chemistry, from [98]; permission conveyed through Copyright Clearance Center, Inc. g)-i). Adapted from [99]
A brief summary of strategies for controlling cell migration and for performing mechanobiology studies reviewed in this subsection is presented in Table 2.
Table 2.
Summary of strategies for controlling cell migration and for performing mechanobiology studies reviewed in this article.
| Phototunable Property | Material | Phototriggered effect |
Observed Properties and Effects | Ref |
|---|---|---|---|---|
| Substrate topography | Azobenzene containing liquid crystal networks | Photoisomerization and mass migration | Nanoroughness increase upon illumination leading to decreased cell speed | [85] |
| Substrate topography | Azobenzene-based molecular glass surface coated with PDMS layer | Photoisomerization and mass migration | Grating photo-inscription and photo-erasure that control cell orientation and migration | [86] |
| Stiffness | Thermo responsive copolymer hydrogel and gold nanorods | Photothermal | Optomechanical stimulation increases cell motility | [87] |
| Stiffness | Four-armed PEG-Dronpa145N hydrogel | Photoisomerization | Real-time modulation of substrate stiffness and collective cell migration | [88] |
| Stiffness | HA modified with methacrylate and coumarin moieties | Photocleavage | Increased endothelial cells migration into the softer hydrogels | [93] |
| Stiffness | Azobenzene crosslinker containing hydrogel | Photoisomerization | In situ tuning of substrate stiffness affecting MSCs adhesion and morphology | [95] |
| Stiffness | Azadibenzocyclooctyne containing click hydrogel | Photocrosslinking | Encapsulation of myoblast in 3D hydrogels and change in cellular localization of mechanoregulatory protein | [96] |
| Stiffness | Azobenzene-bearing hydrogel | Photoisomerization and phase transition | Reversible softening-stiffening affecting gene expression levels of E-cadherin | [97] |
| Stiffness and color | PEG diacrylate hydrogel containing coumarin and mono-dispersed silica NPs | Dimerization and cleavage | In situ manipulation of substrate stiffness and monitoring. Applicable in microfluidics and cell culture microarrays | [98] |
| Stiffness | Patterned thermoresponsive hydrogel containing gold nanorods | Photothermal | Mechanical actuation induces changes in cell migration, localization of mechanosensory protein and secretion and alignment of FN | [99] |
| Stiffness | Hydrogels containing crosslinkers with stimuli-dependent intramolecular association | Reversible association-dissociation | Myoblasts transdifferentiation under cyclic mechanical stimulation | [100] |
2.3. Cell differentiation and stimulation
Cell differentiation, i.e. the process in which cells become more specialized, is the result of the integration of different stimuli in a spatiotemporal manner [101]. Studying how extrinsic mechanical and biochemical cues guide cell fate is not only essential for understanding development and disease, but also for designing new therapeutic strategies [35,102].
Administration and delivery of growth factors [103], small molecules [104], and even ions [105], are among the most common strategies for inducing cell differentiation. In reference [106], Zhang et al. developed NIR-responsive multishell upconverting nanoparticles (UCNPs) constructs for spatiotemporal control of small-molecule release and neural stem cell differentiation (Fig. 4a and 4b). UCNPs are lanthanide-doped optical nanomaterials able to up-convert two or more lower energy-photons into one high energy photon [107]. The multishell structure was optimized to maximize NIR to UV upconversion and was further coated with a mesoporous silica shell that acted as a reservoir of small molecules. The resulting UCNPs were functionalized with a UV photoresponsive polymeric shell containing SP groups as a capping system and then, a colloidal stabilizing polymer layer of polyacrylic acid was further grafted (Fig. 4a). Using this UCNPs and NIR light illumination, retinoic acid, a neuronal differentiation factor, was delivered to human-induced pluripotent stem cell-derived neural stem cells and neuronal differentiation was promoted in a remotely controlled manner (Fig. 4b). Mechanical and biophysical cues can also guide cell differentiation. It is known that, under certain conditions, electrical stimulation can promote cardiac, neuronal or osteogenic commitment. As such, optoelectronic materials that can convert light into electrical signal are very appealing for neuronal cells studies and nerve regeneration as they provide a less invasive and wireless stimulation option. In reference [108], Yuan et al. developed a tri-component polymeric fibrous platform with optoelectronic properties for neuronal differentiation. A mixture of polycaprolactone and photoactive P3HT was electrospunned and biocompatible electroconductive polypyrrole was further polymerized on the nanofibers surface. It was shown that, in comparison to nonfunctionalized nanofibers, tri-component material enhanced neuron-like PC12 cells differentiation and increased cell survival due to reactive oxygen/nitrogen species scavenging of polypyrrole. As substrate topography is another cue known to guide cell behavior, Wu et al. studied the effect of different micro/-nanoscale structures of semiconducting polymer P3HT on neuronal differentiation [109]. Self-assembled nanofibers with 100 nm average diameter, electrospun microfibers with ~ 1 μm diameter and patterned stripes with width of 3, 25, and 50 μm obtained by photolithography were tested against control homogeneous films (Fig. 4c). The combination of topographical and photoconductive stimulation enhanced neuronal differentiation and directed growth of rat pheochromocytoma PC12 cells (Fig. 4d). Other non-metallic photocatalysts such as carbon nitride or graphene oxide have been used as coating of polycaprolactone/gelatin electrospun fibers for neuronal stimulation [110].
Fig. 4.
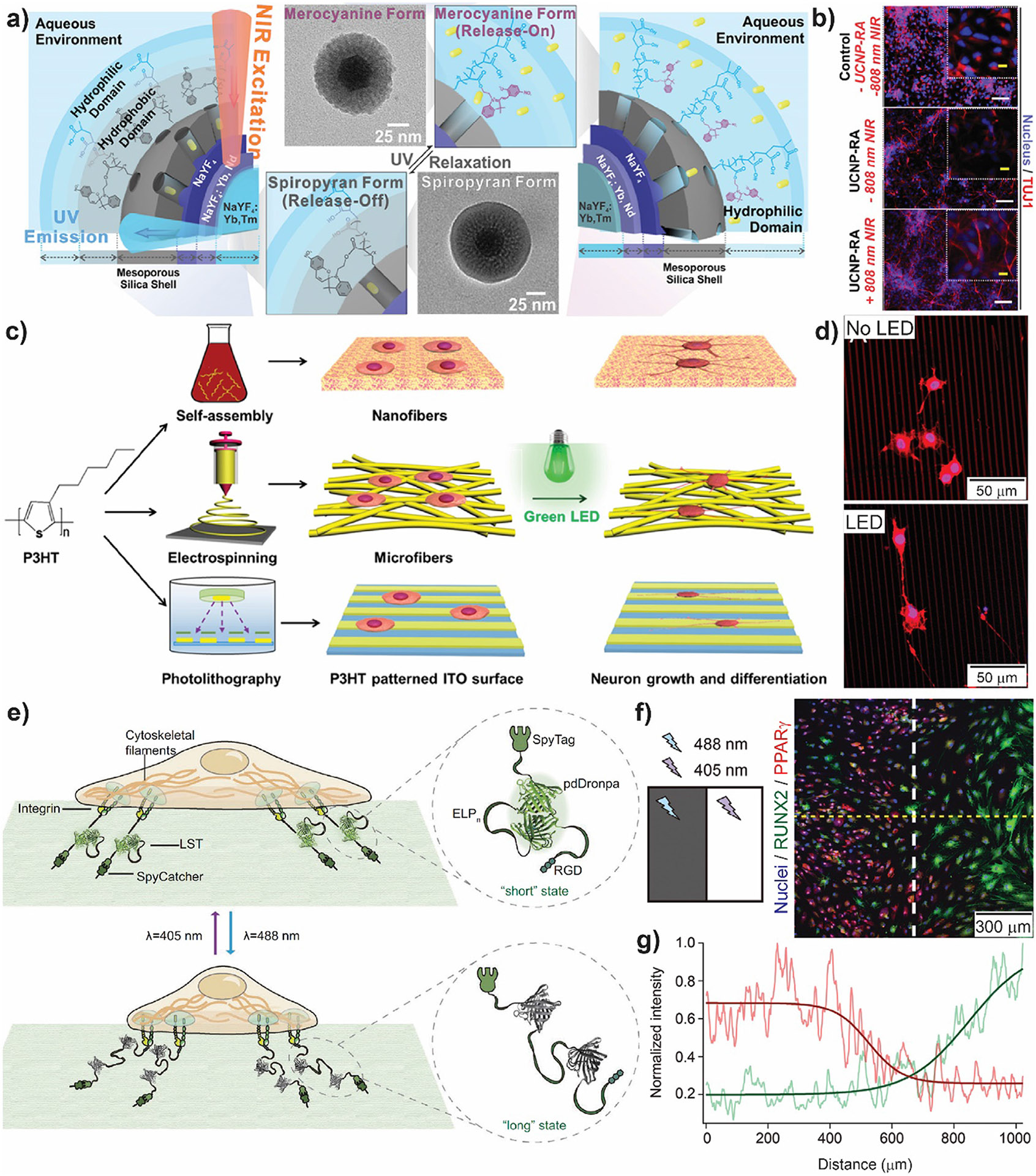
a) Scheme of the NIR-mediated photoswitching of the spiropyran polymer and subsequent hydrophobic-hydrophilic transition on the designed UCNP nanoconstructs to induce on-demand small-molecule release. b) Early neuronal markers expression in control human-induced pluripotent stem cell-derived neural stem cells, and cells exposed to retinoic acid loaded nanoparticles (UCNO-RA) without (−808 nm NIR) and with (808 nm NIR) light stimulation. Blue: nucleus; Red: TUJ1 neuronal marker. Scale bar = 100 μm; inset scale bar = 25 μm. c) Scheme of the preparation of P3HT micro/nanofibers and P3HT-patterned surface for PC12 cell culture. d) Laser scanning confocal microscopy images of neuron-like pheochromocytoma (PC12) cells stained for actin cytoskeleton and cultured on ITO-coated glass surfaces patterned with 3 μm wide stripes of P3HT with and without LED illumination. e) Light-switchable tether (LST) scheme. The LST consists of N-terminal SpyTag to covalently link to SpyCatcher immobilized on glass and C-terminal RGD to bind to integrin. When pdDronpa dimer is formed, repeats of elastin-like peptide (ELPn) are sequestered. Length of LST can be switched between long and short states upon 488 and 405 nm illumination and pdDronpa dissociation and association. f) Spatially control of human MSCs differentiation with light. Scheme of the photomask used to spatially regulate adhesion and differentiation of human MSCs and immunofluorescent images after 10 days differentiation. Nuclei, osteogenic differentiation marker RNX2 and adipogenic differentiation marker PPARγ are shown in blue, green and red respectively. g) Normalized average fluorescent intensities of RNX2 (green) and PPARγ (red) along the horizontal path (yellow dotted line) shown in (f). a)-c) Adapted with permission from [106]. Copyright 2020 American Chemical Society. c)-d) Adapted with permission from [109]. Copyright 2019 American Chemical Society. e)-g) Adapted with permission from [114]. Copyright 2021 Wiley.
Though most engineered scaffolds present fixed mechanical properties to the cells, the stiffness of muscle tissue changes during regeneration. To address this issue, Madl et al. developed a photoresponsive hydrogel system with dynamically-tunable mechanical properties [111]. Multi-armed PEG macromers functionalized with azide or bicyclo [6.1.0] nonyne were crosslinked by biorthogonal SPAAC. In order to enable controlled softening of the hydrogels, UV photocleavable ortho-nitrobenzyl ester moieties were introduced between PEG and a subset of azide groups used in crosslinking. While culturing skeletal muscle stem cells on soft hydrogels with healthy muscle mechanical properties enabled cell proliferation, culturing on stiff hydrogels impaired proliferation and myogenic progression. Experiments with dynamic hydrogels showed that culturing for 3 days on a stiff substrate was enough to impair cell proliferation and block commitment. Biophysical and biochemical cues’ ability for guiding cell fate and commitment can be empowered by controlling spatiotemporal signal presentation. In reference [112], De Martino et al. used azopolymeric photoactive interfaces as a kind of ‘cell gym’ on a chip for presenting/withdrawing morphological signals to living cells. Light-induced topographical changes were obtained by illuminating a pDR1m spin-coated substrate with a 514 nm laser from a single photon confocal microscope. Cyclic cellular and nuclear stretches could be induced by surface topography dynamic changes from a parallel pattern to flat or grid. Culture experiments with human MSCs revealed the possibility of rerouting or reversing stem cell fate by the dynamic modulation of morphological signals. In an intent to emulate natural tissue’s ability to control multidirectional differentiation of MSCs, Yan et al. developed a photocontrolled UCNPs-based substrate functionalized with photocleavable 4-(hydroxymethyl)-3-nitrobenzoic acid modified PEG (marked as P1) and RGD modified PEG (marked as P2) [113]. While the latter can capture MSCs, the former blocks interaction between MSCs and substrate enabling the cells to maintain their stem-cell properties due to the anti-adhesive effect. Cell-matrix interactions can be tuned upon NIR irradiation and photocleavage of P1. Power density of the NIR irradiation correlates with percentage of P1 detachment. Thus, low-power and high-power NIR irradiation led to adipogenic and osteogenic differentiation of MSCs, respectively, due to different cell-substrate forces and cytoskeletal tension. In another approach of cell differentiation by mechanotransduction, Zhang et al. developed light switchable tethers which length can be reversibly modulated by switching light responsive protein pdDronpa in between monomer and dimer states (Fig. 4e) [114]. Upon illumination of 488 and 405 nm light, tether length could be reversibly switched between ‘long’ (175 or 245 nm) and ‘short’ (105 nm) states, respectively, inducing distinct mechanical signals without changing the biochemical conditions. This results in contrasting cellular behaviors, as cells located in the area under irradiation of 405 nm light expressed osteogenic markers and adipogenic markers were observed under 488 nm light excitation (Fig. 4f and 4 g).
Being able to manipulate cell signaling in a precise and minimally invasive manner is crucial for cell basic research and for developing therapies for different diseases such as neurological disorders or cancer [115,116]. For instance, recently, cell stimulation has been achieved by means of magnetic discs that transduced alternating magnetic fields into mechanical forces [17,117], and piezoelectric micromotors that convert ultrasound input into an electrical signal [118]. Even long-range cell stimulation has been reported using a system comprising a circular magnetic array and a nanoscale magnetic torque actuator that can deliver piconewton-scale forces to cells over a working range of ~ 70 cm [119]. Minimally invasive stimulation of cells can be also achieved using 3D scaffolds. Tay et al. reported the synthesis of 3D magnetic hydrogels composed of hyaluronic acid that were able to stimulate mechanosensitive primary dorsal root ganglions in the presence of magnetic fields [120]. It has been reported that low levels of red and NIR light can promote intracellular Ca+2 elevation in neuron and cancer cells [121]. Different optogenetic approaches can be used to gain control over different Ca+2 modulated physiological processes [122]. Moreover, photoactive organic substrates that can transduce energy from light into heat or electricity have been employed to stimulate cells and trigger membrane depolarization [123]. In reference [124], Bossio et al. synthesized P3HT NPs by the re-precipitation method and used their photocatalytic activity to generate intracellular reactive oxygen species (ROS) upon visible light irradiation. The ROS generation did not affect cell viability and triggered intracellular calcium ion flux of Human Embryonic Kidney cells (HEK293). Though organic photovoltaic interfaces present a good performance in light mediated excitation and silencing of neuronal cells, limited adhesion of conjugated polymers in aqueous environments and absorption spectrum restricted to the visible range are clear disadvantages against inorganic materials. To address this issue, Leccardi et al. developed a photovoltaic interface composed of chemically modified organic materials in order to improve adhesion in aqueous environments and optimize photo-electrical stimulation efficiency [125]. The resulting prosthesis presented no cytotoxicity and was able to stimulate explanted degenerated mice retinas upon NIR light illumination.
A brief summary of strategies for controlling cell differentiation and for cell stimulation reviewed in this subsection is presented in Table 3.
Table 3.
Summary of strategies for controlling cell differentiation and for cell stimulation reviewed in this article.
| Mechanism | Material | Phototriggered effect | Observed Properties and Effects | Ref |
|---|---|---|---|---|
| Bioactive molecule release | UCNPs with multishell structure containing SP | Upconversion and photoisomerization | Remotely controlled retinoic acid release promoted neuronal differentiation | [106] |
| Electrical stimulation | Polycaprolactone, P3HT and polypyrrole tricomponent platform | Photovoltaic | Enhanced neuronal differentiation | [108] |
| Topography and electrical stimulation | Different micro/nanoscale structures of P3HT | Photovoltaic | Directed cell growth and enhanced neuronal differentiation | [109] |
| Stiffness | Biorthogonal SPAAC synthetized hydrogels containing photocleavable ortho-nitrobenzyl | Photocleavage | Control over skeletal muscle stem cells fate | [111] |
| Topography | pDR1m substrate | Photoisomerization and mass migration | Rerouting SCs’ fate by dynamic modulation of morphological signals | [112] |
| Mechanical signals | UCNPs substrate functionalized with photocleavable PEG and RGD-PEG | Upconversion and photocleavage | Differentiation of MSCs towards adipocytes or osteoblasts | [113] |
| Mechanical signals | Light switchable tethers containing pdDronpa | Dimerization and cleavage | Expression of osteogenic or adipogenic markers depending on illumination | [114] |
| ROS generation | P3HT NPs | Photocatalysis | Trigger of intracellular calcium ion flux | [124] |
| Electrical stimulation | Interface composed of chemically modified organic materials | Photovoltaic | Stimulation of explanted degenerated mice retinas | [125] |
3. Photoresponsive soft materials in tissue engineering, wound healing and implants
The loss of tissues and organs due to trauma, diseases, congenital defects and an aging world population leads to an increasing demand of organ and tissue repair or replacement [126,127]. By applying the principles of engineering and life sciences the tissue engineering and regenerative medicine field aims to deal with the regeneration or replacement of diseased or damaged tissues and organs [128]. As the development, regeneration and fabrication of a tissue/organ involve very complex processes, the combination of several strategies such as multifunctional scaffolds and biomaterials, drug and growth factors delivery, gene therapies, immunomodulation and external stimulus (i.e. electrical, magnetic, optical) is often required [129]. The spatiotemporal tunability of photoresponsive biomaterials is very appealing in a range of biomedical applications where tissue-like properties and programmable responses are required, such as tissue engineering and drug delivery [130-132].
Along with photoresponsive scaffolds and biomaterials, photobiomodulation (PBM) therapies, i.e. the use of non-ionizing light in the visible and near infrared spectrum for cell modulation, have presented promising results in the tissue engineering field [133]. There are several proposed underlying mechanisms of action for PBM such as activation of light sensitive ion channels and light-mediated effects on important molecules [134]. However, as red and NIR photons are predominantly absorbed by cells’ mitochondria, it is likely that the main mechanism of actions involves light absorption by cytochrome c oxidase and retrograde mitochondrial signaling (i.e., a mechanism by which mitochondria communicate with the cell nucleus affecting gene expression) [134-136]. Visible and NIR light would induce changes in the concentration of signaling molecules such as adenosine triphosphate, cyclic adenosine monophosphate, ROS, calcium and nitric oxide, and the activation of transcription factors. All these activities can lead to cytoprotection, enhanced cell proliferation, migration and differentiation, and modulation of inflammation, with positive effects in tissue repair and regeneration. For instance, it has been shown that an illumination with a diode laser of 830 nm wavelength and 40 mW power significantly enhanced bone repair when associated with bone morphogenetic proteins (BMP) in a critical size femoral defect (CSFD) rat model [137]. It has also been shown the positive effects of laser PBM in cartilage defect in animal models of knee osteoarthritis [138] and in promoting angiogenesis in vitro in a co-culture model of endothelial cells and in vivo in a chick embryo chorioallantoic membrane model [139]. PBM has also been used in combination with stem cell (SC) therapy for temporomandibular joint disc disorders [140]. In a different approach, Khosravipour et al. showed that in vitro PBM preconditioning of human adipose derived stem cells (hADSCs) increased cell viability and bone repair in a CSFD rat model [141]. The combination of human umbilical cord mesenchymal stem cells (hUCMSCs) transplantation and PBM presented a synergistic effect on the recovery of motor function and reduced lesion volume after spinal cord injury (SCI) in a rat model [142]. Biomaterials and PBM may be used together as synergistic strategies for increasing tissue regeneration. Paiva Margi et al. systematically reviewed studies that combined ceramic biomaterials and PBM in the healing process of animal models of bone defects [143]. The authors found that the combination of the appropriate biomaterials and PBM parameters presented enhanced healing effects. Thus, the association of biomaterials and PBM may have huge potential in tissue engineering approaches. The use of photoresponsive soft materials for tissue engineering, regeneration and implants will be discussed in the following subsections.
3.1. Bioactive molecules, Angiogenesis, and immunomodulation
Growth factors (GFs) are bioactive molecules able to control several cellular processes such as proliferation, migration, or differentiation, on targeted cells by specifically binding transmembrane receptors [144]. As GFs play a crucial role in tissue development and regeneration, their use in tissue engineering approaches is promising [145]. However, short-term burst-type diffusion from the delivery site and short half-life due to proteolysis limit GFs effectiveness [103,144]. Therefore, developing biomaterials and strategies that improve GFs stability and control their release is of outmost importance [146]. For instance, Zhao et al. developed a photoresponsive supramolecular HA hydrogel with controlled EGF delivery for wound healing applications [147]. HA (an important component of ECM) chains were conjugated with azobenzene (photoisomerization properties) and β-cyclodextrin (high binding-affinity with trans-azobenzene) groups (Fig. 5a). The hydrogel dynamic network could be controlled by photostimulation, transitioning to a soft or stiff state upon exposure to UV or visible light, respectively (Fig. 5b). EGF release from the loosened hydrogel is fast, thereby increasing its delivery at the wound site (Fig. 5c). Photoresponsive hydrogels with UV irradiation presented good biocompatibility properties assessed through hemolysis assay and through in vitro viability studies in L-929 mouse fibroblasts. Furthermore, illuminated photoresponsive hydrogels presented enhanced wound healing and angiogenesis in rat full-thickness skin defect model with respect to the controls (Fig. 5d). In addition to skin’s protein and cell delivery, photoresponsive hydrogels may be used for tissue engineering approaches of other tissues/organs. In reference [148], the authors employed metal–ligand coordination interactions and photochemistry of cobalamins [149] for developing an injectable, photoresponsive hydrogel capable of delivering cells and neuroprotective proteins to enhance axon regeneration. Via SpyTag/Spy Catcher technology the C-terminal adenosylcobalamin binding domain (CarHc) from the B12-dependent photoreceptor protein (CarH) was fused with a polyhistidine-tag (His6-tag), which can complex with transition metal ions such as Co+2, Cu+2, Ni+2 or Zn+2. The addition of coenzyme B12 or adenosylcobalamin (AdoB12) that binds CarHc induces its tetramerization and leads to self-assembly of the metal–ligand complexes into molecular networks in the dark (sol–gel transition). The C-Co bond within AdoB12 is sensitive to green (522 nm) and white light. Upon light exposure, following cleavage of the C-Co bond, the tetramers dissociate into monomers causing a gel-sol transition and the release of 4′,5′-anhydroadenosine (cleavage product of the coenzyme B12). As Zn+2 is relatively abundant in biological systems, Zn+2−coordinated hydrogels were used for cell encapsulation and in vivo applications. The obtained injectable, self-healing and photodegradable hydrogel was able to encapsulate 3T3 fibroblasts and hMSCs with good viability and was suitable to release the cells within 8 min of light exposure. Leukemia inhibitory factor, a neuroprotective cytokine that facilitates axon growth, laden hydrogels led to prolonged cellular signaling and enhanced axonal regeneration in a mouse optic nerve injury model.
Fig. 5.
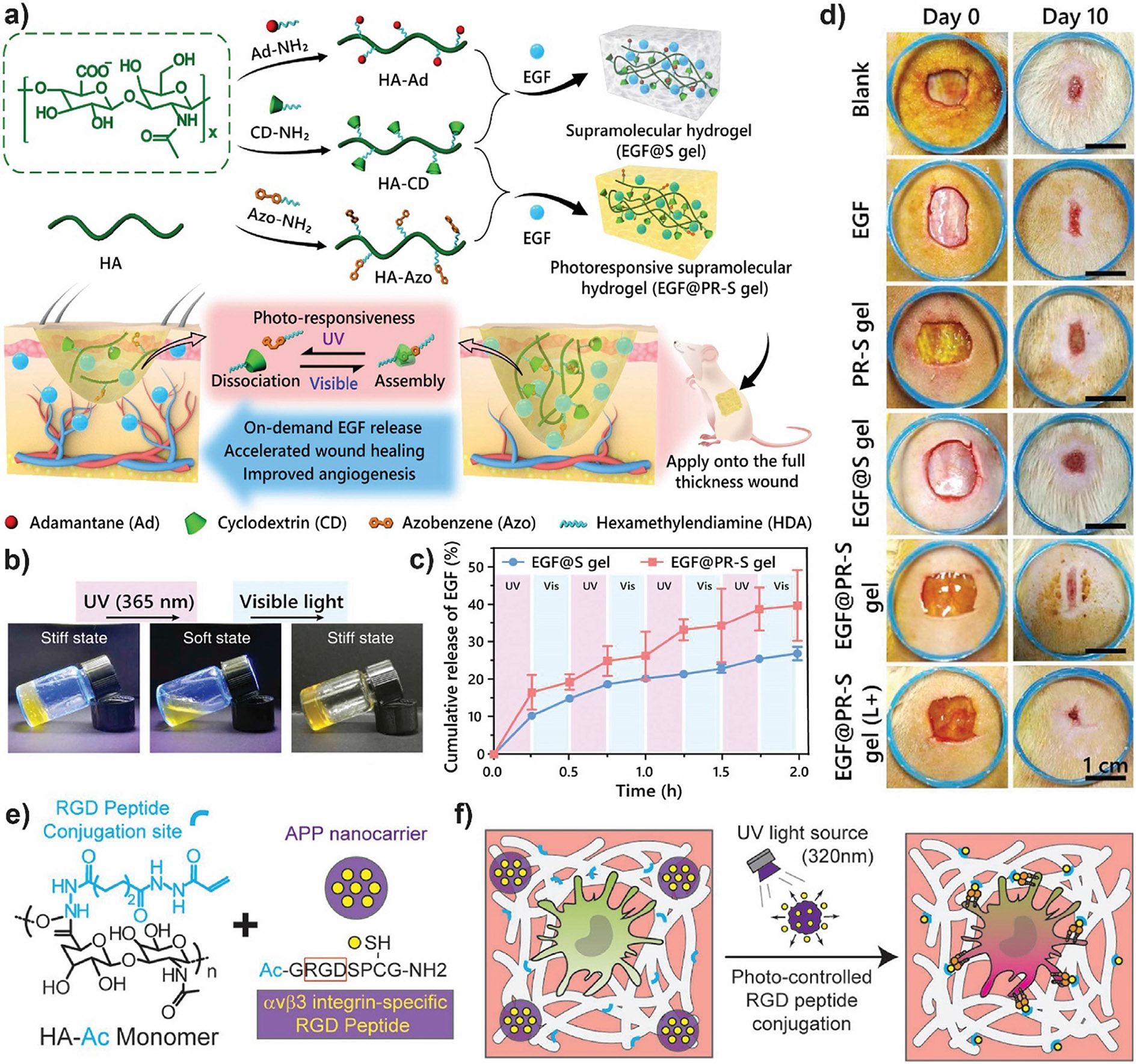
a) Scheme of supramolecular hydrogels’ synthetic route and their application as controlled delivery systems for accelerated wound healing. Adamantane, which also complexes with CD but it is not photoresponsive, was used for the obtention of non-responsive control supramolecular hydrogels (EGF@S gel). b) Photographs of the reversible stiff-soft and soft-stiff state transitions of photoresponsive supramolecular hydrogels (EGF@PR-S) upon exposure to UV and visible light, respectively. c) EGF release profile of EGF@S and EGF@PR-S gels upon alternating UV and ambient visible light irradiation. d) Photographs of full thickness wounds (day 0) and further healing with different treatments (day 10). Blanck; non-treated control; EGF: EGF solution; L+: UV irradiated for 15 min. e) Schematics of acrylate-HA macromers and RGD-loaded photodegradative alkoxylphenacyl-based polycarbonate (APP) nanocarriers (purple. f) Upon UV exposure, RGD peptides (yellow) are released and covalently conjugate to acrylate groups (blue) in crosslinked HA hydrogels (white) to temporally activate αvβ3 integrins (orange) macrophage expressions, leading to macrophage polarization. a)-d) Adapted from [147], Copyright 2020, with permission from Elsevier. e)-f) Adapted with permission from [150]. Copyright 2018 Wiley.
Oxygen diffusion and nutrient supply is a clear limitation for the size and complexity of tissue engineered constructs [151]. As such, angiogenesis, the formation process of new blood vessels, is crucial for supporting functional recovery and graft integration with the host tissue [129]. In that vein, several approaches employing photoresponsive materials have been proposed. In reference [152], Lodola et al. employed polymer-mediated optical stimulation for wireless modulation of endothelial colony-forming cells (ECFCs) proliferation and function. Thin films of P3HT, a biocompatible organic semiconductor widely used for phototransduction applications, were obtained by spin coating, and further coated with fibronectin. ECFCs were isolated from human peripheral blood samples and seeded on top of polymer and control glass substrates. The cultures were optically stimulated by application of milliseconds excitation pulses of a 525 nm LED for 4 to 36 h. The photoexcitation of the P3HT leads to enhanced proliferation and increased tubulogenesis of ECFCs in vitro. The authors propose the Ca+2-permeable transient receptor potential vanilloid 1 (TRPV1) channel and the pro-angiogenic genes expression mediated via NF-κB as the molecular pathway leading to these macroscopic results. In a different approach, Nair et al. employed hydrogels containing a phototriggerable vascular endothelial growth factor (VEGF) peptidomimetic system for light-controlled angiogenesis [153]. The 15 amino acid peptide was functionalized with a 3-(4,5-dimethoxy-2-nitrophenyl)-2-butyl (DMNPB) photoremovable protecting group that temporary inhibits its angiogenic activity. Then, it was incorporated into maleimide cross-linked bioactive PEG hydrogels along with human umbilical vein endothelial cells (HUVECs). By exposing the modified hydrogels to 405 nm light, the formation of new vessel structures at the illuminated sites of the 3D culture was promoted, achieving spatial control of the angiogenic process. Spatiotemporal control of sprouting directionality can also be accomplished without growth factors. In reference [154], Fedele et al. employed an azopolymer photopatterning approach for real-time presentation of topographical cues to HUVECs spheroids. The authors used pDR1m, an azopolymer capable of controlling the material surface profile by a light-induced mass migration, and a confocal microscope to deliver topographical signals during early-stage angiogenesis. The in-situ photo-inscribed pattern was able to guide cell migration, with the spheroid’s cores acquiring a polarized shape and the sprouts following the topographical cues.
The immune system plays a crucial role in controlling wound healing and guiding tissue regeneration [155]. Inflammatory processes may lead to scar formation, fibrosis and structural changes hampering tissue healing and compromising its functionality. Thus, immunomodulatory therapies may prevent tissue/organ damage and induce a pro-regenerative environment to enhance regeneration [129,156]. In that regard, Kang et al. designed a photoresponsive nanocarrier for controlling intracellular calcium levels and, therefore, modulate macrophage polarization towards an activated pro-inflammatory (M1) or anti-inflammatory and pro-healing (M2) phenotype [157]. NIR light has more tissue penetration and presents minimal cytotoxicity in comparison to UV light. The UCNPs were coated with a mesoporous silica shell and were loaded with calcium regulators, i.e., calcium supplier or chelator. The nanocarrier was further chemically modified with a gating structure to gain control over the release of calcium regulators. This was achieved by a serial coupling of a photocleavable 4-(hydroxymethyl)-3-nitrobenzoic acid linker and RGD peptide-bearing molecular cap via cyclodextrin-adamantine host–guest complexation. Moreover, RGD peptide enhances cellular uptake of the nanocarriers through binding to integrin receptors of the cells. Under NIR light excitation, the upconverted light emission from the UCNPs triggered the cleavage of the cap, enabling the on-demand release of calcium regulators. Even when the NIR light was applied through an intervening layer of porcine skin tissue, an elevation or depletion of intracellular calcium ions was induced, promoting a M1 or M2 polarization of macrophages, respectively. It is worth noting that the same group reported the use of a similar photoresponsive nanocarrier system for controlling stem cell differentiation in vivo via remote control of intracellular calcium, an strategy that could accelerate stem cell-based regenerative therapies [158]. Dynamic control of macrophage polarization in vivo could help the treatment of inflammatory diseases and aid tissue regeneration. Macrophage immunomodulation can be also achieved by means of smart biomaterials and controlling cell-ECM interactions. To this end, Wang et al. developed a photoresponsive nanocomposite HA hydrogel with tunable cell adhesion sites [150]. Adhesive peptide RGD were loaded into photodegradative alkoxylphenacyl-based polycarbonate nanocarriers (Fig. 5e). HA was functionalized with acrylate groups to act as binding sites for RGD peptides through a Michael addition between acrylate and cysteine thiol groups. Moreover, HA polymer chains were modified with a cysteine-contained biodegradable matrix metalloproteinase (MMP) crosslinker peptide for encapsulating murine macrophages. The authors showed that upon pulsed UV light exposure, RGD release and conjugation to the HA hydrogel allowed real-time activation of macrophages via ανβ3 integrins, which enhanced anti-inflammatory M2 polarization (Fig. 5f). In a different approach, Zheng et al. designed a photoresponsive hybrid biomaterial which dynamic surface topography for macrophage phenotype modulation [159]. The authors combined two- and four-branched polycaprolactone macromonomers with PEG-modified gold nanorods, casted them into a patterned mold and UV-cured them in the presence of a photoinitiatior. The obtained microgrooved film was then compressed at high temperature with a flat mold and subsequently cooled to obtain a flat film. The obtained shape memory film rapidly recovers the original microgrooved shape under NIR irradiation. The dynamic surface topography can induce the elongation of bone marrow-derived macrophages adhered on the surface, which produces a phenotypic shift from pro-inflammatory (nitric oxide synthase positive, low interleukin-10) to anti-inflammatory, pro-healing (arginase-1 positive, high interleukin-10) [160]. The NIR-triggered dynamic surface topography also modulated macrophage phenotype in an in vivo rat subcutaneous implantation model. The use of photoresponsive immunomodulatory biomaterials may lead to optimization of healing outcomes.
3.2. Shape memory implants and tissue bonding
The ability of shape memory polymers (SMP) to change between a permanent and a temporary shape under certain conditions is promising in biomedical fields, such as tissue engineering and intravascular stents [161,162]. Different kind of stimulus such as temperature, light, solvents, ultrasound, electromagnetic fields, etc., can trigger the transition. Among photoresponsive SMP, two different groups can be considered [163]. The ‘true’ photoresponsive SMP which are based on reversible photochemical crosslinking or photoisomerizable chemical groups, and those SMP that take advantage of photothermal fillers and the heat generated by a light source to trigger a thermoresponsive polymer. Design and development of the first group of polymers is challenging but desired for biomedical applications in which a temperature change is not feasible. The photo-induced dimerization and cleavage at longer and shorter wavelengths, respectively, of cinnamon and anthracene functionalities has been used for developing photoresponsive SMP [164,165]. For instance, in reference Xie et al. developed a light induced shape-memory composite based on poly(l-lactide) (PLA) – PEG copolymer and anthracene groups selectively functionalizing the PEG soft segment [165]. Photoreversible cycloaddition of anthracene groups when illuminated with 365 nm UV light allowed for fixation of a temporary shape generated by an external force and further illumination with 254 nm UV light caused shape recovery due to crosslink cleavage (Fig. 6a). In a different approach, Zhang et al. made use of the photoswitchable glass transition temperature of an azobenzene-containing polymer network for developing a light-triggered athermal shape memory effect [166]. Sequential exposure to UV and visible-light illumination leads to glass transition temperatures below or above room temperature, inducing transition between permanent to temporary shapes (Fig. 6b). On the other hand, shape memory composites have been reported using photothermal fillers. In reference [167], Xie et al. added the natural and biocompatible photothermal substance melanin to polyurethane to obtain a NIR photoresponsive shape memory implant. Polyurethane owns its shape memory behavior to its two segments composition: a hard segment that determines the permanent shape and a soft segment that fixes the temporary shape below the shape memory transition temperature. The melanin/polyurethane composite presented no toxicity in vitro towards hMSCs and mouse fibroblast (L929) cells lines and in vivo in implanted mice. Moreover, a melanin/polyurethane column implanted in the vagina or back subcutis of mice rapidly recovered to its original state within 60 s under a very low NIR laser (Fig. 6c-e). Development of biodegradable SPM composites is crucial for reducing material time inside the body and deleterious effects. Piperazine-based polyurethane presents both thermoresponsivity and degradability. In reference [168], the authors incorporated NIR photothermal black-phosphorus sheets to piperazine-based polyurethane to obtain a SPM with controllable degradation. The composite presented no toxicity in vitro and in vivo, excellent in vivo shape memory capacity, and degrades naturally into non-toxic phosphate and carbon dioxide/water. In order to enhance tissue regeneration, Yang et al. combined cuprorivaite nanosheets into SMP poly (D, l-lactide-co-trimethylene carbonate) [169]. The resulting composites were biodegradable and presented excellent shape memory activity upon NIR irradiation. Sustained release of Cu2+ and ions stimulated the proliferation, migration, and tube formation of HUVECs in vitro. Moreover, the composite was tested as an ‘smart’ suture, NIR laser triggered self-knot in mice incisions and exhibited enhanced wound healing and angiogenesis in vivo in comparison to controls.
Fig. 6.
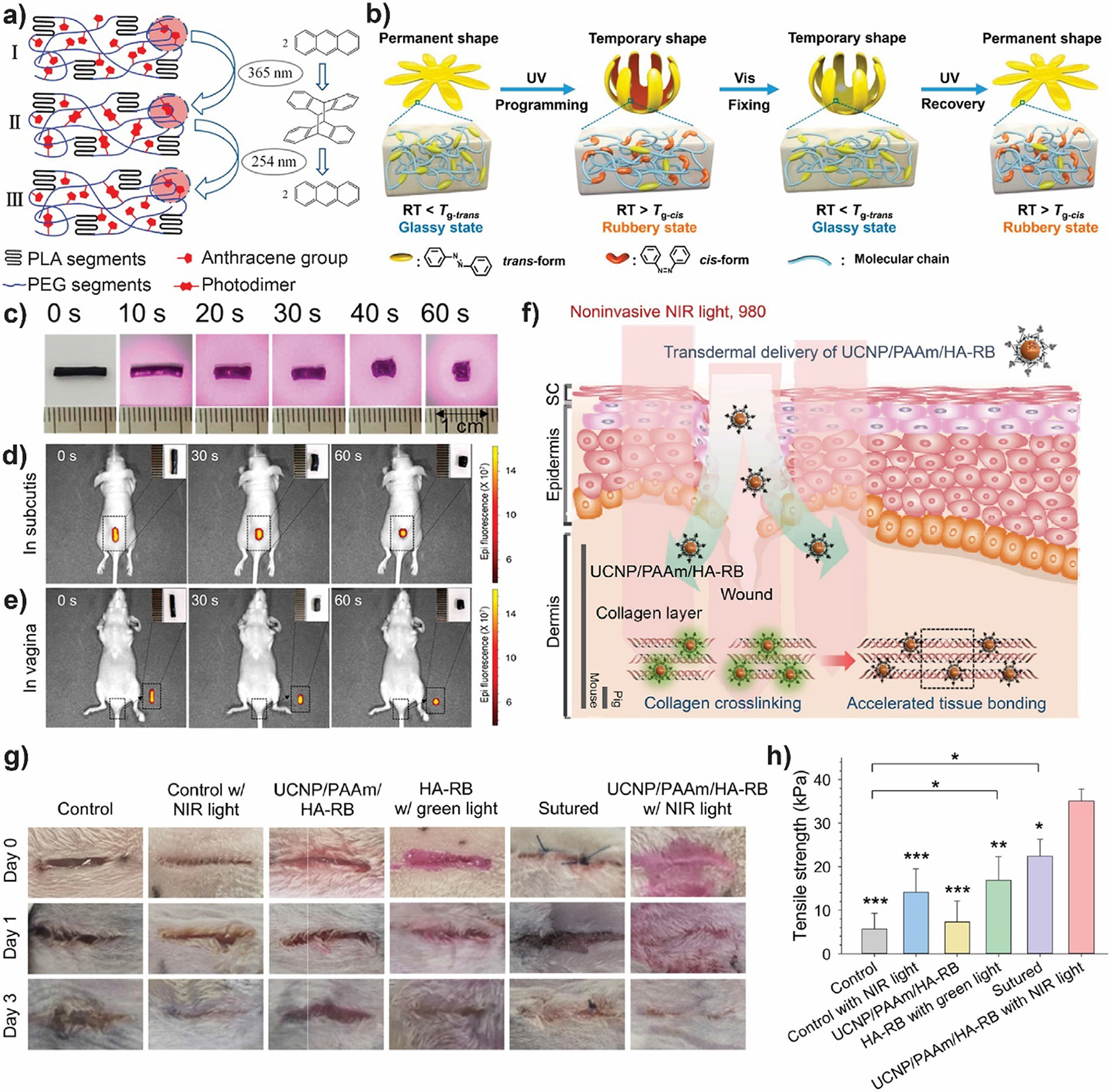
a) Scheme representing the mechanism of light-induced shape-memory effect of PLA-PEG-anthracene composite. PLA crystalline and PEG amorphous regions are represented. b) Shape memory effect of a modeling flower and the mechanism of azobenzene-containing polymer network shape transition. In the permanent shape, azobenzene units are in the trans form and glass transition temperature of the polymer network is above room temperature. After UV irradiation the azobenzene-containing polymer isomerizes to cis form, whose glass temperature is below room temperature and a transition to a temporary shape occurs. This shape can be fixated with visible-light and can be reverted when exposed to UV-light again. c) In vitro shape memory behavior of Cy5.5-labeled melanin/polyurethane (YM/PU) column under the NIR laser. Photographs and fluorescence images displaying the in vivo shape memory behavior of the Cy5.5-labeled YM/PU column in the mouse subcutis (d) and vagina (e) under NIR laser. f) Scheme of the photochemical tissue bonding of injured collagen matrix. Polyallylamine modified UCNP/hyaluronate-rose Bengal conjugate (UCNP/PAAm/HA-RB) complex is delivery transdermally and NIR light is applied for wound healing (gray scale bars = 100 μm, SC: stratum corneum). Images of in vivo photochemical tissue bonding of incised dorsal skin mice (g) and the resulting tensile strength at day 3 (h). Control: phosphate buffered saline (PBS). a) Reprinted with permission from [165]. Copyright 2016 American Chemical Society. b) Reprinted with permission from [166]. Copyright 2019 American Chemical Society. c)-e) Reprinted with permission from [167]. Copyright 2020 American Chemical Society. f)-h) Adapted with permission from [170]. Copyright 2017 American Chemical Society.
Sutures are often needed for wound closure and for the attachment of the increasing number of medical devices that are being implanted in the human body. However, due to drawbacks such as needle trauma, the requirement of skilled surgeons and unsuitability for certain tissues, such as eye or lung, there is an increasing need of new sutureless methods development [171]. In that vein, photochemical tissue bonding (PTB), i.e. the use of photosensitizing substances and light to crosslink proteins on tissue surfaces and form bonds, is receiving increasing attention. PTB can be simply applied by adding a photosensitizer such as rose Bengal and green light, as has been presented by Yao et al. in rat tendon injuries [172]. However, different materials are being developed to fill the wounds and enhance their healing, improve tissue bonding mechanical strength or change illumination parameters. In reference [173] the authors modified collagen, the most abundant protein in skin dermal ECM, with methacrylate moieties. The methacrylated collagen was combined with photosensitizer rose Bengal to obtain a light-activated biomimetic material for tissue sutureless bonding. Under green-light exposure, the activation and crosslinking of methacrylate moieties mediated by rose Bengal excited states leads to hydrogel assembly. By further incorporation of poly-l-lysine (PLL) to the mixture, reduced degradation rate and improved mechanical properties of the resulting bonded tissues was achieved. The performance of the photoactivable formulation was evaluated using an in vivo murine skin wound model. Traditional sutures were used as control. While the sutured wounds showed signs of inflammation, wounds treated with the tissue photobinding formulation showed a close resemble to non-wounded skin. As animal and recombinant proteins are difficult and expensive to isolate/produce, the use of synthetically prepared peptides is appealing as they can be prepared under stringent conditions in a cost-effective manner, they are easy to modify in comparison to full-length proteins and present decreased risks of xenogeneic response. In this vein, McTiernan et al. reported the obtention of adhesive hydrogels by light-triggered self-assembly of collagen-like peptides to acrylate functionalized PEG, with rose Bengal as visible light photoinitiatior and PLL as electron donor. [174] By modifying the components concentration and irradiation times the mechanical properties of the obtained gels could be matched to different tissues. Moreover, in vivo experiments showed that the photocrosslinked collagen-biomimetic matrices were better at supporting epithelial growth and wound healing than conventional wound suture. PTB techniques can be combined with different strategies to promote tissue regeneration or achieve a certain functionality. In reference [175] Ruprai et al. developed biocompatible, biodegradable, photoactivated porous chitosan films able to support bone marrow derived hMSCs viability and multipotency. To mimic the porous structure of the natural ECM that allows cell and nutrients exchange, porous chitosan-based adhesive was obtained by freeze-drying. Furthermore, its degradability was tuned by making a blend of medium molecular weight and water-soluble low molecular weight chitosan. Rose Bengal was used as a photosensitizer. Tissue bonding of the film on sheep small intestine tissue ex vitro was achieved by green light illumination. The tissue bonding strength of the porous and erodible chitosan-based adhesive films could be further improved by incorporating L-3,4-dihydroxyphenylalanine (l-DOPA), a natural catechol [176]. PTB can be combined with soft bioelectronics for different bioapplications such as patches for damaged myocardium. In reference [177] Hoang et al. used laser ablation to introduce pores of different sizes and distances on chitosan films containing rose Bengal. The film surface was further functionalized with conjugated polymer polyaniline. Both the conductivity and the mechanical properties of the obtained film varied as a function of porosity. The patches retained electronic properties under cyclic stretching mimicking the heart contraction cycles. The porous patch incorporated in chitosan adhesives containing rose Bengal could be bonded to samples of tissue cut from sheep small intestine by illumination with green light. To overcome the finite tissue penetration of green light, Han et al. developed a poly(allylamine)-modified UCNPs/Hyaluronate-rose Bengal conjugate complex for transdermal delivery and noninvasive PTB of deep tissue upon NIR light illumination [170]. HA was used as a transdermal delivery carrier due to its hygroscopic behavior that can hydrate skin and for the HA receptors expressed in skin tissues. Thus, conjugate complex could be delivered into a deep and wide area from the boundary of the wound (Fig. 6f). UCNPs were functionalized with poly(allylamine) to provide hydrophilicity and positive charge. Then, they were complexed with negatively charged HA-rose Bengal conjugate by electrostatic interactions. Upon noninvasive NIR light illumination, activation of rose Bengal induced collagen cross-linking and accelerated sutureless skin tissue bonding both ex vivo and in vivo, achieving higher tensile strengths than the sutured tissues (Fig. 6g and 6 h).
A brief summary of strategies for tissue engineering and wound healing reviewed in this section is presented in Table 4.
Table 4.
Summary of strategies for tissue engineering and wound healing reviewed in this article.
| Mechanism | Material | Phototriggered effect | Observed Properties and Effects | Ref |
|---|---|---|---|---|
| EGF release | HA hydrogel containing azobenzene and β-CD | Photoisomerization | Dynamic controllable stiffness, enhanced wound healing and angiogenesis in vivo | [147] |
| Cells and neuroprotective protein delivery | Hydrogels synthetized via metal-directed assembly of His6-tagged proteins | Photocleavage | Injectable, self-healing hydrogel, enhanced axonal regeneration in mouse optic nerve injury model | [148] |
| Electrical stimulation | P3HT films | Photovoltaic | Enhanced proliferation and tubulogenesis of endothelial colony-forming cells | [152] |
| VEGF peptidomimetic release | Hydrogel containing a VEGF peptidomimetic with a photoremovable protecting group | Photocleavage | Spatio-temporal control of new blood vessel formation in the 3D hydrogel | [153] |
| Topography | pDR1m substrate | Photoisomerization and mass migration | Sprouts followed the direction of the generated pattern | [154] |
| Ca2+ regulators delivery | UCNPs coated with mesoporous silica shell and peptide-bearing molecular cap | Upconversion and photocleavage | Control of macrophage polarization towards pro-inflammatory or pro-healing | [157] |
| RGD presentation | Acrylate-HA hydrogel and photodegradative RGD-loaded nanocarriers | Photocleavage | Enhanced anti-inflammatory M2 polarization | [150] |
| Topography | Gold nanorod-containing shape memory polycaprolactone film | Photothermal | Induce elongation and pro-healing polarization of macrophages in vivo | [159] |
| Shape memory implant | Copolymer of PLA and anthracene-functionalized PEG | Dimerization and cleavage | Tunable stiffness and athermal shape memory effect | [165] |
| Shape memory implant | Azobenzene-containing polymer network | Photoisomerization | Athermal shape memory effect by photoswitching of glass transition temperature | [166] |
| Shape memory implant | Melanin/polyurethane Composites | Photothermal | Biocompatible and shape memory effect in vivo using NIR light | [167] |
| Shape memory implant | Piperazine-based polyurethane and black-phosphorus sheets | Photothermal | Biodegradable, biocompatible and shape memory effect in vivo using NIR light | [168] |
| Shape memory implant | Cuprorivaite nanosheets into SMP | Photothermal | Biodegradable, ions release enhances wound healing and angiogenesis in vivo | [169] |
| Tissue bonding | Methacrylated collagen combined with rose Bengal and PLL | Photocrosslinking | Improved mechanical properties and no inflammation signs in skin wound model | [173] |
| Tissue bonding | Collagen-like peptides, acrylate functionalized multi-armed PEG, rose Bengal and PLL | Photocrosslinking | Tunable mechanical properties, improved wound healing in comparison to conventional suture | [174] |
| Tissue bonding and cell delivery | Porous chitosan film with rose Bengal | Photocrosslinking | Cultured hMSCs maintained viability and multipotency | [175] |
| Adhesive bioelectronic patch | Porous chitosan functionalized with polyaniline and containing rose Bengal | Photocrosslinking | Stable mechanical and electronic properties under cyclic stretching | [177] |
| Tissue bonding | Poly(allylamine)-modified UCNPs/Hyaluronate rose Bengal conjugate complex | Upconversion and photocrosslinking | In vivo tissue bonding with higher tensile strengths than sutured tissues | [170] |
4. Photoresponsive soft materials for the delivery of therapeutics
The controlled delivery of therapeutics to a targeted site using nanotechnologies has received great attention in the past decades as an alternative that can enhance the therapeutics’ efficacy and reduce nonspecific toxicity [178,179]. Stimuli-responsive therapeutic delivery systems that can be manipulated externally are highly desired. Light as external stimulus offers precise spatiotemporal control, it is relative safe, and it has minimal interaction with biological tissues [180,181]. Thus, photoresponsive materials have been extensively investigated to enable site-specific therapeutic release that is precisely controlled by light irradiation. Among the photoresponsive materials, polymeric and soft materials have gain attention for their tailorable chemical and physical properties [178]. Different approaches employing photoresponsive polymeric and soft materials for the delivery of drugs, genes, nanoparticles and other biologicals will be discussed in the following subsections.
4.1. Drug delivery
Different external stimuli approaches have been proposed to achieve control over the delivery of drugs such as magnetic, electric, pH, ultrasound, and light stimulation [182]. Light as external stimulus can trigger photoreactions with either the drug vehicle or the drug, as well as trigger photo-radiative phenomena (i. e., photothermal or photoemissions) with light-responsive chemical structures [183,184]. A wide variety of polymeric and soft photoresponsive vehicles have been reported on literature, including nanoparticles, nanogels, micelles, nanofibers, etc. [178]. These photoresponsive systems often rely on different light-switch mechanisms to control drug release such as is photolysis triggered by oxidative stress [185-187], polyelectrolytes protonation/deprotonation in acidic cellular environments [188-190], cleavage of disulfide linkers under reductive conditions [191-193], reduction of hypoxia-responsive moieties,[194,195] thermodynamic conformational changes, upconversion of light, etc. The field of light-triggered drug release using soft materials has been widely investigated, reported and reviewed. Thus, here we will only comment some of the most recent and relevant studies. For instance, Ghani et al. developed a reversible photoresponsive membrane for controlled drug delivery using the SP-MC switch [196]. The photo-responsive interpenetrating polymer network was demonstrated for the UV-light triggered release of dopamine, l-DOPA and prednisone, allowing to control the release of ~ 90–95 % of the payload. An image-guided drug delivery strategy for chemotherapy was designed by Wang et al. [197] Here, a photoresponsive membrane composed of poly (ethylene oxide)-b-poly(spiropyran) (PEO-b-PSPA) diblock copolymer was synthesized. The membrane switched its permeability upon UV and green light irradiation allowing the on-demand release of doxorubicin. Banerjee et al. developed a magnetic and photoresponsive drug delivery system based on functionalized electrospun nanofibers loaded with super-paramagnetic iron oxide nanoparticles [198]. The heat generated from the Nel relaxation effect of magnetic nanoparticles under applied magnetic fields and the laser illumination lead to conformational changes on the thermoresponsive polymer fibers, and consequently the release of therapeutics. A strategy for photoresponsive self-assembly nanocarriers was reported by Cheng et al. The carrier was composed by uracil end-capped PEG and the nucleobase adenine derivative methyl 3-(6-amino-9H-purin-9-yl) propanoate as a model drug. Upon light exposure, the polymeric micelles are disrupted and rapidly release the cargo [199]. Although less common due to challenging synthesis methods, photoresponsive nanocages have been also explored in controlled drug delivery. For instance, Huo et al. prepared polymersomes composed of amphiphilic block copolymers of poly (o-nitrobenzyl acrylate) and poly (N,N’-dimethylacrylamide). Exposure to light irradiation at 365 nm triggered the photocleavage of o-nitrobenzyl groups, which resulted in dissociation of the polymersomes with simultaneous co-release of hydrophilic and hydrophobic cargoes [200]. Another example of photoresponsive nanocarriers are those based on donor-acceptor molecules. Enthilkumar et al, developed a system consisting in photoresponsive conjugated polymer nanoparticles functionalized with donor–acceptor Stenhouse adduct (DASA) and folic acid units for controlled drug delivery and imaging. Upon visible light (λ = 550 nm) irradiation the delivery of both hydrophilic and hydrophobic drugs was triggered [201]. Gupta et al. reported a photodegradable polymeric nanoparticle system composed of an amphiphilic methoxy PEG-b-poly(ε-caprolactone)–co-poly(azido-ε-caprolactone-g-ortho nitrobenzyl retinoic ester) copolymer [202]. In this system retinoic acid was covalently grafted on the surface of the polymeric micelles through a photosensitive o-nitrobenzyl linker to investigate cardiac differentiation. The gradual light-triggered release of retinoic acid allowed a concurrent gradient-like pattern for cardiac differentiation.
Specific targeting of cells remains challenge in the design of drug delivery carriers. A useful approach to overcome the unspecificity of drugs is to modify drug carries with molecular messengers that allow for biorecognition. One of the most exploited specific ligands for cancer targeting is folic acid. In this context, Zhou et al. designed photoresponsive polymersomes electrostatically decorated with folic acid for doxorubicin release. The system showed high cytotoxicity against HeLa cells [203]. Similarly, Gosh et al. designed a doxorubicin -controlled release system employing an amine-PEG wrapped on tetra carboxy zinc phthalocyanine metal–organic framework (ZIF-8) decorated with folic acid. This system showed increased cells death in LNCaP line [204]. Moreover, Yu et al. fabricated glutathione-responsive amphiphiles for specific tumor targeting and imaging-guided chemo-photothermal combination therapy [205]. He et al. synthetized biomimetic polymersomes based on the self-assembling of amphiphilic diblock copolymers encapsulating the hypoxia-activated prodrug AQ4N and UCNPs for combined hypoxia therapy [206]. Biswass et al. reported the light-triggered dual anti-cancer drug delivery from a triblock co-polymer. Chlorambucil was conjugated to the polymer via 3-(3-(hydroxymethyl)-4-nitrophenoxy) propyl acrylate. The polymer prodrug formed spherical micellar nanoparticles that encapsulated a second drug (doxorubicin) in their hydrophobic core. Under UV irradiation both drugs were released [207].
One of the most popular soft nanocarriers for drug delivery are liposomes. Due to their easy fabrication, size control, cargo loading versatility and biocompatibility, liposomes represent a great option for photoresponsive drug delivery. For instance, Zheng et al. utilized the phospholipid 1,2-dipalmitoyl-sn-glycero-3-phosphocholine to fabricate thermosensitive liposomes loaded with the photosensitizer zinc phthalocyanine substituted by tetra-ethylene glycol for molecular imaging, chemo, photodynamic and photothermal therapy. In vivo experiments demonstrated that the doxorubicin loaded liposomes accumulated in the tumor, enhancing deleterious cancer effects and diminishing liver adsorption [208]. Along the same lines Shi et al., introduced photo-activated nanoliposomes for light-controlled chemotherapy with doxorubicin [209]. Ilhami et al. also encapsulated doxorubicin and the pro-photosensitizer 5-aminolevulinic acid within adenine-functionalized supramolecular micelles to achieve effective photo-chemotherapy [210]. Multiresponsive drug delivery systems have been explored to enhance chemotherapy. In that vein, Bai et al. developed self-assembled supramolecular prodrug complexes with β-cyclodextrin–acylhydrazone–doxorubicin and the targeting of azobenzene-terminated poly[2-(dimethylamino) ethyl methacrylate] as a building block. The system displayed dual responsive behavior upon UV-light irradiation and pH changes [211]. Likewise Chen et al. reported a photo, pH, and redox responsive nanogel of poly(acrylic acid-co-spiropyran methacrylate) crosslinked by disulfide-containing N,N-bis(acryloyl)cystamine loaded with doxorubicin [212]. Liu et al. also demonstrated the suitability of a photo and pH-triggered liposome system for doxorubicin delivery [213].
Combination therapies with light-triggered drug release using soft materials is often targeted for synergistic therapeutic effects. Batta et al. reported chitosan-tripolyphosphate nanoparticles decorated with chlorin e6 and encapsulating doxorubicin [214]. The nanoformulation was prepared by ionotropic gelation and had significant anti-proliferative activity against MCF-7 breast cancer cells after NIR irradiation. A sequential strategy was successfully designed by He et al., which consisted in a biodegradable backbone containing a photosensitizer ruthenium complex and the anti-cancer drug paclitaxel. Upon red light irradiation, the ruthenium complex is cleaved producing singlet oxygen molecules that triggered paclitaxel release (Fig. 7b).[215] The combined chemo-photodynamic therapy presented enhanced in vivo antitumor efficacy in 4T1 tumor-bearing mice (Fig. 7c). A great advance was reported by Xu et al., where they bioengineered living neutrophils as a novel type of “photoactive neutrophil”. The neutrophil system was loaded with the nanocomplex retinoic acid/chlorin e6. The irradiated neutrophils released the nanocomplex and after bypassing the tumor barrier, retinoic acid induced mitochondrial membrane disruption in combination with photodynamic therapy. The designed photoactive neutrophil produced tumor reduction without severe side effects [216]. Xu et al. developed a 3D pyramid-shaped RNA nanocage for light-controlled drug release using paclitaxel as a model drug. The RNA nanocage disintegration was achieved by introducing photocleavable spacers along the RNA chain. Exposure to UV-light triggered paclitaxel release showing high cytotoxicity against breast cancer cells [217]. In the same vein, Yang et al. used a nuclei acid to fabricate a new DNA aptamer-grafted photoresponsive hyperbranched polymer. The polymer self-assembled into nanoparticles. UV-light irradiation of the system allowed drug release due to nanoparticle disaggregation. The doxorubicin loaded DNA aptamer nanoparticles exhibited selective photo-triggered cytotoxicity towards cancer cells [218]. Guo et al. fabricated a multi-responsive PEG-based block copolymer through Cu(I)-catalyzed azide–alkyne cycloaddition click polymerization. Nile red was encapsulated as a model drug to study drug release in different environments. The system showed a photo, oxidative, and reductive response due to the o-nitrobenzyl groups, peroxalate ester bonds, disulfide bonds, and triazole units, which triggered the stimuli-specific responses [219] . Rafique et al. investigated a soft system coloaded with doxorubicin and chlorin e6 onto mesoporous-silica-coated UCNPs for antitumor combination therapy. On-demand controlled release was switched under NIR irradiation and the dual light-triggered release in a synergistic therapeutic effect that enhanced cell death [220]. Fan et al. developed a promising photoresponsive and degradable hollow mesoporous organosilica nanocarrier. The organosilica was bonded to organoalkoxysilanes and wrapped with graphene oxide quantum dots. Light irradiation leads to organosilica nanoparticles degradation, resulting in enhanced local doxorubicin release in vivo [221]. One more example of photoresponsive mesoporous silica drug delivery system was showed by Beňová et al., where p-coumaric acid derivatives were used as photo-switchable ligands for the delivery of the non-steroidal anti-inflammatory drug naproxen [222]. Another coumarin derivative light-triggered drug delivery system was proposed by Liu et al. This system consisted in a coumarin derivative hydrogelator which photocleavage at the C─N bond in 7-amino coumarin upon 365 nm light irradiation [223].
Fig. 7.
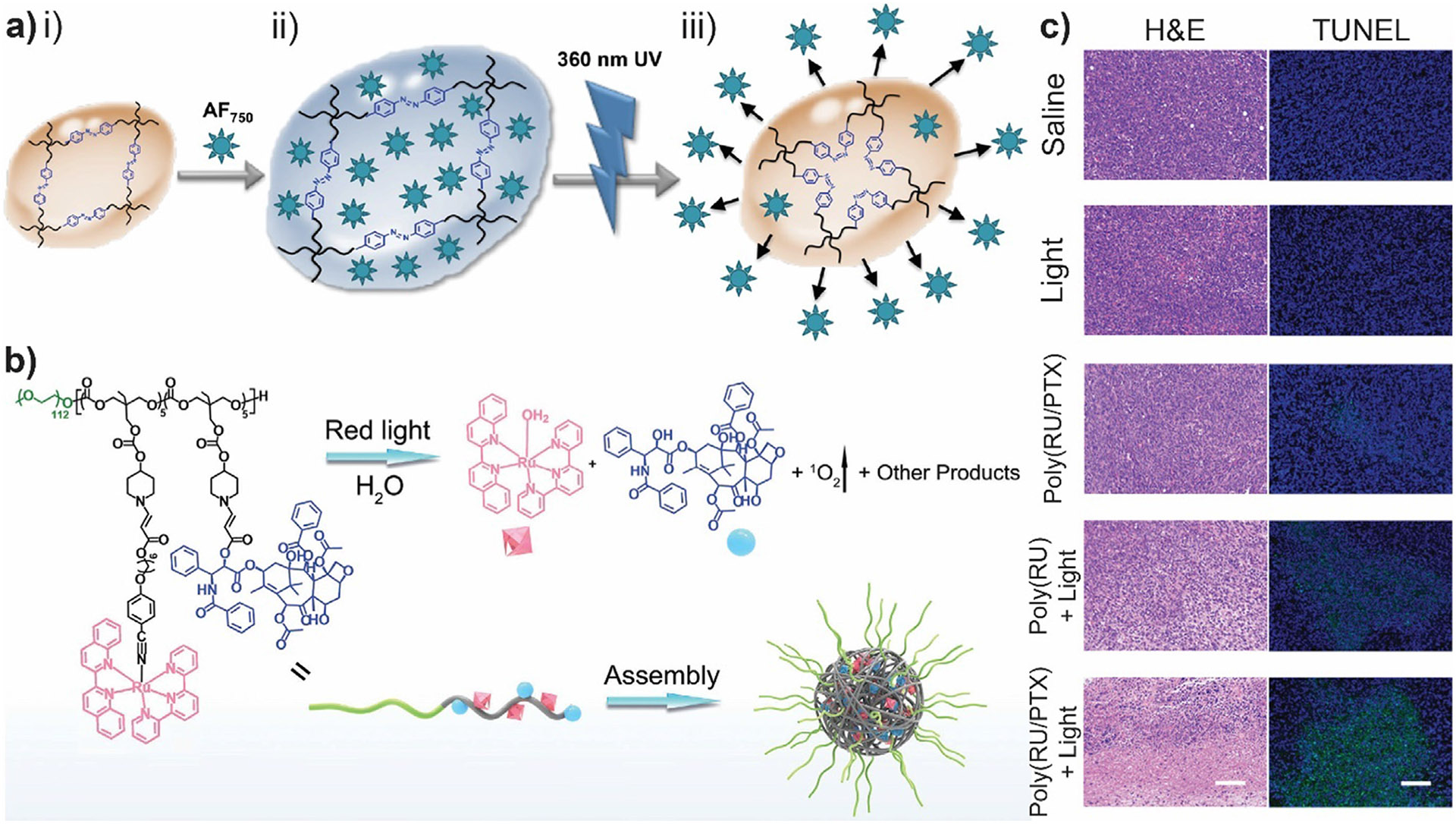
a) Swelling and contraction of photoresponsive hydrogel composed of azobenzoic acid and four-armed PEG. i) Dry hydrogel with trans-azobenzene. ii) Swollen hydrogel loaded with Alexa Fluor 750. iii) After UV illumination, photoisomerization leads to hydrogel contraction and Alexa Fluor 750 release. b) Chemical structure of the biodegradable, biocompatible, amphiphilic block copolymer with paclitaxel (PTX) and Ru complexes (poly (Ru/PTX)). Cleavage of the Ru complex, generation of 1O2 and release of anticancer drug PTX can be induced with red light irradiation. c) Ex vivo histological analyses of tumor sections after different treatments by hematoxylin and eosin (H&E) and (TUNEL) terminal deoxynucleotidyl transferase mediated UTP end labeling staining. Scale bar: 100 μm. Significant tumor cell nuclear ablation and higher level of apoptosis (green) in tumor tissues from mice in the poly (Ru/PTX) can be observed in H&E and TUNEL staining, respectively. Adapted with permission from [224]. Copyright 2018 American Chemical Society. Adapted from [215]
Hydrogels have been also used as drug carriers for phototriggered release. An approach for photoresponsive microneedle arrays was proposed by Amer et al. The system consist in the modification of the microneedle implant adherence upon light irradiation [225]. The microneedle arrays were fabricated with a photoresponsive polyvinyl alcohol and SP-conjugated N-isopropylacrylamide hydrogel. By phototiggering the deswelling of the microneedles, a significant decrease in extraction force was observed. In the same vein, Li et al. reported phototriggered implantable polymeric microneedles for transdermal analgesia delivery [226]. The system consisted in iron oxide nanoparticles coated with polydopamine, polyvinylpyrrolidone and polycaprolactone. The microneedles were loaded with lidocaine hydrochloride and administrated into porcine skin. NIR irradiation penetrated the skin and enhanced lidocaine hydrochloride release from the microneedles. Another photoresponsive design was reported by Rastogi et al., in which a hydrogel was prepared by the crosslinking of di-NHS ester of azo-benzoic acid with four-armed amine-terminated PEG. The hydrogel was loaded with the fluorescent dye Alexa Fluor 750 and its release was triggered by UV-light exposure. UV-light induced photoisomerization and the reduction of the hydrogel size as consequence. Hydrogel size reduction may be a result of the inherently smaller footprint of the cis azobenzene conformation, as well as dipole–dipole interactions between the polar cis azobenzene and the polymer network (Fig. 7a) [224].
4.2. Gene delivery
Gene therapy relies on the modification of the genome by delivering recombinant nuclei acids (DNA or RNA) that add, replace, or remove specific genetic sequences. Despite the recent advances in gene engineering, the safe and specific delivery of genes into targeted cells remains a challenge. Several approaches for the non-viral delivery of genes have been investigated, including the use of stimuli responsive materials as they provide options for on-demand gene release, targeting, and non-harmful immune or genotoxic off-target side effects, etc. [227].
In regard to photoresponsive gene delivery, Dang et al. designed gold nanoshells conjugated to methoxy-PEG-thiol and micoRNA-34a, a tumor suppressor for triple-negative breast cancer that is degraded by nucleases and cannot passively enter cells [228]. The light-responsive nanocarrier enhanced the microRNA-34a stability and on-demand release following irradiation, resulting in a decrease on breast cancer cells viability, proliferation and migration. Lin et al. designed photoresponsive hollow silica nanoparticles loaded with chlorin e6 and a plasmid encoding caspase-8 gene attached to a polymer. Upon light irradiation at 405 nm, the system released the gene and generated ROS which showed antitumor effects both in vitro and in vivo [229]. A photo-responsive cationic block copolymer has shown potential in the development of robust approaches for gene therapy and regenerative medicine. The versatile system disassembles upon light irradiation facilitating the intracellular release of small interfering RNA (siRNA), messenger RNA, and proteins [230]. In this line, Greco et al. employed mPEG-b-poly(5-(3-(amino) propoxy)-2-nitrobenzyl methacrylate) polymers complexed with siRNA for photo-switching release. The nanocarrier exhibited good spatiotemporal control over cell-to-cell gene expression and low off-target effects [231]. Duan et al. reported the development of photodegradable, highly branched poly (β-amino ester) nanoparticles with strong nucleic acid binding capacity for DNA and siRNA delivery. The nanocarrier showed higher transfection efficiencies in multiple mammalian cells, and lower cytotoxicity compared to commercial available PEI 25 k and Lipofectamine 2000 reagents [232]. Vascular endothelial growth factor (VEGF) is the chief regulator in neovascular lesions. Li et al. proposed a strategy for the sequential light-triggered gene delivery of a CRISPR/dCas9 system. Poly(caprolactone)-PEG nanoparticles were loaded with a sgRNA that targeted the VEGF gene, and with arabidopsis flavoprotein cryptochrome 2-cib1 plasmids conjugated to CRISP/dCas9. Upon light exposure the cib1 side of the plasmid fuses with CRISPR/dCas9, while the cryptochrome 2 side fuses with the histone of the sgRNA target. The linked plasmid construct acts on the VEGF gene triggering its transcriptional inhibition. Enhanced therapeutic efficiency was shown when the CRISPR nanosystem was designed as photoresponsive [233].
Often, photoresponsive gene delivery systems have been coupled to photothermal and/or photodynamic therapy to enhance therapeutic outcomes. Wang et al. developed a biomimetic gene delivery strategy to treat glioblastoma tumors. The team designed a lipoprotein-based system by fusing the tumor-penetrating lipoprotein tLyP-1 to the apolipoprotein A-I-mimicking peptide. Indocyanine green was encapsulated and the siRNA target HIF-1α was anchored on the surface for targeting. The tLyP-1 facilitated blood brain barrier permeability, HIF-1α siRNA induced hypoxia into the tumor, and indocyanine green acted as the photo-triggered molecule to release siRNA and produce ROS and local hyperthermia. The smart multi-stimuli nanocarrier was demonstrated as brain-cancer therapy [234]. Zhang et al. fabricated photosensitive nanocapsules comprised of core–shell UCNPs coated with a mesoporous silica layer and PEG was grafted using a photo-cleavable linker. The silica coating was loaded with the potosensitizer hypocrellin and a siRNA against polo-like kinase 1. Upon light exposure, PEG-siRNA was released and ROS were generated. Through this approach gene silencing efficiency was enhanced and cell proliferation was suppressed in vitro and in vivo [235]. Wu et al. reported poly (lactic-co-glycolic acid) nanoparticles surface modified with a disulfide-containing alkyl modified polyethylenimine. Doxorubicin was encapsulated through an o-nitrobenzyl ester linker, and siRNA of P-gp protein encapsulated onto the cationic polymeric shell. The polymer coating on the surface displayed reductive and photoresponsive behaviors. P-gp siRNA is released by enrichment of the reducing agent glutathione, and doxorubicin is released upon light exposure, demonstrating the sequential reduction/photo-triggered gene and chemotherapy strategy [236]. Lin et al. synthesized a photo-sensitive metal organic framework (MOF) for siRNA release accompanied by MOFs dissociation into protonatable 2-methylimidazalo and osmotic rupturing Zn2+ ions. The release of siRNA and the dissociation products cooperatively contributed to endo/lysosomal rupture (~ 90 % efficiency). Photosensitive MOFs exhibited high control on siRNA [237]. DNAzyme is often used for gene silencing. To improve its efficiency Gong et al. proposed a nanocarrier to encapsulate DNAzyme and together with MnO2 and indocyanine green. Upon light exposure DNAzyme is released and simultaneously MnO2 is decomposed into Mn2+ ions, which supplemented with O2 generation to enhance DNAzyme activity [238]. To overcome the challenges associated with light penetration into tissues and tumors, this being wavelengths ranging from the UV to NIR I region, novel material systems responsive to NIR II have been proposed [239-241]. Such photoresponsive materials include soft materials such as semiconducting polymers [242,243], or molybdenum polyoxometalate [244,245], and the combination of soft materials with D–A conjugated small molecular dyes [246], copper-based materials [247,248], or gold nanostructures [249,250], to name a few. In this vein, Lin et al. proposed a photothermal gene delivery system based on poly[2(dimethylamino) ethyl methacrylate] – cooper sulfide – silicon dioxide nanoparticles loaded with a plasmid encoding IL-12 gene. This hybrid nanoformulation was demonstrated for simultaneous photothermal and immunotherapy in vitro and in vivo [251], Moreover, photoresponsive systems for viral gene delivery have been also investigated. Wang et al. reported an unnatural azido-amino acid aided caging–uncaging strategy to control the transduction capability of a lentiviral vector in vitro and in vivo [252]. Horner et al. engineered a similar approach, using an adenoviral vector photoresponsive to red light [253].
4.3. Nanoparticle and biomacromolecules delivery
In the recent years, the FDA has been approving novel therapies that involve the use of biological macromolecules as therapeutic agents to treat different types of cancers, degenerative or inherited diseases. Some of those biomacromolecules are cytokines, antibodies, pro-apoptotic proteins, grown factors, and ribosomes [254-257]. Novel approaches for the delivery of biomacromolecules are focused on developing formulations highly stable, with good drug availability, and with targeting and on demand release capabilities. Additionally, non-biological nanoparticles made of polymers, metals, semiconductors, composites, and other materials, have shown effectiveness not only as carriers but as therapeutic agents themselves. Some drawbacks of the use of nanoparticles as therapeutic agents include the poor membrane permeability, cytotoxicity, and solubility in biological systems [258]. In this section we will summarize recent advances in photosensitive soft materials for the delivery of large therapeutics such as is the case of nanoparticles and biomacromolecules. For instance, Ballesteros et al. developed a system composed by a photoresponsive nanogel containing silver nanoparticles immobilized on the surface of electrospun polycaprolactone nanofibers mats. Silver nanoparticles are released when the nanofiber is irradiated with UV-light. Silver nanoparticles generate antibacterial activity against S. aureus and E. coli [259]. On the same line, a photoresponsive nanogel was fabricated based on aniline- and chitosan-containing silver nanoparticles with antibacterial properties to increase E. coli death [260]. Among the different light-responsive materials, conjugated polymers are emerging candidates with successful application in gene therapy. In this vein, Wang et al. reported photoresponsive conjugated polymer nanoparticles for remote control of gene expression. Upon irradiation the conjugated polymer dissipate heat, which serves as activation trigger for protein-70 promoter which begins transcription of downstream EGFP gene in the living cells [261].
In the case of biomacromolecule release Wang et al. synthesized protein-based photo-responsive hydrogels by covalently polymerizing the photoreceptor C-terminal adenosylcobalamin binding domain proteins using the genetically encoded SpyTagSpy-Catcher chemistry. The system was used for the release of bulky globular proteins, such as mCherry [262]. Song et al. designed a UV-light photoresponsive polypeptide-glycosylated poly(amidoamine) dendron amphiphiles for ovalbumin release. The polymersome was also pH sensitive self-assembling at pH 7.4 and disassembling at pH 5 [263]. Wang et al. designed nanoparticles for local light-triggered penetrating peptide release for choroidal neovascularization inhibition. After intravenous injection in mice and ocular light-irradiation, the nanosystem accumulated in the neovascular lesions [264]. In the same line, Peng et al. constructed a nanosystem with active tumor-targeting, enhanced penetration, and photoresponsive drug release behavior. The system encapsulated and delivered the programmed cell death-ligand 1 to enhance tumor immunotherapy. Tumor growth was inhibited by 97.5 % in the first 24, and total inhibition was achieved by the combinatorial release of a photosintesizer (IR820) for photothermal therapy [265]. Fujimoto et al. demonstrated the aggregation of liposomes using ultrafast DNA photocrosslinking with 3-cyanovinylcarbazole nucleoside. This technology has potential for biomacromolecules release [266]. Dai et al. reported a photoresponsive hydrogel by integrating 4arm-PEG and azobenzene into cellulose nanofibrils for the controlled release of bovine serum albumin. Protein release in this system was reported to be 5-fold higher under UV light irradiation [267]. Yang et al. fabricated an enzyme and photoresponsive nanogel system through UV-light chemical cross-linking of cinnamyloxy groups with PEGylated HA. The CD44-targeted nanogels exhibited higher killing efficiency in CD44-positive A549 cells than to CD44-negative HepG2 cells [268]. Lim et al. engineered a nanosytem loaded with chlorin e6-conjugated di-block PEG-PLL copolymer and membrane disruptive D-(KLAKLAK)2 peptide for photoresponsive combinational chemotherapy [269]. Kozaki et al. proposed a screening system for intracellular functional peptides. It combined a photocleavable peptide array system with cell-penetrating peptides (CPPs). A high capacity to induce cell death by the modified peptides was found [270]. Dong et al. fabricated nanoparticles based on the PA-C1b peptide labeled with sulfo-cyanine7 and loaded into mesoporous silica nanoparticles wrapped by graphene oxide and conjugated to folic acid. The light-mediated peptide release was tested for cancer therapy and for antimicrobial treatment [271]. Wan et al. developed a nucleus-targeting self-assembled nanoparticle system built on IR780 conjugated with TAT peptide and loaded with doxorubicin. The system showed both improved cellular internalization and enhanced photodynamic and photothermal efficacy. In a mice bearing breast cancer model, intratumoral injection of the nanoparticles with local laser irradiation achieved synergistic effects for breast tumor ablation and recurrence [263]. Designed a hybrid microparticles consisting of silk fibroin, gelatin, agarose, and NIR-responsive black phosphorus quantum dots were loaded with growth factors and antibacterial peptides. Upon NIR-irradiation the microparticles released in a controlled manner the growth factors and promoted neovascularization in in vitro and in vivo wound healing models [272]. Finally, cells can be also considered as particulate biomacromolecues. In that vein, Narayan et al. demonstrated the synthesis of genetically engineered Silk-Elastin-Like Protein photo-responsive hydrogels for the encapsulation and release of L929 murine fibroblasts from 3D cultures [273].
4.4. Photodynamic, photothermal and other light-based therapies
In previous sections we have introduced photodynamic therapy (PDT) and photothermal therapy (PTT) as synergistic approaches to aid photosensitive soft materials in the manipulation of cell functions, the regeneration of tissues, and the delivery of therapeutics. In most of the previously discussed combinatorial strategies, inorganic nanoparticles or molecules have been integrated into the soft systems to allow their combination with PDT and/or PTT. This section will be dedicated to review the scarce but highly relevant research done in the development of soft materials for PDT, PTT, and other light-based therapies.
PDT requires three basic components: a photosensitizer, light, and oxygen. These components are non-cytotoxic individually but when combined react to produce highly cytotoxic products, such as ROS, singlet oxygen or triplet oxygen [274]. Such cytotoxic products lead to cell apoptosis or necrosis, and to inflammatory responses that trigger the immunological activation of specialized cells [274]. For instance, Sánchez-Ramírez et al. synthesized poly (lactic-co-glycolic) nanoparticles loaded with carboplatin and indocyanine green. These nanoparticles were used against the ovarian cancer cell line SKOV-3, founding an enhanced cytotoxic effect upon light irradiation [275]. Pan et al. synthesized NH2-terminated diethylene glycol ligands tethered to cis-silicon phthalocyanine for the self-assembly of monodisperse nanospheres. These nanospheres exhibited enhanced red-shifted absorption and emission in the NIR range (750–850 nm), which resulted in an enhanced tumor inhibition effect against in vitro and in vivo models using A549 cells [276]. Liang et al. developed an oxygen self-supplemented PDT nanocarrier based on the ultrasonic dispersion of perfluorooctyl bromide liquid into porphyrin grafted lipid nanoparticles. Both in vitro and in vivo results demonstrated that the obtained lipid-based nanosystem could act as a prominent oxygen reservoir and effectively replenish oxygen into the hypoxic tumors with no need for external stimulation, and dramatically inhibited tumor growth and liver metastasis in an HT-29 colon cancer mouse model [277]. Similar to all medical treatments and therapies, PDT has its own limitations. Short lifetime and diffusion distance of ROS may weaken PDT efficiency [278]. Targeting mitochondria, which in tumor cells are particularly sensitive to redox reactions, may help overcome this limitation and increase tumor killing ability. For PDT to be effective, it is necessary to administer a relatively high dose of photosensitizer. Targeting strategies and physical forces (e.g., electric or magnetic field) can be used to increase drug accumulation in the tumor tissue, decreasing the necessary dose of photosensitizer. [279]. As patients undergoing PDT have to avoid direct sunlight until the drug is eliminated from the body to prevent skin and ocular lesions, developing activatable photosensitizer would be very beneficial. Photosensitizer quenching may hamper their performance, thus preventing their aggregation by dispersing them in functional materials is a viable option for enhancing PDT efficacy [278]. As photosensitizer accumulation in tumor cells may be hindered in some situations, electroporation can be used to increase the intracellular concentration of drug. The efficacy of PDT depends on the oxygen concentration and solid tumors tend to present hypoxic microenvironments [279]. To overcome this limitation, several strategies are being studied such as carrying oxygen to the tumor site or catalyzing hydrogen peroxide inside tumor tissue to generate oxygen.
On the other hand, PTT is based on the hyperthermia effect of certain materials when exposed to specific light wavelengths [280-285]. Although there is a wide variety of photosensitive materials investigated in PTT, here we focus on the soft materials. For example, Ko et al. developed a photoresponsive self-gelling hydrogel formed from HA and gallic acid coordination with ferric ions. The hydrogel could behave as an injectable and could also be adhered to the skin. In the injectable form, upon NIR irradiation the hydrogel was capable to suppress lung metastasis in 4T1-Luc orthotopic breast tumors. When adhere to the skin of mice bearing a xenograft of human epithelial carcinoma cells and NIR-light was applied, tumor ablation was achieved. The application of the hydrogel to skin A375 melanoma-xenografted tumor sites followed by NIR irradiation also resulted in complete tumor ablation [286]. Tang et al. reported a near-infrared (NIR) dye croconaine (CR780) engineered with RGD peptide and PEG, which self-assembled into uniform nanoparticles. These tumor-targeted multimodal nanotheranostic assembly was radiolabeled with 125I to allow for tumor ablation and inhibition of tumor relapse [287]. Among the different light-responsive materials, conjugated polymers are emerging candidates with successful application in the PTT and gene therapy. Chen et al. demonstrated that the combination of mild hyperthermia with CAR T cells therapy can potentially increase the therapeutic index of solid tumors. In this work chondroitin sulfate proteoglycan-4-specific CAR T cells were administered along with poly(lactide-co-glycolide) nanoparticles carrying indocyanine green into Nod scid gamma mice engrafted with the human melanoma cell line WM115. The combination therapy displayed superior antitumor activity after NIR exposure [288]. More recent research has been published supporting the potential in the combination of PTT and CAR T cell therapies for different tumor cancer models [289-291]. In a similar context, Jiang et al. fabricated red blood cell membrane-camouflaged melanin nanoparticles as a platform for in vivo antitumor PTT. These nanoparticles exhibited significantly higher PTT efficacy than that of bare melanin nanoparticles in A549 tumor-bearing mice [292]. Zhou et al. fabricated a dual peptide decorated melanin-like nanoparticle for tumor-targeted and autophagy-promoted PTT. In vivo experiments revealed that the tumor-targeted and autophagy promotion-associated PTT efficiently regressed tumors at temperatures around 43 °C [293]. PTT has been explored as an antibiotic-free strategy to treat bacterial infections. In this context, Han et al. reported hydrogels composed of quaternary ammonium and double-bond modified chitosan and metal–organic framework particles. The hydrogel was capable to inhibit 99.97 % and 99.93 % of bacterial growth for Staphylococcus aureus and Escherichia coli, respectively [294]. A critical factor limiting the use of PTT is thermal damage of normal tissues. To overcome this limitation, one alternative is to improve the tumor-specific targeting of photothermal reagents [278]. This can be done by using nanocarriers with tunable physicochemical properties or with targeting ligands. Another strategy involves the reduction of the heat resistance of tumor cells by inhibiting the synthesis of heat shock proteins in the tumor followed by low-temperature PTT. Both strategies aim to achieve tumor suppression while reducing thermal damage to normal tissues. Monotherapies, such as PTT or PDT, even in scenarios with high therapeutic effect, may not be enough to eliminate all tumor cells. This can lead to tumor recurrence and metastasis. Combining PTT and PDT with other treatment approaches such as immunotherapies or chemotherapies can provide synergistic therapeutical effects [295,296].
Photobiomodulation therapy (PBMT) emerges as utilitarian alternative for controlling and managing the interactions between light, biomolecules, photo-responsive materials and drugs. Several strategies have been proposed using PBMT for the treatment of diabetic ulcers, blood disorders, coronary artery disease, wound healing, inflammation, chronic pain, neurodegenerative disorders, etc. [297]. For instance, the association of Alzheimer disease with the aggregation of β-amyloid peptides is well known and a wide diversity of drugs and materials have been tested for to avoid amyloidosis with no success [298]. Recently, PBMT has been studied for Alzheimer disease treatment. Light exposure has been found to trigger numerous mechanism that led ultimately to amyloid aggregation obstruction. Specific light wavelengths are capable to trigger chemical and physical transformations in the cellular microenvironment such as oxygen reactive species production, heat release, chemical cleavage or peptide conjugative reactions. Those transformations interact with β-amyloid peptides provoking their degradation, crosslinking and other chemical alterations that knock out the aggregates and the oligomers [299-301]. The additional use of photosensitizers can improve the suppression of amyloidosis in a simple, non-toxic and cheap manner [302]. More recently, it has been explored the aiding use of fluorescent molecules and photo-responsive materials to block β-amyloid accumulation [303]. Other approaches based on PBMT have been emerged as prominent modalities to control and modulate the immunological response of tumoral cells. In this case, the use of materials is critical to bridge the light action with immune activation. For instance, it has been reported that bacteria loaded into red blood cells membrane particles is able to release melanin. Melanin release triggers macrophage recruitment and accelerates the phenotype change of these cells to detonate an immune response. Simultaneously, melanin serves as photothermal agent for PTT. These combinatorial immunomodulatory and PTT approach inhibited melanoma and colon cancer using in vivo models [283]. Another interesting PBMT approach is for the photoinhibition of angiogenesis in tumors. In this case, polydopamine crosslinked collagen/silk fibroin composites were prepared to deliver thrombin for blocking blood vessels. The system showed a sustained effect to prevent the recurrence and metastasis of an in vivo model of triple negative breast cancer [304]. In the same line, NIR-II responsive hydrogels were constructed by the incorporation of nitric oxide precursors and 2D tungsten oxide nanosheets within a hydrogel. This hydrogel demonstrated angiogenesis inhibition and anti-recurrence tumor efficacy [305]. Finally, ana strategy for light controlled proteolysis targeting chimeras has been developed. Here, upon light irradiation targeted proteins promote proteasomes degradation with a high spatiotemporal control [306]. While PBMT and phototherapies in general present tremendous potential for minimally invasive topical or superficial treatments, poor tissue penetration of light represents a serious limitation for extending the application to deep tissues. While ultrasound can penetrate above 10 cm of soft tissues [307] and magnetic tissue penetration is only limited by the magnetic field generator’s range [308], UV–vis light has a penetration depth of only a few millimeters or less in soft tissues and NIR just a few centimeters [309]. Several light delivery strategies are being developed to activate photoresponsive biomaterials in vivo such as optical waveguides and implanted micro-LEDs [310,311]. Though with these technologies is possible to overcome poor light penetration of tissues, it is worth noting that the implantation of these devices requires invasive interventions. A brief summary of phototherapies reviewed in this section is presented in Table 5.
Table 5.
Summary of phototherapies reviewed in this article.
| Therapy | Material | Observed Properties and Effects |
Ref |
|---|---|---|---|
| PDT, PTT and chemotherapy | Poly(lactic-co-glycolic)/chitosan NPs loaded with carboplatin and indocyanine green | Biodegradable and biocompatible NPs for multimodal cancer therapy | [275] |
| PDT and PTT | NH2-terminated diethylene glycol ligands tethered to cis-silicon phthalocyanine forming nanospheres | Multimodal therapy and phototheranostic agent, tumor-sensitive system due to pH-dependent response | [276] |
| Oxygen self-supplemented PDT | Ultrasonic dispersion of perfluorooctyl bromide liquid into porphyrin grafted lipid nanoparticles | Oxygen replenish into hypoxic tumors, can act as phototheranostic agent, inhibits tumor growth and metastasis | [277] |
| PTT | Hydrogel based on HA and gallic acid coordinated with ferric ions | Tumor ablation in vivo both in injectable and skin adherable forms | [286] |
| PTT | Croconaine dye and RGD engineered radiolabeled PEG self-assembled into NPs | Multimodal nanotheranostic assembly, tumor ablation and inhibition of tumor relapse | [287] |
| PTT and cell therapy | CAR T cells administered along with indocyanine green-loaded poly (lactide-co-glycolide) NPs | Combination therapy presents superior antitumor activity | [288] |
| PTT | Red blood cell membrane-camouflaged melanin nanoparticles | Enhanced blood retention and tumor accumulation. Higher PTT efficacy than bare melanin NPs | [292] |
| PTT | Dual peptide decorated melanin-like NPs | Tumor-targeting and autophagy-promotion synergistically enhances tumor regression | [293] |
| PTT | Prussian blue and modified chitosan hydrogel | Bacteria capture and kill | [294] |
| PTT and immunotherapy | Melanin-releasing bacteria loaded into red blood cells membrane | Recruitment and anti-tumor polarization of macrophages along with PTT inhibit tumor growth | [283] |
| PTT and anti-angiogenic therapy | Polydopamine crosslinked collagen/silk fibroin composites loaded with thrombin | Thrombin blocking blood vessels and PTT prevention of angiogenesis synergistically inhibits tumor relapse | [304] |
| Anti-angiogenic therapy | Hydrogel containing nitric oxide precursors and 2D tungsten oxide nanosheets | Tumor growth and recurrence inhibition | [305] |
5. Challenges, perspectives and conclusions
Here, we reviewed the different photomedicine approaches that have been developed using soft materials. Approaches to modulate cell functions, for tissue engineering and regenerative medicine, for controlled drug delivery and for phototherapy comment on the complexity of the photo-triggered effects on soft materials systems and on their therapeutic relevance. Photosensitive soft nano- and microstructures are capable to emulate nature’s soft matter dynamic behavior offering multiple possibilities with high spatiotemporal control for the manipulation of cellular signaling and behavior, which has a clear potential as alternative treatments for various health conditions. All the soft materials systems discussed here rely on light-triggered mechanisms such as isomerization, cleavage, crosslinking, hyperthermia, activation reactions or electroconduction, that through different paths interfere with biological functioning at the cellular or tissue level. Photosensitive soft materials and biointerfaces have been explored to manipulate cellular responses such as adhesion, spreading, morphology, migration, and differentiation. These systems have been providing us with novel tools to interrogate cells and improve our understanding of basic cell biology including mechanics and signaling. Moreover, the ability to manipulate cell behaviors using photosensitive soft materials and light as a trigger has allowed the investigation of alternative less invasive therapeutic solutions to modulate cell growth, differentiation, and migration. Synthetic soft material interfaces have been widely exploited to mimic the ECM because of their analogue physical and chemical properties. It is important to note that most of the materials and studies presented here used one stimulus to modulate cell behavior, whether it was mechanical, electrical or a change in wettability of the substrate. However, in physiological context, cells are under a complex and dynamic environment with several chemical and physical signals [129,312]. The design of future photoresponsive materials should aim to achieve precise control over multiple environmental properties and be able to provide multiple stimuli independently and with large spatiotemporal precision [21]. For instance, by combining a molecule exhibiting photoisomerization at a certain wavelength with other molecule exhibiting photovoltaic effect at a different wavelength, independent control over material structure and cell electrical stimulation could be achieved by using the appropriate light. Besides, photoresponsive materials could be combined with materials able to respond to other exogenous (ultrasound, electricity or magnetic fields) or endogenous (pH, reactive oxygen species, pressure) signals to obtain multiresponsiveness [313,314]. The integration of different functional units using engineering approaches will allow us to control multiple factors separately and to better mimic tissues’ complex environments.
It is worth noting that light and photoresponsive soft materials offer tremendous opportunities as building blocks in modern bioengineering technologies. Photopolimerization, i.e., light-induced chain-growth and crosslinking of functional monomers into polymers, can be used in light-based 3D-printing techniques to rapidly obtain complex, high resolution biomedical microdevices and photonics materials [315-318]. Moreover, light and photoresponsive materials offer great opportunities in the four-dimensional (4D) bioprinting technologies, in which the concept of time is integrated as a fourth dimension with traditional 3D bioprinting [319]. Due to light high spatial and temporal precision, it can act as a remote source of energy to induce rapid and localized transformation of material structure and chemistry. Photoresponsive polymers or polymer composites can be used as bioinks to print dynamic materials with light-triggered shape memory effect, self-healing or tunable biodegradability [320-323]. Another bioengineering technology in which photoresponsive materials could generate a large impact is the area of microfluidics, lab-on-a-chip and organ-on-a-chip. Light-based bioprinting of 3D structures with controlled shape and architecture may find interesting applications in hydrogel-based organ-on-a-chip devices [324,325]. Though microfluidic devices may be small, fluidic controllers are often large, inaccurate, or expensive. The integration of microscale photoresponsive soft polymer actuators within microfluidic devices may serve as an inexpensive and highly accurate alternative [326-328]. The micromanipulation offered by these light-controlled actuators may be useful in fluid handling and circulation of culture media in organ-on-a-chip devices [329]. Photonic hydrogels has been proposed for non-invasive real-time control and investigation of the extracellular stiffness in cell incubation systems such as organ-on-a-chip devices [98]. Finally, photoresponsive soft materials are being used for the development of autonomous and highly controlled light-driven soft robots [330]. For instance, light responsiveness and molecular anisotropy have been used to create soft robots able to crawl and jump or elicit complex underwater locomotion [331,332].
Photoresponsive soft scaffolds have provided a unique approach for remote-triggered restoration and replacement of different biological tissues. These photosensitive soft microstructures have been investigated also when combined with drugs, other photosensitive molecules, or nanoparticles to endow light-triggered multifunctionalities and improve tissue engineering and regenerative medicine outcomes. Photosensitive soft nanomaterials have been developed for the wireless on-demand delivery of therapeutics. Photosensitive soft drug delivery systems can be surface engineered to target specific organs/tissues and can be loaded with multiple therapeutics. In these systems, NIR light provides a unique contact less option that can reach few centimeters into the body with high precision to control the delivery of drugs, genes, nanoparticles, or energy. The combination of multiple therapeutic delivery approaches has resulted in enhanced effects. Although with less incidence, photoresponsive soft materials have been explored as mediators of PDT, PTT, and other light-based therapies. The overall goal for the development of photoresponsive soft biointerfaces and materials is to provide minimally invasive therapeutic options that can be externally controlled with high precision.
Nevertheless, the most critical drawback of all photomedicines relies on the light and the biological tissues wavelength-dependent optical properties. Short wavelength light is less desired because it penetrates only 0.5–2.5 mm into biological tissues with up to a 40 % of light scattering. NIR light can penetrate 8–10 mm into biological tissues without scattering [333], still being insufficient to pass into deep tissues or through bone and cartilage tissues. Moreover, the majority of the light responsive motifs have low sensitivity to NIR light which results in high optical power density requirements for their activation [179]. Although light stimulation provides high spatial control, the micrometer incident light can trigger nonspecific and undesired photoreactions in surrounding healthy cells and tissues. Other impairments on the clinical translation of photomedicines are the design of biodegradable and biocompatible photosensitive materials and their controlled large-scale production.
To overcome light penetration and specificity limitations, UCNPs have been proposed. Specifically, UCNPs systems for transforming NIR light into UV/visible light to enhance the effect of PTT and PDT. However, UCNPs biocompatibility, biodegradability, and safety remains to be addressed [334]. The evident need of external stimuli with higher penetration into biological tissues has led to the development of NIR II-triggered strategies. NIR II approaches are in their earlier stages of development, but the limited knowledge in the field suggests their great potential to be utilized in photomedicines when combined with NIR II photosensitive materials [335]. It is clear that there is a big gap between fundamental and translational research that needs to be addressed to improve our understanding of photosensitive soft material’s cytotoxicity, biodegradability, and long-term effects. It is expected that the interactive collaboration between material scientists and chemists, biologists, and clinicians will unlock photomedicines future clinical translation.
Acknowledgements
In honor and memory of Dr. Jorge Romero, who has been part of this work in a special way and holds a special place in our hearts.
This work was partially supported by the NIH National Institute of General Medical Sciences (NIGMS), Grant SC1GM130542. Manuel Eduardo Martinez-Cartagena thanks the FONCYT (Mexico) for financial support through the projects COAH-2020-C14-B007 and CB2016-287954-Q.
Abbreviations:
- BMP
Bone Morphogenetic Protein
- BSA
Bovine Serum Albumin
- β-CD
β-cyclodextrin
- CSFD
Critical Size Femoral Defect
- ECM
Extracellular Matrix
- ECs
Endothelial Cells
- EGF
Epidermal Growth Factor
- GF
Growth Factor
- HA
Hyaluronic Acid
- HUVECs
Human Umbilical Vein Endothelial Cells
- LED
Light-Emitting Diode
- MC
Merocyanine
- MMP
Matrix Metalloproteinase
- MSC
Mesenchymal Stem Cell
- NIR
Near-Infrared
- PBMT
Photobiomodulation therapy
- pDR1m
poly-Disperse Red1-methacrylate
- PEG
Polyethylene Glycol
- PBS
Phosphate Buffered Saline
- PLA
Poly(l-Lactide)
- PLL
Poly-l-Lysine
- PMMA
Poly(methyl methacrylate)
- PTB
Photochemical Tissue Bonding
- RGD
Arg-Gly-Asp, arginine-glycine-aspartate
- SAMs
Self-Assembled Monolayers
- SEM
Scanning electron microscopy
- SM
Stem Cell
- SMP
Shape Memory Polymer
- SP
Spiropyran
- UCNPs
Upconversion Nanoparticles
- UV
Ultraviolet
- VEGF
Vascular Endothelial Growth Factor
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- [1].Rus D, Tolley MT, Design, fabrication and control of soft robots, Nature 521 (2015)467–475. [DOI] [PubMed] [Google Scholar]
- [2].Seliktar D, Designing Cell-Compatible Hydrogels for Biomedical Applications, Science 336 (2012) 1124–1128. [DOI] [PubMed] [Google Scholar]
- [3].Quake SR, Scherer A, From Micro- to Nanofabrication with Soft Materials, Science 290 (2000) 1536–1540. [DOI] [PubMed] [Google Scholar]
- [4].Boelke J, Hecht S, Designing Molecular Photoswitches for Soft Materials Applications, Advanced Optical Materials 7 (2019) 1900404. [Google Scholar]
- [5].Whitesides GM, Soft Robotics, Angewandte Chemie International Edition 57 (2018) 4258–4273. [DOI] [PubMed] [Google Scholar]
- [6].Guntnur RT, Muzzio N, Morales M, Romero G, Phase transition characterization of poly(oligo(ethylene glycol)methyl ether methacrylate) brushes using the quartz crystal microbalance with dissipation, Soft Matter (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Badeau BA, DeForest CA, Programming Stimuli-Responsive Behavior into Biomaterials, Annu. Rev. Biomed. Eng 21 (2019) 241–265. [DOI] [PubMed] [Google Scholar]
- [8].Carayon I, Gaubert A, Mousli Y, Philippe B, Electro-responsive hydrogels: macromolecular and supramolecular approaches in the biomedical field, Biomaterials Science 8 (2020) 5589–5600. [DOI] [PubMed] [Google Scholar]
- [9].Kanaan AF, Piedade AP, Electro-responsive polymer-based platforms for electrostimulation of cells, Materials Advances 3 (2022) 2337–2353. [Google Scholar]
- [10].Qu J, Liang Y, Shi M, Guo B, Gao Y, Yin Z, Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release, Int. J. Biol. Macromol 140 (2019) 255–264. [DOI] [PubMed] [Google Scholar]
- [11].Zheng XS, Tan C, Castagnola E, Cui XT, Electrode Materials for Chronic Electrical Microstimulation, Advanced Healthcare Materials 10 (2021) 2100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P, Wireless magnetothermal deep brain stimulation, Science 347 (2015) 1477–1480. [DOI] [PubMed] [Google Scholar]
- [13].Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A, Remote control of ion channels and neurons through magnetic-field heating of nanoparticles, Nat Nano 5 (2010) 602–606. [DOI] [PubMed] [Google Scholar]
- [14].Shen Y, Wu C, Uyeda TQP, Plaza GR, Liu B, Han Y, Lesniak MS, Cheng Y, Elongated Nanoparticle Aggregates in Cancer Cells for Mechanical Destruction with Low Frequency Rotating Magnetic Field, Theranostics 7 (2017) 1735–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Duyn JH, Studying brain microstructure with magnetic susceptibility contrast at high-field, NeuroImage 168 (2018) 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ho DN, Chapter 15 - Magnetic Resonance Imaging and Alternating Magnetic Fields, in: Chen X, Wong S (Eds.), Cancer Theranostics, Academic Press, Oxford, 2014, pp. 255–268. [Google Scholar]
- [17].Collier C, Muzzio N, Thevi Guntnur R, Gomez A, Redondo C, Zurbano R, Schuller IK, Monton C, Morales R, Romero G, Wireless Force-Inducing Neuronal Stimulation Mediated by High Magnetic Moment Microdiscs, Adv Healthc Mater 11 (2022) e2101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ghanouni P, Pauly KB, Elias WJ, Henderson J, Sheehan J, Monteith S, Wintermark M, Transcranial MRI-Guided Focused Ultrasound: A Review of the Technologic and Neurologic Applications, American Journal of Roentgenology 205 (2015) 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Legon W, Bansal P, Tyshynsky R, Ai L, Mueller JK, Transcranial focused ultrasound neuromodulation of the human primary motor cortex, Scientific Reports 8 (2018) 10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li Q, Schenning APHJ, Bunning TJ, Light-Responsive Smart Soft Matter Technologies, Advanced Optical Materials 7 (2019) 1901160. [Google Scholar]
- [21].Gelmi A, Schutt CE, Stimuli-Responsive Biomaterials: Scaffolds for Stem Cell Control, Advanced Healthcare Materials 10 (2021) 2001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fomina N, Sankaranarayanan J, Almutairi A, Photochemical mechanisms of light-triggered release from nanocarriers, Advanced drug delivery reviews 64 (2012) 1005–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sun J, Wang F, Zhang H, Liu K, Azobenzene-Based Photomechanical Biomaterials, Advanced NanoBiomed Research 1 (2021) 2100020. [Google Scholar]
- [24].Xiong Q, Lim Y, Li D, Pu K, Liang L, Duan H, Photoactive Nanocarriers for Controlled Delivery, Adv. Funct. Mater 30 (2020) 1903896. [Google Scholar]
- [25].Liu J, Kang W, Wang W, Photocleavage-based Photoresponsive Drug Delivery†, Photochemistry and Photobiology 98 (2022) 288–302. [DOI] [PubMed] [Google Scholar]
- [26].Dong Y, Jin G, Hong Y, Zhu H, Lu TJ, Xu F, Bai D, Lin M, Engineering the Cell Microenvironment Using Novel Photoresponsive Hydrogels, ACS Applied Materials & Interfaces 10 (2018) 12374–12389. [DOI] [PubMed] [Google Scholar]
- [27].Karisma VW, Wu W, Lei M, Liu H, Nisar MF, Lloyd MD, Pourzand C, Zhong JL, UVA-Triggered Drug Release and Photo-Protection of Skin, Frontiers in Cell and Developmental Biology 9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xiao L, Chen X, Yang X, Sun J, Geng J, Recent Advances in Polymer-Based Photothermal Materials for Biological Applications, ACS Applied Polymer Materials 2 (2020) 4273–4288. [Google Scholar]
- [29].Wu J, Zheng Y, Jiang S, Qu Y, Wei T, Zhan W, Wang L, Yu Q, Chen H, Two-in-One Platform for High-Efficiency Intracellular Delivery and Cell Harvest: When a Photothermal Agent Meets a Thermoresponsive Polymer, ACS Applied Materials & Interfaces 11 (2019) 12357–12366. [DOI] [PubMed] [Google Scholar]
- [30].Liu C, Guo X, Ruan C, Hu H, Jiang B-P, Liang H, Shen X-C, An injectable thermosensitive photothermal-network hydrogel for near-infrared-triggered drug delivery and synergistic photothermal-chemotherapy, Acta Biomater. 96 (2019) 281–294. [DOI] [PubMed] [Google Scholar]
- [31].Meng Z, Hou W, Zhou H, Zhou L, Chen H, Wu C, Therapeutic Considerations and Conjugated Polymer-Based Photosensitizers for Photodynamic Therapy, Macromol. Rapid Commun 39 (2018) 1700614. [DOI] [PubMed] [Google Scholar]
- [32].Peddinti BST, Morales-Gagnon N, Pourdeyhimi B, Scholle F, Spontak RJ, Ghiladi RA, Photodynamic Coatings on Polymer Microfibers for Pathogen Inactivation: Effects of Application Method and Composition, ACS Applied Materials & Interfaces 13 (2021) 155–163. [DOI] [PubMed] [Google Scholar]
- [33].Han M, Srivastava SB, Yildiz E, Melikov R, Surme S, Dogru-Yuksel IB, Kavakli IH, Sahin A, Nizamoglu S, Organic Photovoltaic Pseudocapacitors for Neurostimulation, ACS Applied Materials & Interfaces 12 (2020) 42997–43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kim J, Seo J, Jung D, Lee T, Ju H, Han J, Kim N, Jeong J, Cho S, Seol JH, Lee J, Active photonic wireless power transfer into live tissues, Proceedings of the National Academy of Sciences 117 (2020) 16856–16863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Walma DAC, Yamada KM, The extracellular matrix in development, Development, 147 (2020) dev175596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hussey GS, Dziki JL, Badylak SF, Extracellular matrix-based materials for regenerative medicine, Nature Reviews Materials 3 (2018) 159–173. [Google Scholar]
- [37].Nicolas J, Magli S, Rabbachin L, Sampaolesi S, Nicotra F, Russo L, 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate, Biomacromolecules 21 (2020) 1968–1994. [DOI] [PubMed] [Google Scholar]
- [38].Frantz C, Stewart KM, Weaver VM, The extracellular matrix at a glance, J. Cell Sci 123 (2010) 4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].van Dijk M, Goransson SA, Stromblad S, Cell to extracellular matrix interactions and their reciprocal nature in cancer, Exp. Cell Res 319 (2013) 1663–1670. [DOI] [PubMed] [Google Scholar]
- [40].Mohamed MA, Fallahi A, El-Sokkary AMA, Salehi S, Akl MA, Jafari A, Tamayol A, Fenniri H, Khademhosseini A, Andreadis ST, Cheng C, Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology, Prog. Polym. Sci 98 (2019) 101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rosales AM, Anseth KS, The design of reversible hydrogels to capture extracellular matrix dynamics, Nature Reviews Materials 1 (2016) 15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mertgen AS, Trossmann VT, Guex AG, Maniura-Weber K, Scheibel T, Rottmar M, Multifunctional Biomaterials: Combining Material Modification Strategies for Engineering of Cell-Contacting Surfaces, ACS Appl Mater Interfaces 12 (2020) 21342–21367. [DOI] [PubMed] [Google Scholar]
- [43].Hippler M, Lemma ED, Bertels S, Blasco E, Barner-Kowollik C, Wegener M, Bastmeyer M, 3D Scaffolds to Study Basic Cell Biology, Adv. Mater 31 (2019) e1808110. [DOI] [PubMed] [Google Scholar]
- [44].Iozzo RV, Gubbiotti MA, Extracellular matrix: The driving force of mammalian diseases, Matrix Biol. 71–72 (2018) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bacakova L, Filova E, Parizek M, Ruml T, Svorcik V, Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants, Biotechnol. Adv 29 (2011) 739–767. [DOI] [PubMed] [Google Scholar]
- [46].Zhang S, Xing M, Li B, Biomimetic Layer-by-Layer Self-Assembly of Nanofilms, Nanocoatings, and 3D Scaffolds for Tissue Engineering, Int. J. Mol. Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Qiu S, Ji J, Sun W,Pei J, He J, Li Y,Li JJ, Wang G, Recent advances in surface manipulation using micro-contact printing for biomedical applications, Smart Materials in Medicine 2 (2021) 65–73. [Google Scholar]
- [48].Su Y, Toftdal MS, Le Friec A, Dong M, Han X, Chen M, 3D Electrospun Synthetic Extracellular Matrix for Tissue Regeneration, Small Science 1 (2021) 2100003. [Google Scholar]
- [49].Zheng Y, Farrukh A, del Campo A, Optoregulated Biointerfaces to Trigger Cellular Responses, Langmuir 34 (2018) 14459–14471. [DOI] [PubMed] [Google Scholar]
- [50].Thang DC, Wang Z, Lu X, Xing B, Precise cell behaviors manipulation through light-responsive nano-regulators: recent advance and perspective, Theranostics 9 (2019) 3308–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Khalili AA, Ahmad MR, A Review of Cell Adhesion Studies for Biomedical and Biological Applications, Int. J. Mol. Sci 16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Muzzio N, Azzaroni O, Moya S, Pasquale M, Concepts for Designing Tailored Thin Film Surfaces with Potential Biological Applications, Multilayer Thin Films - Versatile Applications for Materials Engineering, IntechOpen; 2020. [Google Scholar]
- [53].Hou Y, Xie W, Yu L, Camacho LC, Nie C, Zhang M, Haag R, Wei Q, Surface Roughness Gradients Reveal Topography-Specific Mechanosensitive Responses in Human Mesenchymal Stem Cells, Small 16 (2020) 1905422. [DOI] [PubMed] [Google Scholar]
- [54].Muzzio NE, Pasquale MA, Marmisolle WA, von Bilderling C, Cortez ML, Pietrasanta LI, Azzaroni O, Self-assembled phosphate-polyamine networks as biocompatible supramolecular platforms to modulate cell adhesion, Biomater Sci 6 (2018) 2230–2247. [DOI] [PubMed] [Google Scholar]
- [55].Muzzio NE, Pasquale MA, Rios X, Azzaroni O, Llop J, Moya SE, Adsorption and Exchangeability of Fibronectin and Serum Albumin Protein Corona on Annealed Polyelectrolyte Multilayers and Their Consequences on Cell Adhesion, Advanced Materials Interfaces 6 (2019) 1900008. [Google Scholar]
- [56].Hao Y, Cui H, Meng J, Wang S, Photo-responsive smart surfaces with controllable cell adhesion, J. Photochem. Photobiol. A: Chem 355 (2018) 202–211. [Google Scholar]
- [57].Wu L, Di Cio S, Azevedo HS, Gautrot JE, Photoconfigurable, Cell-Remodelable Disulfide Cross-linked Hyaluronic Acid Hydrogels, Biomacromolecules 21 (2020) 4663–4672. [DOI] [PubMed] [Google Scholar]
- [58].Karimipour K, Keyvan Rad J, Shirvalilou S, Khoei S, Mahdavian AR, Spiropyran-based photoswitchable acrylic nanofibers: A stimuli-responsive substrate for light controlled C6 glioma cells attachment/detachment, Colloids Surf. B. Biointerfaces 203 (2021) 111731. [DOI] [PubMed] [Google Scholar]
- [59].He D, Arisaka Y, Masuda K, Yamamoto M, Takeda N, A photoresponsive soft interface reversibly controls wettability and cell adhesion by conformational changes in a spiropyran-conjugated amphiphilic block copolymer, Acta Biomater. 51 (2017) 101–111. [DOI] [PubMed] [Google Scholar]
- [60].Zhang J, Ma W, He X-P, Tian H, Taking Orders from Light: Photo-Switchable Working/Inactive Smart Surfaces for Protein and Cell Adhesion, ACS Applied Materials & Interfaces 9 (2017) 8498–8507. [DOI] [PubMed] [Google Scholar]
- [61].Li G, Wang H, Zhu Z, Fan J-B, Tian Y, Meng J, Wang S, Photo-Irresponsive Molecule-Amplified Cell Release on Photoresponsive Nanostructured Surfaces, ACS Applied Materials & Interfaces 11 (2019) 29681–29688. [DOI] [PubMed] [Google Scholar]
- [62].Ravichandran G, Rengan AK, Aptamer-Mediated Nanotheranostics for Cancer Treatment: A Review, ACS Applied Nano Materials 3 (2020) 9542–9559. [Google Scholar]
- [63].Huang F, Chen M, Zhou Z, Duan R, Xia F, Willner I, Spatiotemporal patterning of photoresponsive DNA-based hydrogels to tune local cell responses, Nature Communications 12 (2021) 2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Milos F, Tullii G, Gobbo F, Lodola F, Galeotti F, Verpelli C, Mayer D, Maybeck V, Offenhäusser A, Antognazza MR, High Aspect Ratio and Light-Sensitive Micropillars Based on a Semiconducting Polymer Optically Regulate Neuronal Growth, ACS Applied Materials & Interfaces 13 (2021) 23438–23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Izuta S, Yamaguchi S, Kosaka T, Okamoto A, Reversible and Photoresponsive Immobilization of Nonadherent Cells by Spiropyran-Conjugated PEG–Lipids, ACS Applied Bio Materials 2 (2019) 33–38. [DOI] [PubMed] [Google Scholar]
- [66].Nagata K, Kurebayashi T, Imato K, Takeda N, Photoresponsive fiber scaffolds with a core–sheath nanostructure for regulating cell behaviors, Journal of Materials Chemistry B 6 (2018) 2052–2056. [DOI] [PubMed] [Google Scholar]
- [67].Ma D, Zhou N, Zhang T, Hu K, Ma X.e., Gu N, Photoresponsive smart hydrogel microsphere via host-guest interaction for 3D cell culture, Colloids Surf. Physicochem. Eng. Aspects 522 (2017) 97–104. [Google Scholar]
- [68].Liu Z, Meyers MA, Zhang Z, Ritchie RO, Functional gradients and heterogeneities in biological materials: Design principles, functions, and bioinspired applications, Prog. Mater Sci 88 (2017) 467–498. [Google Scholar]
- [69].Datta P, Vyas V, Dhara S, Chowdhury AR, Barui A, Anisotropy Properties of Tissues: A Basis for Fabrication of Biomimetic Anisotropic Scaffolds for Tissue Engineering, J. Bionic Eng 16 (2019) 842–868. [Google Scholar]
- [70].Hadden WJ, Young JL, Holle AW, McFetridge ML, Kim DY, Wijesinghe P, Taylor-Weiner H, Wen JH, Lee AR, Bieback K, Vo B-N, Sampson DD, Kennedy BF, Spatz JP, Engler AJ, Choi YS, Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels, Proceedings of the National Academy of Sciences 114 (2017) 5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ender AM, Kaygisiz K, Räder H-J, Mayer FJ, Synatschke CV, Weil T, Cell-Instructive Surface Gradients of Photoresponsive Amyloid-like Fibrils, ACS Biomaterials Science & Engineering 7 (2021) 4798–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Norris SCP, Tseng P, Kasko AM, Direct Gradient Photolithography of Photodegradable Hydrogels with Patterned Stiffness Control with Submicrometer Resolution, ACS Biomaterials Science & Engineering 2 (2016) 1309–1318. [DOI] [PubMed] [Google Scholar]
- [73].Rape AD, Zibinsky M, Murthy N, Kumar S, A synthetic hydrogel for the high-throughput study of cell–ECM interactions, Nature Communications 6 (2015) 8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Vaquero S, Bossio C, Bellani S, Martino N, Zucchetti E, Lanzani G, Antognazza MR, Conjugated polymers for the optical control of the electrical activity of living cells, Journal of Materials Chemistry B 4 (2016) 5272–5283. [DOI] [PubMed] [Google Scholar]
- [75].Jarzębska NT, Yamaguchi S, Izuta S, Kosaka T, Yamahira S, Nagamune T, Okamoto A, Photo-responsive materials with strong cell trapping ability for light-guided manipulation of nonadherent cells, Biomaterials, Science 7 (2019) 4514–4518. [DOI] [PubMed] [Google Scholar]
- [76].Yamaguchi S, Takasaki Y, Yamahira S, Nagamune T, Photo-Cleavable Peptide-Poly(Ethylene Glycol) Conjugate Surfaces for Light-Guided Control of Cell Adhesion, Micromachines 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Li X, Matino L, Zhang W, Klausen L, McGuire AF, Lubrano C, Zhao W, Santoro F, Cui B, A nanostructure platform for live-cell manipulation of membrane curvature, Nat. Protoc 14 (2019) 1772–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].De Martino S, Zhang W, Klausen L, Lou H-Y, Li X, Alfonso FS, Cavalli S, Netti PA, Santoro F, Cui B, Dynamic Manipulation of Cell Membrane Curvature by Light-Driven Reshaping of Azopolymer, Nano Lett. 20 (2020) 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rossano L, Cimmino C, Cavalli S, Ventre M, Netti PA, Regulating Fibroblast Shape and Mechanics through Photoresponsive Surfaces with Concentric Circular Topographic Patterns, Advanced Materials Interfaces 5 (2018) 1800890. [Google Scholar]
- [80].Thüroff F, Goychuk A, Reiter M, Frey E, Bridging the gap between single-cell migration and collective dynamics, eLife 8 (2019) e46842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Norden C, Lecaudey V, Collective cell migration: general themes and new paradigms, Curr. Opin. Genet. Dev 57 (2019) 54–60. [DOI] [PubMed] [Google Scholar]
- [82].Muzzio NE, Horowitz CM, Azzaroni O, Moya SE, Pasquale MA, Tilted mammalian cell colony propagation dynamics on patterned substrates, Chaos, Solitons & Fractals 146 (2021) 110841. [Google Scholar]
- [83].Shellard A, Mayor R, All Roads Lead to Directional Cell Migration, Trends Cell Biol. 30 (2020) 852–868. [DOI] [PubMed] [Google Scholar]
- [84].Wong M, Gilmour D, Going your own way: Self-guidance mechanisms in cell migration, Curr. Opin. Cell Biol 72 (2021) 116–123. [DOI] [PubMed] [Google Scholar]
- [85].Koçer G, ter Schiphorst J, Hendrikx M, Kassa HG, Leclère P, Schenning APHJ, Jonkheijm P, Light-Responsive Hierarchically Structured Liquid Crystal Polymer Networks for Harnessing Cell Adhesion and Migration, Adv. Mater 29 (2017) 1606407. [DOI] [PubMed] [Google Scholar]
- [86].Isomäki M, Fedele C, Kääriäinen L, Mäntylä E, Nymark S, Ihalainen TO, Priimagi A, Light-Responsive Bilayer Cell Culture Platform for Reversible Cell Guidance, Small Science, n/a (2021) 2100099. [Google Scholar]
- [87].Liu J, Shang J, Chen Y, Tian Y, Yang Q, Chen M, Xiong B, Zhang X-B, A surface-engineered NIR light-responsive actuator for controllable modulation of collective cell migration, Journal of Materials Chemistry B 7 (2019) 5528–5534. [DOI] [PubMed] [Google Scholar]
- [88].Wu X, Huang W, Wu W-H, Xue B, Xiang D, Li Y, Qin M, Sun F, Wang W, Zhang W-B, Cao Y, Reversible hydrogels with tunable mechanical properties for optically controlling cell migration, Nano Research 11 (2018) 5556–5565. [Google Scholar]
- [89].Muzzio NE, Carballido M, Pasquale MA, Gonzalez PH, Azzaroni O, Arvia AJ, Morphology and dynamics of tumor cell colonies propagating in epidermal growth factor supplemented media, Phys. Biol 15 (2018) 046001. [DOI] [PubMed] [Google Scholar]
- [90].Heinrich MA, Alert R, LaChance JM, Zajdel TJ, Košmrlj A, Cohen DJ, Size-dependent patterns of cell proliferation and migration in freely-expanding epithelia, eLife 9 (2020) e58945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Yamamoto S, Okada K, Sasaki N, Chang AC, Yamaguchi K, Nakanishi J, Photoactivatable Hydrogel Interfaces for Resolving the Interplay of Chemical, Mechanical, and Geometrical Regulation of Collective Cell Migration, Langmuir 35 (2019) 7459–7468. [DOI] [PubMed] [Google Scholar]
- [92].Yamada KM, Sixt M, Mechanisms of 3D cell migration, Nature Reviews Molecular Cell Biology 20 (2019) 738–752. [DOI] [PubMed] [Google Scholar]
- [93].Cao W, Li X, Zuo X, Gao C, Migration of endothelial cells into photo-responsive hydrogels with tunable modulus under the presence of pro-inflammatory macrophages, Regenerative Biomaterials 6 (2019) 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Özkale B, Sakar MS, Mooney DJ, Active biomaterials for mechanobiology, Biomaterials 267 (2021) 120497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lee IN, Dobre O, Richards D, Ballestrem C, Curran JM, Hunt JA, Richardson SM, Swift J, Wong LS, Photoresponsive Hydrogels with Photoswitchable Mechanical Properties Allow Time-Resolved Analysis of Cellular Responses to Matrix Stiffening, ACS Applied Materials & Interfaces 10 (2018) 7765–7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Brown TE, Silver JS, Worrell BT, Marozas IA, Yavitt FM, Günay KA, Bowman CN, Anseth KS, Secondary Photocrosslinking of Click Hydrogels To Probe Myoblast Mechanotransduction in Three Dimensions, Journal of the American Chemical Society 140 (2018) 11585–11588. [DOI] [PubMed] [Google Scholar]
- [97].Homma K, Chang AC, Yamamoto S, Tamate R, Ueki T, Nakanishi J, Design of azobenzene-bearing hydrogel with photoswitchable mechanics driven by photo-induced phase transition for in vitro disease modeling, Acta Biomater. 132 (2021) 103–113. [DOI] [PubMed] [Google Scholar]
- [98].Li S, Zeng Y, Hou W, Wan W, Zhang J, Wang Y, Du X, Gu Z, Photo-responsive photonic hydrogel: in situ manipulation and monitoring of cell scaffold stiffness, Materials Horizons 7 (2020) 2944–2950. [Google Scholar]
- [99].Chandorkar Y, Castro Nava A, Schweizerhof S, Van Dongen M, Haraszti T, Köhler J, Zhang H, Windoffer R, Mourran A, Möller M, De Laporte L, Cellular responses to beating hydrogels to investigate mechanotransduction, Nature Communications 10 (2019) 4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Liu L, Shadish JA, Arakawa CK, Shi K, Davis J, DeForest CA, Cyclic Stiffness Modulation of Cell-Laden Protein-Polymer Hydrogels in Response to User-Specified Stimuli Including Light, Advanced Biosystems 2 (2018) 1800240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ng IC, Pawijit P, Tan J, Yu H, Anatomy and Physiology for Biomaterials Research and Development, in: Narayan R (Ed.), Encyclopedia of Biomedical Engineering, Elsevier, Oxford, 2019, pp. 225–236. [Google Scholar]
- [102].Facklam AL, Volpatti LR, Anderson DG, Biomaterials for Personalized Cell Therapy, Adv. Mater 32 (2020) 1902005. [DOI] [PubMed] [Google Scholar]
- [103].Mitchell AC, Briquez PS, Hubbell JA, Cochran JR, Engineering growth factors for regenerative medicine applications, Acta Biomater. 30 (2016) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zentelytė A, Žukauskaitė D, Jacerytė I, Borutinskaitė VV, Navakauskienė R, Small Molecule Treatments Improve Differentiation Potential of Human Amniotic Fluid Stem Cells, Frontiers in Bioengineering and Biotechnology 9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Escobar A, Muzzio NE, Martínez-Villacorta ÁM, Abarrategi A, Bindini E, Grzelczak M, Bordoni AV, Angelomé PC, Moya SE, Mesoporous titania coatings with carboxylated pores for complexation and slow delivery of strontium for osteogenic induction, Appl. Surf. Sci 510 (2020) 145172. [Google Scholar]
- [106].Zhang Y, Wiesholler LM, Rabie H, Jiang P, Lai J, Hirsch T, Lee K-B, Remote Control of Neural Stem Cell Fate Using NIR-Responsive Photoswitching Upconversion Nanoparticle Constructs, ACS Applied Materials & Interfaces 12 (2020) 40031–40041. [DOI] [PubMed] [Google Scholar]
- [107].Wen S, Zhou J, Zheng K, Bednarkiewicz A, Liu X, Jin D, Advances in highly doped upconversion nanoparticles, Nature Communications 9 (2018) 2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Yuan B, Aziz MRF, Li S, Wu J, Li D, Li R-K, An electro-spun tri-component polymer biomaterial with optoelectronic properties for neuronal differentiation, Acta Biomater. (2021). [DOI] [PubMed] [Google Scholar]
- [109].Wu Y, Peng Y, Bohra H, Zou J, Ranjan VD, Zhang Y, Zhang Q, Wang M, Photoconductive Micro/Nanoscale Interfaces of a Semiconducting Polymer for Wireless Stimulation of Neuron-Like Cells, ACS Applied Materials & Interfaces 11 (2019) 4833–4841. [DOI] [PubMed] [Google Scholar]
- [110].Zhang Z, Xu R, Wang Z, Dong M, Cui B, Chen M, Visible-Light Neural Stimulation on Graphitic-Carbon Nitride/Graphene Photocatalytic Fibers, ACS Applied Materials & Interfaces 9 (2017) 34736–34743. [DOI] [PubMed] [Google Scholar]
- [111].Madl CM, Flaig IA, Holbrook CA, Wang YX, Blau HM, Biophysical matrix cues from the regenerating niche direct muscle stem cell fate in engineered microenvironments, Biomaterials 275 (2021) 120973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].De Martino S, Cavalli S, Netti PA, Photoactive Interfaces for Spatio-Temporal Guidance of Mesenchymal Stem Cell Fate, Advanced Healthcare Materials 9 (2020) 2000470. [DOI] [PubMed] [Google Scholar]
- [113].Yan Z, Qin H, Ren J, Qu X, Photocontrolled Multidirectional Differentiation of Mesenchymal Stem Cells on an Upconversion Substrate, Angew. Chem. Int. Ed 57 (2018) 11182–11187. [DOI] [PubMed] [Google Scholar]
- [114].Zhang J, Wong SHD, Wu X, Lei H, Qin M, Shi P, Wang W, Bian L, Cao Y, Engineering Photoresponsive Ligand Tethers for Mechanical Regulation of Stem Cells, Adv. Mater 33 (2021) 2105765. [DOI] [PubMed] [Google Scholar]
- [115].Calvo-Rodriguez M, Bacskai BJ, Mitochondria and Calcium in Alzheimer’s Disease: From Cell Signaling to Neuronal Cell Death, Trends Neurosci. 44 (2021) 136–151. [DOI] [PubMed] [Google Scholar]
- [116].Büsselberg D, Florea A-M, Targeting Intracellular Calcium Signaling ([Ca2+] i) to Overcome Acquired Multidrug Resistance of Cancer Cells: A Mini-Overview, Cancers (Basel) 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Gregurec D, Senko AW, Chuvilin A, Reddy PD, Sankararaman A, Rosenfeld D, Chiang P-H, Garcia F, Tafel I, Varnavides G, Ciocan E, Anikeeva P, Magnetic Vortex Nanodiscs Enable Remote Magnetomechanical Neural Stimulation, ACS Nano 14 (2020) 8036–8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Liu L, Chen B, Liu K,Gao J, Ye Y, Wang Z, Qin N, Wilson DA, Tu Y, Peng F, Wireless Manipulation of Magnetic/Piezoelectric Micromotors for Precise Neural Stem-Like Cell Stimulation, Adv. Funct. Mater 30 (2020) 1910108. [Google Scholar]
- [119].Lee J-U, Shin W, Lim Y, Kim J, Kim WR, Kim H, Lee J-H, Cheon J, Non-contact long-range magnetic stimulation of mechanosensitive ion channels in freely moving animals, Nature Materials 20 (2021) 1029–1036. [DOI] [PubMed] [Google Scholar]
- [120].Tay A, Sohrabi A, Poole K, Seidlits S, Di Carlo D, A 3D Magnetic Hyaluronic Acid Hydrogel for Magnetomechanical Neuromodulation of Primary Dorsal Root Ganglion Neurons, Adv. Mater 30 (2018) 1800927. [DOI] [PubMed] [Google Scholar]
- [121].Golovynska I, Golovynskyi S, Stepanov YV, Stepanova LI, Qu J, Ohulchanskyy TY, Red and near-infrared light evokes Ca2+ influx, endoplasmic reticulum release and membrane depolarization in neurons and cancer cells, J. Photochem. Photobiol. B: Biol 214 (2021) 112088. [DOI] [PubMed] [Google Scholar]
- [122].Nguyen NT, Ma G, Zhou Y, Jing J, Optogenetic approaches to control Ca2+-modulated physiological processes, Current Opinion in Physiology 17 (2020) 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Hopkins J, Travaglini L, Lauto A, Cramer T, Fraboni B, Seidel J, Mawad D, Photoactive Organic Substrates for Cell Stimulation: Progress and Perspectives, Advanced Materials Technologies 4 (2019) 1800744. [Google Scholar]
- [124].Bossio C, Abdel Aziz I, Tullii G, Zucchetti E, Debellis D, Zangoli M, Di Maria F, Lanzani G, Antognazza MR, Photocatalytic Activity of Polymer Nanoparticles Modulates Intracellular Calcium Dynamics and Reactive Oxygen Species in HEK-293 Cells, Frontiers in Bioengineering and Biotechnology 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Airaghi Leccardi MJI, Chenais NAL, Ferlauto L, Kawecki M, Zollinger EG, Ghezzi D, Photovoltaic organic interface for neuronal stimulation in the near-infrared, Communications Materials 1 (2020) 21. [Google Scholar]
- [126].Frey BM, Zeisberger SM, Hoerstrup SP, Tissue Engineering and Regenerative Medicine - New Initiatives for Individual Treatment Offers, Transfus. Med. Hemother 43 (2016) 318–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Dzobo K, Thomford NE, Senthebane DA, Shipanga H, Rowe A, Dandara C, Pillay M, Motaung K, Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine, Stem Cells Int. 2018 (2018) 2495848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Qu H, Fu H, Han Z, Sun Y, Biomaterials for bone tissue engineering scaffolds: a review, RSC Advances 9 (2019) 26252–26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Muzzio N, Moya S, Romero G, Multifunctional Scaffolds and Synergistic Strategies in Tissue Engineering and Regenerative Medicine, Pharmaceutics 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ruskowitz ER, DeForest CA, Photoresponsive biomaterials for targeted drug delivery and 4D cell culture, Nature Reviews Materials 3 (2018) 17087. [Google Scholar]
- [131].Zhu H, Yang H, Ma Y, Lu TJ, Xu F, Genin GM, Lin M, Spatiotemporally Controlled Photoresponsive Hydrogels: Design and Predictive Modeling from Processing through Application, Adv. Funct. Mater 30 (2020) 2000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Bisoyi HK, Urbas AM, Li Q, Soft Materials Driven by Photothermal Effect and Their Applications, Photoactive Functional Soft Materials (2019) 1–44. [Google Scholar]
- [133].Hamblin MR, Photobiomodulation or low-level laser therapy, Journal of Biophotonics 9 (2016) 1122–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].de Freitas LF, Hamblin MR, Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy, IEEE J Sel Top Quantum Electron 22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Dompe C, Moncrieff L, Matys J, Grzech-Leśniak K, Kocherova I, Bryja A, Bruska M, Dominiak M, Mozdziak P, Skiba TH, Shibli JA, Angelova Volponi A, Kempisty B, Dyszkiewicz-Konwińska M, Photobiomodulation—Underlying Mechanism and Clinical Applications, Journal of Clinical Medicine, 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Walker BR, Moraes CT, Nuclear-Mitochondrial Interactions, Biomolecules 12 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Gerbi M.E.M.d.M., Miranda JM, Arruda J.A.A.d., Moreno LMM, Carneiro VSM, Brasilino NC, Menezes RF, Brugnera A Junior, Pinheiro ALB, Photobiomodulation Therapy in Bone Repair Associated with Bone Morphogenetic Proteins and Guided Bone Regeneration: A Histomorphometric Study, Photomed. Laser Surg, 36 (2018) 581–588. [DOI] [PubMed] [Google Scholar]
- [138].Xiang A, Deng H, Cheng K, Liu H, Lin L, Qu X, Liu S, Shen X, Laser photobiomodulation for cartilage defect in animal models of knee osteoarthritis: a systematic review and meta-analysis, Lasers Med. Sci 35 (2020) 789–796. [DOI] [PubMed] [Google Scholar]
- [139].Winter R, Dungel P, Reischies FMJ, Rohringer S, Slezak P, Smolle C, Spendel S, Kamolz L-P, Ghaffari-Tabrizi-Wizsy N, Schicho K, Photobiomodulation (PBM) promotes angiogenesis in-vitro and in chick embryo chorioallantoic membrane model, Sci. Rep 8 (2018) 17080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Karic V, Chandran R, Abrahamse H, Photobiomodulation and Stem Cell Therapy for Temporomandibular Joint Disc Disorders, Photobiomodulation, Photomedicine, and Laser Surgery 38 (2020) 398–408. [DOI] [PubMed] [Google Scholar]
- [141].Khosravipour A, Amini A, Masteri Farahani R, Zare F, Mostafavinia A, Fallahnezhad S, Akbarzade S, Ava p., Asgari M, Mohammadbeigi A, Rezaei F, Ghoreishi SK, Chien S, Bayat M, Preconditioning adipose-derived stem cells with photobiomodulation significantly increased bone healing in a critical size femoral defect in rats, Biochemical and Biophysical Research Communications 531 (2020) 105–111. [DOI] [PubMed] [Google Scholar]
- [142].Chen H, Wang Y, Tu W, Wang H, Yin H, Sha H, Li Y, Effects of photobiomodulation combined with MSCs transplantation on the repair of spinal cord injury in rat, J. Cell. Physiol 236 (2021) 921–930. [DOI] [PubMed] [Google Scholar]
- [143].Magri AMP, Parisi JR, de Andrade ALM, Rennó ACM, Bone substitutes and photobiomodulation in bone regeneration: A systematic review in animal experimental studies, Journal of Biomedical Materials Research Part A 109 (2021) 1765–1775. [DOI] [PubMed] [Google Scholar]
- [144].Ren X, Zhao M, Lash B, Martino MM, Julier Z, Growth Factor Engineering Strategies for Regenerative Medicine Applications, Frontiers in Bioengineering and Biotechnology 7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Lee K, Silva EA, Mooney DJ, Growth factor delivery-based tissue engineering: general approaches and a review of recent developments, J R Soc Interface 8 (2011) 153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Park JW, Hwang SR, Yoon IS, Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration, Molecules 22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Zhao W, Li Y, Zhang X, Zhang R, Hu Y, Boyer C, Xu F-J, Photo-responsive supramolecular hyaluronic acid hydrogels for accelerated wound healing, Journal of Controlled Release 323 (2020) 24–35. [DOI] [PubMed] [Google Scholar]
- [148].Jiang B, Liu X, Yang C, Yang Z, Luo J, Kou S, Liu K, Sun F, Injectable, photoresponsive hydrogels for delivering neuroprotective proteins enabled by metal-directed protein assembly, Science Advances, 6 eabc4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Cooper CL, Panitz N, Edwards TA, Goyal P, Role of the CarH photoreceptor protein environment in the modulation of cobalamin photochemistry, Biophys. J 120 (2021) 3688–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Wang H, Morales R-T-T, Cui X, Huang J, Qian W, Tong J, Chen W, A Photoresponsive Hyaluronan Hydrogel Nanocomposite for Dynamic Macrophage Immunomodulation, Advanced Healthcare Materials 8 (2019) 1801234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Mastrullo V, Cathery W, Velliou E, Madeddu P, Campagnolo P, Angiogenesis in Tissue Engineering: As Nature Intended?, Frontiers in Bioengineering and Biotechnology 8 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Lodola F, Rosti V, Tullii G, Desii A, Tapella L, Catarsi P, Lim D, Moccia F, Antognazza MR, Conjugated polymers optically regulate the fate of endothelial colony-forming cells, Science Advances, 5 eaav4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Nair RV, Farrukh A, del Campo A, Light-Regulated Angiogenesis via a Phototriggerable VEGF Peptidomimetic, Advanced Healthcare Materials 10 (2021) 2100488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Fedele C, De Gregorio M, Netti PA, Cavalli S, Attanasio C, Azopolymer photopatterning for directional control of angiogenesis, Acta Biomater. 63 (2017) 317–325. [DOI] [PubMed] [Google Scholar]
- [155].Moore EM, Maestas DR Jr., Comeau HY, Elisseeff JH, The Immune System and Its Contribution to Variability in Regenerative Medicine, Tissue Eng Part B Rev 27 (2021) 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Villarreal-Leal RA, Healey GD, Corradetti B, Biomimetic immunomodulation strategies for effective tissue repair and restoration, Adv. Drug Del. Rev 179 (2021) 113913. [DOI] [PubMed] [Google Scholar]
- [157].Kang H, Zhang K, Wong DSH, Han F, Li B, Bian L, Near-infrared light-controlled regulation of intracellular calcium to modulate macrophage polarization, Biomaterials 178 (2018) 681–696. [DOI] [PubMed] [Google Scholar]
- [158].Kang H, Zhang K, Pan Q, Lin S, Wong DSH, Li J, Lee W-Y-W, Yang B, Han F, Li G, Li B, Bian L, Remote Control of Intracellular Calcium Using Upconversion Nanotransducers Regulates Stem Cell Differentiation In Vivo, Adv. Funct. Mater 28 (2018) 1802642. [Google Scholar]
- [159].Zheng X, Xin L, Luo Y, Yang H, Ye X, Mao Z, Zhang S, Ma L, Gao C, Near-Infrared-Triggered Dynamic Surface Topography for Sequential Modulation of Macrophage Phenotypes, ACS Applied Materials & Interfaces 11 (2019) 43689–43697. [DOI] [PubMed] [Google Scholar]
- [160].Rath M, Müller I, Kropf P, Closs EI, Munder M, Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages, Front. Immunol 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Pfau MR, Grunlan MA, Smart scaffolds: shape memory polymers (SMPs) in tissue engineering, Journal of Materials Chemistry B 9 (2021) 4287–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Yakacki CM, Gall K, Shape-Memory Polymers for Biomedical Applications, in: Lendlein A (Ed.), Shape-Memory Polymers, Springer, Berlin Heidelberg, Berlin, Heidelberg, 2010, pp. 147–175. [Google Scholar]
- [163].Delaey J, Dubruel P, Van Vlierberghe S, Shape-Memory Polymers for Biomedical Applications, Adv. Funct. Mater 30 (2020) 1909047. [Google Scholar]
- [164].Leng J, Li S, Yang M, Hu J, Double-layer hydrogel with photoresponsive shape memory features for controllable catalysis, J. Mater. Res 34 (2019) 1795–1804. [Google Scholar]
- [165].Xie H, He M-J, Deng X-Y, Du L, Fan C-J, Yang K-K, Wang Y-Z, Design of Poly(l-lactide)–Poly(ethylene glycol) Copolymer with Light-Induced Shape-Memory Effect Triggered by Pendant Anthracene Groups, ACS Applied Materials & Interfaces 8 (2016) 9431–9439. [DOI] [PubMed] [Google Scholar]
- [166].Zhang X, Zhu C, Xu B, Qin L, Wei J, Yu Y, Rapid, Localized, and Athermal Shape Memory Performance Triggered by Photoswitchable Glass Transition Temperature, ACS Applied Materials & Interfaces 11 (2019) 46212–46218. [DOI] [PubMed] [Google Scholar]
- [167].Xie W, Yan F, Pakdel E, Sharp J, Liu D, Wang X, Zhan S, Sun L, Natural Melanin/Polyurethane Composites as Highly Efficient Near-Infrared-Photoresponsive Shape Memory Implants, ACS Biomaterials Science & Engineering 6 (2020) 5305–5314. [DOI] [PubMed] [Google Scholar]
- [168].Xie H,Shao J, Ma Y,Wang J, Huang H, Yang N, Wang H, Ruan C, Luo Y, Wang Q-Q, Chu PK, Yu X-F, Biodegradable near-infrared-photoresponsive shape memory implants based on black phosphorus nanofillers, Biomaterials 164 (2018) 11–21. [DOI] [PubMed] [Google Scholar]
- [169].Yang C, Zheng R, Younis MR, Shao J, Fu L-H, Zhang D-Y, Lin J, Li Z, Huang P, NIR-II light-responsive biodegradable shape memory composites based on cuprorivaite nanosheets for enhanced tissue reconstruction, Chem. Eng. J 419 (2021) 129437. [Google Scholar]
- [170].Han S, Hwang BW, Jeon EY, Jung D, Lee GH, Keum DH, Kim KS, Yun SH, Cha HJ, Hahn SK, Upconversion Nanoparticles/Hyaluronate–Rose Bengal Conjugate Complex for Noninvasive Photochemical Tissue Bonding, ACS Nano 11 (2017) 9979–9988. [DOI] [PubMed] [Google Scholar]
- [171].Ark M, Cosman PH, Boughton P, Dunstan CR, Review: Photochemical Tissue Bonding (PTB) methods for sutureless tissue adhesion, Int. J. Adhes. Adhes 71 (2016) 87–98. [Google Scholar]
- [172].Yao Z, Wang X, Zhang W, Liu Y, Ni T, Photochemical tissue bonding promotes the proliferation and migration of injured tenocytes through ROS/RhoA/NF-κB/Dynamin 2 signaling pathway, J. Cell. Physiol 233 (2018) 7047–7056. [DOI] [PubMed] [Google Scholar]
- [173].Pupkaite J, Ahumada M, McLaughlin S, Temkit M, Alaziz S, Seymour R, Ruel M, Kochevar I, Griffith M, Suuronen EJ, Alarcon EI, Collagen-Based Photoactive Agent for Tissue Bonding, ACS Applied Materials & Interfaces 9 (2017) 9265–9270. [DOI] [PubMed] [Google Scholar]
- [174].McTiernan CD, Cortes DC, Lazurko C, Amrani S, Rosales-Rojas R, Zuñiga-Bustos M, Sedlakova V, Poblete H, Stamplecoskie K, Suuronen EJ, Alarcon EI, Light-Activated Peptide-Based Materials for Sutureless Wound Closure, ACS Applied Materials & Interfaces 11 (2019) 45007–45015. [DOI] [PubMed] [Google Scholar]
- [175].Ruprai H, Romanazzo S, Ireland J, Kilian K, Mawad D, George L, Wuhrer R, Houang J, Ta D, Myers S, Lauto A, Porous Chitosan Films Support Stem Cells and Facilitate Sutureless Tissue Repair, ACS Applied Materials & Interfaces 11 (2019) 32613–32622. [DOI] [PubMed] [Google Scholar]
- [176].Ruprai H, Shanu A, Mawad D, Hook JM, Kilian K, George L, Wuhrer R, Houang J, Myers S, Lauto A, Porous chitosan adhesives with L-DOPA for enhanced photochemical tissue bonding, Acta Biomater. 101 (2020) 314–326. [DOI] [PubMed] [Google Scholar]
- [177].Hoang A-P, Ruprai H, Fidanovski K, Eslami M, Lauto A, Daniels J, Mawad D, Porous and sutureless bioelectronic patch with retained electronic properties under cyclic stretching, Applied Materials Today 15 (2019) 315–322. [Google Scholar]
- [178].Martín Giménez VM, Arya G, Zucchi IA, Galante MJ, Manucha W, Photo-responsive polymeric nanocarriers for target-specific and controlled drug delivery, Soft Matter 17 (2021) 8577–8584. [DOI] [PubMed] [Google Scholar]
- [179].Zhou Y, Ye H, Chen Y, Zhu R, Yin L, Photoresponsive Drug/Gene Delivery Systems, Biomacromolecules 19 (2018) 1840–1857. [DOI] [PubMed] [Google Scholar]
- [180].Khatun Z, Nurunnabi M, Nafiujjaman M, Reeck GR, Khan HA, Cho KJ, Lee Y-K, A hyaluronic acid nanogel for photo–chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin, Nanoscale 7 (2015) 10680–10689. [DOI] [PubMed] [Google Scholar]
- [181].Kaur G, Chang SLY, Bell TDM, Hearn MTW, Saito K, Bioinspired core-crosslinked micelles from thymine-functionalized amphiphilic block copolymers: Hydrogen bonding and photo-crosslinking study, Journal of Polymer Science Part A: Polymer Chemistry 49 (2011) 4121–4128. [Google Scholar]
- [182].Rapp TL, DeForest CA, Targeting drug delivery with light: A highly focused approach, Adv. Drug Del. Rev 171 (2021) 94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Wells LA, Lasowski F, Fitzpatrick SD, Sheardown H, Responding to Change: Thermo- and Photoresponsive Polymers as Unique, Biomaterials 38 (2010) 487–509. [DOI] [PubMed] [Google Scholar]
- [184].Tang Z, Gao H, Chen X, Zhang Y, Li A, Wang G, Advanced multifunctional composite phase change materials based on photo-responsive materials, Nano Energy 80 (2021) 105454. [Google Scholar]
- [185].Yin W, Ke W, Chen W, Xi L, Zhou Q, Mukerabigwi JF, Ge Z, Integrated block copolymer prodrug nanoparticles for combination of tumor oxidative stress amplification and ROS-responsive drug release, Biomaterials 195 (2019) 63–74. [DOI] [PubMed] [Google Scholar]
- [186].Saravanakumar G, Kim J, Kim WJ, Reactive-Oxygen-Species-Responsive Drug Delivery Systems: Promises and Challenges, Advanced Science 4 (2017) 1600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Lee SH, Gupta MK,Bang JB, Bae H, Sung H-J, Current Progress in Reactive Oxygen Species (ROS)-Responsive Materials for Biomedical Applications, Advanced Healthcare Materials 2 (2013) 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Zhou T, Zhou X, Xing D, Controlled release of doxorubicin from graphene oxide based charge-reversal nanocarrier, Biomaterials 35 (2014) 4185–4194. [DOI] [PubMed] [Google Scholar]
- [189].Zhang Y, Teh C, Li M, Ang CY, Tan SY, Qu Q, Korzh V, Zhao Y, Acid-Responsive Polymeric Doxorubicin Prodrug Nanoparticles Encapsulating a Near-Infrared Dye for Combined Photothermal-Chemotherapy, Chem. Mater 28 (2016) 7039–7050. [Google Scholar]
- [190].Xu C, Yan Y, Tan J, Yang D, Jia X, Wang L, Xu Y, Cao S, Sun S, Biodegradable Nanoparticles of Polyacrylic Acid-Stabilized Amorphous CaCO3 for Tunable pH-Responsive Drug Delivery and Enhanced Tumor Inhibition, Adv. Funct. Mater 29 (2019) 1808146. [Google Scholar]
- [191].Chi Y, Yin X, Sun K, Feng S, Liu J, Chen D, Guo C, Wu Z, Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models, Journal of Controlled Release 261 (2017) 113–125. [DOI] [PubMed] [Google Scholar]
- [192].Sun B, Luo C, Yu H, Zhang X, Chen Q, Yang W, Wang M, Kan Q, Zhang H, Wang Y, He Z, Sun J, Disulfide Bond-Driven Oxidation- and Reduction-Responsive Prodrug Nanoassemblies for Cancer Therapy, Nano Lett. 18 (2018) 3643–3650. [DOI] [PubMed] [Google Scholar]
- [193].Chen J, Ding J, Wang Y, Cheng J, Ji S, Zhuang X, Chen X, Sequentially Responsive Shell-Stacked Nanoparticles for Deep Penetration into Solid Tumors, Adv. Mater 29 (2017) 1701170. [DOI] [PubMed] [Google Scholar]
- [194].Li Y, Lu A, Long M, Cui L, Chen Z, Zhu L, Nitroimidazole derivative incorporated liposomes for hypoxia-triggered drug delivery and enhanced therapeutic efficacy in patient-derived tumor xenografts, Acta Biomater. 83 (2019) 334–348. [DOI] [PubMed] [Google Scholar]
- [195].Liu H, Xie Y, Zhang Y, Cai Y, Li B, Mao H, Liu Y, Lu J, Zhang L, Yu R, Development of a hypoxia-triggered and hypoxic radiosensitized liposome as a doxorubicin carrier to promote synergetic chemo-/radio-therapy for glioma, Biomaterials 121 (2017) 130–143. [DOI] [PubMed] [Google Scholar]
- [196].Ghani M, Heiskanen A, Kajtez J, Rezaei B, Larsen NB, Thomsen P, Kristensen A, Žukauskas A, Alm M, Emnéus J, On-Demand Reversible UV-Triggered Interpenetrating Polymer Network-Based Drug Delivery System Using the Spiropyran-Merocyanine Hydrophobicity Switch, ACS Applied Materials & Interfaces 13 (2021) 3591–3604. [DOI] [PubMed] [Google Scholar]
- [197].Wang X, Hu J, Liu G, Tian J, Wang H, Gong M, Liu S, Reversibly Switching Bilayer Permeability and Release Modules of Photochromic Polymersomes Stabilized by Cooperative Noncovalent Interactions, Journal of the American Chemical Society 137 (2015) 15262–15275. [DOI] [PubMed] [Google Scholar]
- [198].Banerjee A, Jariwala T, Baek Y-K, To DTH,Tai Y,Liu J, Park H, Myung NV, Nam J, Magneto- and opto-stimuli responsive nanofibers as a controlled drug delivery system, Nanotechnology 32 (2021) 505101. [DOI] [PubMed] [Google Scholar]
- [199].Cheng C-C, Gebeyehu BT, Huang S-Y, Abebe Alemayehu Y, Sun Y-T, Lai Y-C, Chang Y-H, Lai J-Y, Lee D-J, Entrapment of an adenine derivative by a photo-irradiated uracil-functionalized micelle confers controlled self-assembly behavior, Journal of Colloid and Interface Science 552 (2019) 166–178. [DOI] [PubMed] [Google Scholar]
- [200].Hou W, Liu R, Bi S, He Q, Wang H, Gu J, Photo-Responsive Polymersomes as Drug Delivery System for Potential Medical Applications, Molecules 25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Senthilkumar T, Zhou L, Gu Q, Liu L, Lv F, Wang S, Conjugated Polymer Nanoparticles with Appended Photo-Responsive Units for Controlled Drug Delivery, Release, and Imaging, Angew. Chem. Int. Ed 57 (2018) 13114–13119. [DOI] [PubMed] [Google Scholar]
- [202].Gupta MK, Balikov DA, Lee Y, Ko E, Yu C, Chun YW, Sawyer DB, Kim WS, Sung H-J, Gradient release of cardiac morphogens by photo-responsive polymer micelles for gradient-mediated variation of embryoid body differentiation, Journal of Materials Chemistry B 5 (2017) 5206–5217. [DOI] [PubMed] [Google Scholar]
- [203].Zhou Y, Chen R, Yang H, Bao C, Fan J, Wang C, Lin Q, Zhu L, Light-responsive polymersomes with a charge-switch for targeted drug delivery, Journal of Materials Chemistry B 8 (2020) 727–735. [DOI] [PubMed] [Google Scholar]
- [204].Ghosh S, Gul AR, Xu P, Lee SY, Rafique R, Kim YH, Park TJ, Target delivery of photo-triggered nanocarrier for externally activated chemo-photodynamic therapy of prostate cancer, Materials Today Chemistry 23 (2022) 100688. [Google Scholar]
- [205].Yu F, Zhu M, Li N, Ao M, Li Y, Zhong M, Yuan Q, Chen H, Fan Z, Wang Y, Hou Z, Qi Z, Shen Y, Chen XD, Imaging-guided synergistic targeting-promoted photo-chemotherapy against cancers by methotrexate-conjugated hyaluronic acid nanoparticles, Chem. Eng. J 380 (2020) 122426. [Google Scholar]
- [206].He Y, Guo S, Zhang Y, Liu Y, Ju H, Near-Infrared Photo-controlled Permeability of a Biomimetic Polymersome with Sustained Drug Release and Efficient Tumor Therapy, ACS Applied Materials & Interfaces 13 (2021) 14951–14963. [DOI] [PubMed] [Google Scholar]
- [207].Biswas G, Jena BC, Maiti S, Samanta P, Mandal M, Dhara D, Photoresponsive Block Copolymer Prodrug Nanoparticles as Delivery Vehicle for Single and Dual Anticancer Drugs, ACS Omega 2 (2017) 6677–6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Zheng K, Liu H, Liu X, Jiang L, Li L, Wu X, Guo N, Ding C, Huang M, Photo-triggered release of doxorubicin from liposomes formulated by amphiphilic phthalocyanines for combination therapy to enhance antitumor efficacy, Journal of Materials Chemistry B 8 (2020) 8022–8036. [DOI] [PubMed] [Google Scholar]
- [209].Shi J, Su Y, Liu W, Chang J, Zhang Z, A nanoliposome-based photoactivable drug delivery system for enhanced cancer therapy and overcoming treatment resistance, Int J Nanomedicine 12 (2017) 8257–8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Ilhami FB, Peng K-C, Chang Y-S, Alemayehu YA, Tsai H-C, Lai J-Y, Chiao Y-H, Kao C-Y, Cheng C-C, Photo-Responsive Supramolecular Micelles for Controlled Drug Release and Improved Chemotherapy, Int. J. Mol. Sci 22(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Bai Y, Liu CP, Song X, Zhuo L, Bu H, Tian W, Photo- and pH- Dual-Responsive β-Cyclodextrin-Based Supramolecular Prodrug Complex Self-Assemblies for Programmed Drug Delivery, Chemistry – An Asian Journal 13 (2018) 3903–3911. [DOI] [PubMed] [Google Scholar]
- [212].Chen S, Bian Q, Wang P, Zheng X, Lv L, Dang Z, Wang G, Photo, pH and redox multi-responsive nanogels for drug delivery and fluorescence cell imaging, Polymer Chemistry 8 (2017) 6150–6157. [Google Scholar]
- [213].Liu C, Ewert KK, Wang N, Li Y, Safinya CR, Qiao W, A multifunctional lipid that forms contrast-agent liposomes with dual-control release capabilities for precise MRI-guided drug delivery, Biomaterials 221 (2019) 119412. [DOI] [PubMed] [Google Scholar]
- [214].Bhatta A, Krishnamoorthy G, Marimuthu N, Dihingia A, Manna P, Biswal HT, Das M, Krishnamoorthy G, Chlorin e6 decorated doxorubicin encapsulated chitosan nanoparticles for photo-controlled cancer drug delivery, Int. J. Biol. Macromol 136 (2019) 951–961. [DOI] [PubMed] [Google Scholar]
- [215].Guo B, Fan R, Shen S, Xue Y, Zhu Z, Xu RX, A photo-responsive membrane for tailored drug delivery with spatially and temporally controlled release, Journal of Materials Chemistry B 9 (2021) 8615–8625. [DOI] [PubMed] [Google Scholar]
- [216].Xu Y, Zhang X, Hu G, Wu X, Nie Y, Wu H, Kong D, Ning X, Multistage targeted “Photoactive neutrophil” for enhancing synergistic photo-chemotherapy, Biomaterials 279 (2021) 121224. [DOI] [PubMed] [Google Scholar]
- [217].Xu C, Li H, Zhang K, Binzel DW, Yin H, Chiu W, Guo P, Photo-controlled release of paclitaxel and model drugs from RNA pyramids, Nano Research 12 (2019) 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Yang L, Sun H, Liu Y, Hou W, Yang Y, Cai R, Cui C, Zhang P, Pan X, Li X, Li L, Sumerlin BS, Tan W, Self-Assembled Aptamer-Grafted Hyperbranched Polymer Nanocarrier for Targeted and Photoresponsive Drug Delivery, Angew. Chem. Int. Ed 57 (2018) 17048–17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Guo Q, Liu J, Yang H, Lei Z, Synthesis of Photo, Oxidative, and Reductive Triple-Stimuli-Responsive Block Copolymer Micelles as Nanocarriers for Controlled Release, Langmuir (2021). [DOI] [PubMed] [Google Scholar]
- [220].Rafique R, Gul AR, Lee IG, Baek SH, Kailasa SK, Iqbal N, Cho EJ, Lee M, Park TJ, Photo-induced reactions for disassembling of coloaded photosensitizer and drug molecules from upconversion-mesoporous silica nanoparticles: An effective synergistic cancer therapy, Materials Science and Engineering: C 110 (2020) 110545. [DOI] [PubMed] [Google Scholar]
- [221].Fan J, Zhang Z, Wang Y, Lin S, Yang S, Photo-responsive degradable hollow mesoporous organosilica nanoplatforms for drug delivery, Journal of Nanobiotechnology 18 (2020) 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Beňová E, Zeleňák V, Halamová D, Almáši M, Petrul’ová V, Psotka M, Zeleňáková A, Bačkor M, Hornebecq V, A drug delivery system based on switchable photo-controlled p-coumaric acid derivatives anchored on mesoporous silica, Journal of Materials Chemistry B 5 (2017) 817–825. [DOI] [PubMed] [Google Scholar]
- [223].Liu Q, Wang H, Li G, Liu M, Ding J, Huang X, Gao W, Huayue W, A photocleavable low molecular weight hydrogel for light-triggered drug delivery, Chin. Chem. Lett 30 (2019) 485–488. [Google Scholar]
- [224].Rastogi SK, Anderson HE, Lamas J, Barret S, Cantu T, Zauscher S, Brittain WJ, Betancourt T, Enhanced Release of Molecules upon Ultraviolet (UV) Light Irradiation from Photoresponsive Hydrogels Prepared from Bifunctional Azobenzene and Four-Arm Poly(ethylene glycol), ACS Applied Materials & Interfaces 10 (2018) 30071–30080. [DOI] [PubMed] [Google Scholar]
- [225].Amer M, Ni X, Xian M, Chen RK, Photo-Responsive Hydrogel Microneedles With Interlocking Control for Easy Extraction in Sustained Ocular Drug Delivery, Journal of Engineering and Science in Medical Diagnostics and Therapy 5 (2021). [Google Scholar]
- [226].Li Y, Liao X, Zheng B, Studies on local anesthetic lidocaine hydrochloride delivery via photo-triggered implantable polymeric microneedles as a patient-controlled transdermal analgesia system, J. Biomater. Sci. Polym. Ed 1–19 (2021). [DOI] [PubMed] [Google Scholar]
- [227].Hu J-J, Jiang W, Yuan L, Duan C, Yuan Q, Long Z, Lou X, Xia F, Recent advances in stimuli-responsive theranostic systems with aggregation-induced emission characteristics, Aggregate 2 (2021) 48–65. [Google Scholar]
- [228].Dang MN, Gomez Casas C, Day ES, Photoresponsive miR-34a/Nanoshell Conjugates Enable Light-Triggered Gene Regulation to Impair the Function of Triple-Negative Breast Cancer Cells, Nano Lett. 21 (2021) 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Lin X, Wu M, Li M, Cai Z, Sun H, Tan X, Li J, Zeng Y, Liu X, Liu J, Photo-responsive hollow silica nanoparticles for light-triggered genetic and photodynamic synergistic therapy, Acta Biomater. 76 (2018) 178–192. [DOI] [PubMed] [Google Scholar]
- [230].Epps TH, Vi T, Sullivan MO, Design and development of a robust photo-responsive block copolymer framework for tunable nucleic acid delivery and efficient gene silencing, Polym. J 50 (2018) 711–723. [Google Scholar]
- [231].Au - Greco CT, Au - Epps IIITH, Au - Sullivan MO, Predicting Gene Silencing Through the Spatiotemporal Control of siRNA Release from Photo-responsive Polymeric Nanocarriers, JoVE, (2017) e55803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Duan S, Cao D, Li X, Zhu H, Lan M, Tan Z, Song Z, Zhu R, Yin L, Chen Y, Topology-assisted, photo-strengthened DNA/siRNA delivery mediated by branched poly(β-amino ester)s via synchronized intracellular kinetics, Biomaterials Science 8 (2020) 290–301. [DOI] [PubMed] [Google Scholar]
- [233].Li J, Hao Y, Pan H, Zhang Y, Cheng G, Liu B,Chang J, Wang H, CRISPR-dcas9 Optogenetic Nanosystem for the Blue Light-Mediated Treatment of Neovascular Lesions, ACS Applied Bio Materials 4 (2021) 2502–2513. [DOI] [PubMed] [Google Scholar]
- [234].Wang R, Wang X, Li J, Di L, Zhou J, Ding Y, Lipoprotein-biomimetic nanostructure enables tumor-targeted penetration delivery for enhanced photo-gene therapy towards glioma, Bioactive Materials (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Zhang Y, Ren K, Zhang X, Chao Z, Yang Y, Ye D, Dai Z, Liu Y, Ju H, Photo-tearable tape close-wrapped upconversion nanocapsules for near-infrared modulated efficient siRNA delivery and therapy, Biomaterials 163 (2018) 55–66. [DOI] [PubMed] [Google Scholar]
- [236].Wu M, Li J, Lin X, Wei Z, Zhang D, Zhao B, Liu X, Liu J, Reduction/photo dual-responsive polymeric prodrug nanoparticles for programmed siRNA and doxorubicin delivery, Biomaterials Science 6 (2018) 1457–1468. [DOI] [PubMed] [Google Scholar]
- [237].Lin G, Zhang Y, Zhang L, Wang J, Tian Y, Cai W, Tang S, Chu C, Zhou J, Mi P, Chen X, Liu G, Metal-organic frameworks nanoswitch: Toward photo-controllable endo/lysosomal rupture and release for enhanced cancer RNA interference, Nano Research 13 (2020) 238–245. [Google Scholar]
- [238].Gong X, Li R, Wang J, Wei J, Ma K, Liu X, Wang F, A Smart Theranostic Nanocapsule for Spatiotemporally Programmable Photo-Gene Therapy, Angew. Chem. Int. Ed 59 (2020) 21648–21655. [DOI] [PubMed] [Google Scholar]
- [239].Liu Y, Bhattarai P, Dai Z, Chen X, Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer, Chem. Soc. Rev 48 (2019) 2053–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Wang Q, Dai Y, Xu J, Cai J, Niu X, Zhang L, Chen R, Shen Q, Huang W, Fan Q, All-in-One Phototheranostics: Single Laser Triggers NIR-II Fluorescence/Photoacoustic Imaging Guided Photothermal/Photodynamic/Chemo Combination Therapy, Adv. Funct. Mater 29 (2019) 1901480. [Google Scholar]
- [241].Zheng D, Yu P, Wei Z, Zhong C, Wu M, Liu X, RBC Membrane Camouflaged Semiconducting Polymer Nanoparticles for Near-Infrared Photoacoustic Imaging and Photothermal Therapy, Nano-Micro Letters 12 (2020) 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Wei Z, Xin F, Zhang J, Wu M, Qiu T, Lan Y, Qiao S, Liu X, Liu J, Donor–acceptor conjugated polymer-based nanoparticles for highly effective photoacoustic imaging and photothermal therapy in the NIR-II window, Chem. Commun 56 (2020) 1093–1096. [DOI] [PubMed] [Google Scholar]
- [243].Cao Z, Feng L, Zhang G, Wang J, Shen S, Li D, Yang X, Semiconducting polymer-based nanoparticles with strong absorbance in NIR-II window for in vivo photothermal therapy and photoacoustic imaging, Biomaterials 155 (2018) 103–111. [DOI] [PubMed] [Google Scholar]
- [244].Liu G, Zhu J, Guo H, Sun A, Chen P, Xi L, Huang W, Song X, Dong X, Mo2C-Derived Polyoxometalate for NIR-II Photoacoustic Imaging-Guided Chemodynamic/Photothermal Synergistic Therapy, Angew. Chem. Int. Ed 58 (2019) 18641–18646. [DOI] [PubMed] [Google Scholar]
- [245].Yang H, Liu R, Xu Y, Qian L, Dai Z, Photosensitizer Nanoparticles Boost Photodynamic Therapy for Pancreatic Cancer Treatment, Nano-Micro Letters 13 (2021) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Shao W, Wei Q, Wang S, Li F, Wu J, Ren J, Cao F, Liao H, Gao J, Zhou M, Ling D, Molecular engineering of D–A–D conjugated small molecule nanoparticles for high performance NIR-II photothermal therapy, Materials Horizons 7 (2020) 1379–1386. [Google Scholar]
- [247].Wu X, Suo Y, Shi H, Liu R, Wu F, Wang T, Ma L, Liu H, Cheng Z, Deep-Tissue Photothermal Therapy Using Laser Illumination at NIR-IIa Window, Nano-Micro Letters 12 (2020) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Zhang D, Xu H, Zhang X, Liu Y, Wu M, Li J, Yang H, Liu G, Liu X, Liu J, Yuan Z, Self-Quenched Metal-Organic Particles as Dual-Mode Therapeutic Agents for Photoacoustic Imaging-Guided Second Near-Infrared Window Photochemotherapy, ACS Applied Materials & Interfaces 10 (2018) 25203–25212. [DOI] [PubMed] [Google Scholar]
- [249].Zhang W, Cai K, Li X, Zhang J, Ma Z, Foda MF, Mu Y, Dai X, Han H, Au Hollow Nanorods-Chimeric Peptide Nanocarrier for NIR-II Photothermal Therapy and Real-time Apoptosis Imaging for Tumor Theranostics, Theranostics 9 (2019) 4971–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Vijayaraghavan P, Liu C-H, Vankayala R, Chiang C-S, Hwang KC, Designing Multi-Branched Gold Nanoechinus for NIR Light Activated Dual Modal Photodynamic and Photothermal Therapy in the Second Biological Window, Adv. Mater 26 (2014) 6689–6695. [DOI] [PubMed] [Google Scholar]
- [251].Lin X, Wang X,Li J, Cai L, Liao F, Wu M, Zheng D, Zeng Y, Zhang Z, Liu X,Wang J, Yao C, Localized NIR-II photo-immunotherapy through the combination of photothermal ablation and in situ generated interleukin-12 cytokine for efficiently eliminating primary and abscopal tumors, Nanoscale 13 (2021) 1745–1758. [DOI] [PubMed] [Google Scholar]
- [252].Wang Y, Li S, Tian Z, Sun J, Liang S, Zhang B, Bai L, Zhang Y, Zhou X, Xiao S, Zhang Q, Zhang L, Zhang C, Zhou D, Generation of a caged lentiviral vector through an unnatural amino acid for photo-switchable transduction, Nucleic Acids Res. 47 (2019) e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Hörner M, Jerez-Longres C, Hudek A, Hook S, Yousefi OS, Schamel Wolfgang WA, Hörner C, Zurbriggen Matias D, Ye H, Wagner Hanna J, Weber W, Spatiotemporally confined red light-controlled gene delivery at single-cell resolution using adeno-associated viral vectors, Science Advances, 7 eabf0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Holt LJ, Herring C, Jespers LS, Woolven BP, Tomlinson IM, Domain antibodies: proteins for therapy, Trends Biotechnol. 21 (2003) 484–490. [DOI] [PubMed] [Google Scholar]
- [255].Selkoe DJ, Alzheimer’s Disease: Genes, Proteins, and Therapy, Physiol. Rev 81 (2001) 741–766. [DOI] [PubMed] [Google Scholar]
- [256].Keppler D, Multidrug Resistance Proteins (MRPs, ABCCs): Importance for Pathophysiology and Drug Therapy, in: Fromm MF, Kim RB (Eds.), Drug Transporters, Springer, Berlin Heidelberg, Berlin, Heidelberg, 2011, pp. 299–323. [DOI] [PubMed] [Google Scholar]
- [257].Shepard HM, Phillips GL, Thanos CD, Feldmann M, Developments in therapy with monoclonal antibodies and related proteins, Clin. Med. (Northfield Il.) 17 (2017) 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].He C, Tang Z, Tian H, Chen X, Co-delivery of chemotherapeutics and proteins for synergistic therapy, Adv. Drug Del. Rev 98 (2016) 64–76. [DOI] [PubMed] [Google Scholar]
- [259].Ballesteros CAS, Correa DS, Zucolotto V, Polycaprolactone nanofiber mats decorated with photoresponsive nanogels and silver nanoparticles: Slow release for antibacterial control, Materials Science and Engineering: C 107 (2020)110334. [DOI] [PubMed] [Google Scholar]
- [260].Ballesteros CAS, Bernardi JC, Correa DS, Zucolotto V, Controlled Release of Silver Nanoparticles Contained in Photoresponsive Nanogels, ACS Applied Bio Materials 2 (2019) 644–653. [DOI] [PubMed] [Google Scholar]
- [261].Wang Y, Li S, Zhang P, Bai H, Feng L, Lv F, Liu L, Wang S, Photothermal-Responsive Conjugated Polymer Nanoparticles for Remote Control of Gene Expression in Living Cells, Adv. Mater 30 (2018) 1705418. [DOI] [PubMed] [Google Scholar]
- [262].Wang R, Yang Z, Luo J, Hsing IM, Sun F, B12-dependent photoresponsive protein hydrogels for controlled stem cell/protein release, Proceedings of the National Academy of Sciences 114 (2017) 5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Wan G, Cheng Y, Song J, Chen Q, Chen B, Liu Y, Ji S, Chen H, Wang Y, Nucleus-targeting near-infrared nanoparticles based on TAT peptide-conjugated IR780 for photo-chemotherapy of breast cancer, Chem. Eng. J 380 (2020) 122458. [Google Scholar]
- [264].Wang Y, Liu C-H, Ji T, Mehta M, Wang W, Marino E, Chen J, Kohane DS, Intravenous treatment of choroidal neovascularization by photo-targeted nanoparticles, Nature Communications 10 (2019) 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Peng J, Yang Q, Xiao Y, Shi K, Liu Q, Hao Y, Yang F, Han R, Qian Z, Tumor Microenvironment Responsive Drug-Dye-Peptide Nanoassembly for Enhanced Tumor-Targeting, Penetration, and Photo-Chemo-Immunotherapy, Adv. Funct. Mater 29 (2019) 1900004. [Google Scholar]
- [266].Fujimoto K, Ichikawa M, Nakamura S, Photoinduced aggregation of liposome modified with DNA containing ultrafast DNA photo-cross-linker, J. Chem. Technol. Biotechnol 97 (2022) 295–298. [Google Scholar]
- [267].Dai L,Lu J, Kong F, Liu K, Wei H, Si C, Reversible photo-controlled release of bovine serum albumin by azobenzene-containing cellulose nanofibrils-based hydrogel, Advanced Composites and Hybrid Materials 2 (2019) 462–470. [Google Scholar]
- [268].Yang HY, Meng Du J, Jang M-S, Mo XW, Sun XS, Lee DS, Lee JH, Fu Y, CD44-Targeted and Enzyme-Responsive Photo-Cross-Linked Nanogels with Enhanced Stability for In Vivo Protein Delivery, Biomacromolecules 22 (2021) 3590–3600. [DOI] [PubMed] [Google Scholar]
- [269].Lim C, Kang JK, Won WR, Park JY, Han SM, Le TN, Kim JC,Her J, Shin Y, Oh KT, Co-delivery of D-(KLAKLAK)2 Peptide and Chlorin e6 using a Liposomal Complex for Synergistic Cancer Therapy, Pharmaceutics 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Kozaki I, Shimizu K, Honda H, Effective modification of cell death-inducing intracellular peptides by means of a photo-cleavable peptide array-based screening system, Journal of Bioscience and Bioengineering 124 (2017) 209–214. [DOI] [PubMed] [Google Scholar]
- [271].Dong W, Wen J, Li Y, Wang C, Sun S, Shang D, Targeted antimicrobial peptide delivery in vivo to tumor with near infrared photoactivated mesoporous silica nanoparticles, Int. J. Pharm 588 (2020) 119767. [DOI] [PubMed] [Google Scholar]
- [272].Zhang H, Zhang Z, Zhang H, Chen C, Zhang D, Zhao Y, Protein-Based Hybrid Responsive Microparticles for Wound Healing, ACS Applied Materials & Interfaces 13 (2021) 18413–18422. [DOI] [PubMed] [Google Scholar]
- [273].Narayan OP, Mu X, Hasturk O, Kaplan DL, Dynamically tunable light responsive silk-elastin-like proteins, Acta Biomater. 121 (2021) 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J, Photodynamic therapy of cancer: An update, CA Cancer J. Clin 61 (2011) 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Sánchez-Ramírez DR, Domínguez-Ríos R, Juárez J, Valdés M, Hassan N, Quintero-Ramos A, del Toro-Arreola A, Barbosa S, Taboada P, Topete A, Daneri-Navarro A, Biodegradable photoresponsive nanoparticles for chemo-, photothermal- and photodynamic therapy of ovarian cancer, Materials Science and Engineering: C 116 (2020) 111196. [DOI] [PubMed] [Google Scholar]
- [276].Pan J, Ouyang A, Fang W, Cheng G, Liu W, Wang F, Zhao D, Le K, Jiang J, cis-Silicon phthalocyanine conformation endows J-aggregated nanosphere with unique near-infrared absorbance and fluorescence enhancement: a tumor sensitive phototheranostic agent with deep tissue penetrating ability, Journal of Materials Chemistry B 8 (2020) 2895–2908. [DOI] [PubMed] [Google Scholar]
- [277].Liang X, Chen M, Bhattarai P, Hameed S, Dai Z, Perfluorocarbon@Porphyrin Nanoparticles for Tumor Hypoxia Relief to Enhance Photodynamic Therapy against Liver Metastasis of Colon Cancer, ACS Nano 14 (2020) 13569–13583. [DOI] [PubMed] [Google Scholar]
- [278].Deng X, Shao Z, Zhao Y, Solutions to the Drawbacks of Photothermal and Photodynamic Cancer Therapy, Advanced Science 8 (2021) 2002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Gunaydin G, Gedik ME, Ayan S, Photodynamic Therapy—Current Limitations and Novel Approaches, Frontiers in Chemistry 9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Xu H-L, ZhuGe D-L, Chen P-P, Tong M-Q, Lin M-T, Jiang X, Zheng Y-W, Chen B, Li X-K, Zhao Y-Z, Silk fibroin nanoparticles dyeing indocyanine green for imaging-guided photo-thermal therapy of glioblastoma, Drug Deliv. 25 (2018) 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Sweeney EE,Cano-Mejia J, Fernandes R, Photothermal Therapy Generates a Thermal Window of Immunogenic Cell Death in Neuroblastoma, Small 14 (2018) 1800678. [DOI] [PubMed] [Google Scholar]
- [282].Hao M, Kong C, Jiang C, Hou R, Zhao X, Li J, Wang Y, Gao Y, Zhang H, Yang B, Jiang J, Polydopamine-coated Au-Ag nanoparticle-guided photothermal colorectal cancer therapy through multiple cell death pathways, Acta Biomater. 83 (2019) 414–424. [DOI] [PubMed] [Google Scholar]
- [283].Chen Q, Liu C, Liu C, Zhong D, Hua S, He J, Wang K, Zhou M, Wrapping Porphyromonas gingivalis for tumor microenvironment immunomodulation and photothermal immunotherapy, Nano Today 41 (2021) 101311. [Google Scholar]
- [284].Zhi D, Yang T, O’Hagan J, Zhang S, Donnelly RF, Photothermal therapy, Journal of Controlled Release 325 (2020) 52–71. [DOI] [PubMed] [Google Scholar]
- [285].Fernandes N, Rodrigues CF, Moreira AF, Correia IJ, Overview of the application of inorganic nanomaterials in cancer photothermal therapy, Biomaterials, Science 8 (2020) 2990–3020. [DOI] [PubMed] [Google Scholar]
- [286].Ko S, Park JY, Oh Y-K, A Microbial Siderophore-Inspired Self-Gelling Hydrogel for Noninvasive Anticancer Phototherapy, Cancer Res. 79 (2019) 6178. [DOI] [PubMed] [Google Scholar]
- [287].Tang L, Sun X, Liu N, Zhou Z, Yu F, Zhang X, Sun X, Chen X, Radiolabeled Angiogenesis-Targeting Croconaine Nanoparticles for Trimodality Imaging Guided Photothermal Therapy of Glioma, ACS Applied Nano Materials 1 (2018)1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Chen Q, Hu Q, Dukhovlinova E, Chen G, Ahn S, Wang C, Ogunnaike EA, Ligler FS, Dotti G, Gu Z, Photothermal Therapy Promotes Tumor Infiltration and Antitumor Activity of CAR T Cells, Adv. Mater 31 (2019) 1900192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Miller IC, Zamat A, Sun L-K, Phuengkham H, Harris AM, Gamboa L, Yang J, Murad JP, Priceman SJ, Kwong GA, Enhanced intratumoural activity of CAR T cells engineered to produce immunomodulators under photothermal control, Nature, Biomedical Engineering 5 (2021) 1348–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Ma W, Zhu D, Li J, Chen X, Xie W, Jiang X, Wu L, Wang G, Xiao Y, Liu Z, Wang F, Li A, Shao D, Dong W, Liu W, Yuan Y, Coating biomimetic nanoparticles with chimeric antigen receptor T cell-membrane provides high specificity for hepatocellular carcinoma photothermal therapy treatment, Theranostics 10 (2020) 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Han Y, Pan H, Li W, Chen Z, Ma A, Yin T, Liang R, Chen F, Ma Y, Jin Y, Zheng M, Li B, Cai L, T Cell Membrane Mimicking Nanoparticles with Bioorthogonal Targeting and Immune Recognition for Enhanced Photothermal Therapy, Advanced Science 6 (2019) 1900251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Jiang Q, Luo Z, Men Y, Yang P, Peng H, Guo R, Tian Y, Pang Z, Yang W, Red blood cell membrane-camouflaged melanin nanoparticles for enhanced photothermal therapy, Biomaterials 143 (2017) 29–45. [DOI] [PubMed] [Google Scholar]
- [293].Zhou Z, Yan Y, Wang L, Zhang Q, Cheng Y, Melanin-like nanoparticles decorated with an autophagy-inducing peptide for efficient targeted photothermal therapy, Biomaterials 203 (2019) 63–72. [DOI] [PubMed] [Google Scholar]
- [294].Han D, Li Y, Liu X, Li B, Han Y, Zheng Y, Yeung KWK, Li C, Cui Z, Liang Y, Li Z, Zhu S, Wang X, Wu S, Rapid bacteria trapping and killing of metal-organic frameworks strengthened photo-responsive hydrogel for rapid tissue repair of bacterial infected wounds, Chemical Engineering Journal 396 (2020) 125194. [Google Scholar]
- [295].Zou J, Li L, Yang Z, Chen X, Phototherapy meets immunotherapy: a win–win strategy to fight against cancer, Nanophotonics 10 (2021) 3229–3245. [Google Scholar]
- [296].Luo D, Carter KA, Miranda D, Lovell JF, Chemophototherapy: An Emerging Treatment Option for Solid Tumors, Advanced Science 4 (2017) 1600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Chung H, Dai T, Sharma SK, Huang Y-Y, Carroll JD, Hamblin MR, The Nuts and Bolts of Low-level Laser (Light) Therapy, Ann. Biomed. Eng 40 (2012) 516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Glenner GG, Wong CW, Alzheimer’s disease and Down’s syndrome: Sharing of a unique cerebrovascular amyloid fibril protein, Biochemical and Biophysical Research Communications 122 (1984) 1131–1135. [DOI] [PubMed] [Google Scholar]
- [299].Lee BI, Chung YJ, Park CB, Photosensitizing materials and platforms for light-triggered modulation of Alzheimer’s β-amyloid self-assembly, Biomaterials 190–191 (2019) 121–132. [DOI] [PubMed] [Google Scholar]
- [300].Liu W, Dong X, Liu Y, Sun Y, Photoresponsive materials for intensified modulation of Alzheimer’s amyloid-β protein aggregation: A review, Acta Biomater. 123 (2021) 93–109. [DOI] [PubMed] [Google Scholar]
- [301].Li C, Wang J, Liu L, Alzheimer’s Therapeutic Strategy: Photoactive Platforms for Suppressing the Aggregation of Amyloid β Protein, Frontiers in Chemistry 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Lee BI, Suh YS, Chung YJ, Yu K, Park CB, Shedding Light on Alzheimer’s β-Amyloidosis: Photosensitized Methylene Blue Inhibits Self-Assembly of β-Amyloid Peptides and Disintegrates Their Aggregates, Sci. Rep 7 (2017) 7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Wang X, Wang C, Chan H-N, Ashok I, Krishnamoorthi SK, Li M, Li H-W, Wong MS, Amyloid-β oligomer targeted theranostic probes for in vivo NIR imaging and inhibition of self-aggregation and amyloid-β induced ROS generation, Talanta 224 (2021) 121830. [DOI] [PubMed] [Google Scholar]
- [304].Wang H, Jin Y, Tan Y, Zhu H, Huo W, Niu P, Li Z, Zhang J, Liang X-J, Yang X, Photo-responsive hydrogel facilitates nutrition deprivation by an ambidextrous approach for preventing cancer recurrence and metastasis, Biomaterials 275 (2021) 120992. [DOI] [PubMed] [Google Scholar]
- [305].Zhao S, Zhang L, Deng L, Ouyang J, Xu Q, Gao X, Zeng Z, Liu Y-N, NIR-II Responsive Hydrogel as an Angiogenesis Inhibition Agent for Tumor Microenvironment Reprogramming, Small 17 (2021) 2103003. [DOI] [PubMed] [Google Scholar]
- [306].Liu J, Chen H, Ma L, He Z, Wang D, Liu Y, Lin Q, Zhang T, Gray N, Kaniskan HÜ, Jin J, Wei W, Light-induced control of protein destruction by opto-PROTAC, Science Advances, 6 eaay5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Ouyang J, Tang Z, Farokhzad N, Kong N, Kim NY, Feng C, Blake S, Xiao Y, Liu C, Xie T, Tao W, Ultrasound mediated therapy: Recent progress and challenges in nanoscience, Nano Today 35 (2020) 100949. [Google Scholar]
- [308].Sitti M, Wiersma DS, Pros and Cons: Magnetic versus Optical Microrobots, Adv. Mater 32 (2020) 1906766. [DOI] [PubMed] [Google Scholar]
- [309].Pearson S, Feng J, del Campo A, Lighting the Path: Light Delivery Strategies to Activate Photoresponsive Biomaterials In Vivo, Adv. Funct. Mater 31 (2021) 2105989. [Google Scholar]
- [310].Wang J, Dong J, Optical Waveguides and Integrated Optical Devices for Medical Diagnosis, Health Monitoring and Light Therapies, Sensors 20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].McKendry JJD, Gu E, McAlinden N, Laurand N, Mathieson K, Dawson MD, Chapter Two - Micro-LEDs for biomedical applications, in: Jiang H, Lin J (Eds.) Semiconductors and Semimetals, Elsevier; 2021, pp. 57–94. [Google Scholar]
- [312].Tian C, Zhang J, Gu J, Li W, Cao Y, Light controlled biomaterials for regulating cell migration and differentiation, Smart Materials in Medicine 3 (2022) 209–216. [Google Scholar]
- [313].Wang H, Gao L, Fan T, Zhang C, Zhang B, Al-Hartomy OA, Al-Ghamdi A, Wageh S, Qiu M, Zhang H, Strategic Design of Intelligent-Responsive Nanogel Carriers for Cancer Therapy, ACS Applied Materials & Interfaces 13 (2021) 54621–54647. [DOI] [PubMed] [Google Scholar]
- [314].Bril M, Fredrich S, Kurniawan NA, Stimuli-responsive materials: A smart way to study dynamic cell responses, Smart Materials in Medicine 3 (2022) 257–273. [Google Scholar]
- [315].Hwang HH, Zhu W, Victorine G, Lawrence N, Chen S, 3D-Printing of Functional Biomedical Microdevices via Light- and Extrusion-Based Approaches, Small Methods 2 (2018) 1700277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Yu C, Schimelman J, Wang P, Miller KL, Ma X, You S, Guan J, Sun B, Zhu W, Chen S, Photopolymerizable Biomaterials and Light-Based 3D Printing Strategies for Biomedical Applications, Chem. Rev 120 (2020) 10695–10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Nocentini S, Martella D, Parmeggiani C, Wiersma DS, 3D Printed Photoresponsive Materials for Photonics, Advanced Optical Materials 7 (2019) 1900156. [Google Scholar]
- [318].Zhao Z, Tian X, Song X, Engineering materials with light: recent progress in digital light processing based 3D printing, Journal of Materials Chemistry C 8 (2020) 13896–13917. [Google Scholar]
- [319].Taylor JM, Luan H, Lewis JA, Rogers JA, Nuzzo RG, Braun PV, Biomimetic and Biologically Compliant Soft Architectures via 3D and 4D Assembly Methods: A Perspective, Adv. Mater 34 (2022) 2108391. [DOI] [PubMed] [Google Scholar]
- [320].Wan Z, Zhang P, Liu Y, Lv L, Zhou Y, Four-dimensional bioprinting: Current developments and applications in bone tissue engineering, Acta Biomater. 101 (2020) 26–42. [DOI] [PubMed] [Google Scholar]
- [321].Arif ZU, Khalid MY, Zolfagharian A, Bodaghi M, 4D bioprinting of smart polymers for biomedical applications: recent progress, challenges, and future perspectives, React. Funct. Polym 179 (2022) 105374. [Google Scholar]
- [322].Sonatkar J, Kandasubramanian B, Ismail SO, 4D printing: Pragmatic progression in biofabrication, Eur. Polym. J 169 (2022) 111128. [Google Scholar]
- [323].Arif ZU, Khalid MY, Ahmed W, Arshad H, A review on four-dimensional (4D) bioprinting in pursuit of advanced tissue engineering applications, Bioprinting 27 (2022) e00203. [Google Scholar]
- [324].Levato R, Lim KS, Li W, Asua AU, Peña LB, Wang M, Falandt M, Bernal PN, Gawlitta D, Zhang YS, Woodfield TBF, Malda J, High-resolution lithographic biofabrication of hydrogels with complex microchannels from low-temperature-soluble gelatin bioresins, Materials Today Bio 12 (2021) 100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Guimarães CF, Ahmed R, Marques AP, Reis RL, Demirci U, Engineering Hydrogel-Based Biomedical Photonics: Design, Fabrication, and Applications, Adv. Mater 33 (2021) 2006582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].ter Schiphorst J, Saez J, Diamond D, Benito-Lopez F, Schenning APHJ, Light-responsive polymers for microfluidic applications, Lab on a Chip 18 (2018) 699–709. [DOI] [PubMed] [Google Scholar]
- [327].Delaney C, McCluskey P, Coleman S, Whyte J, Kent N, Diamond D, Precision control of flow rate in microfluidic channels using photoresponsive soft polymer actuators, Lab on a Chip 17 (2017) 2013–2021. [DOI] [PubMed] [Google Scholar]
- [328].Choi E, Jeong HH, Qiu T, Fischer P, Palagi S, Soft Continuous Surface for Micromanipulation driven by Light-controlled Hydrogels, 2019 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS), 2019, pp. 1–6. [Google Scholar]
- [329].Chen L-J, Li Q, Soft Photoactuators in Microfluidics, Photoactive Functional Soft Materials (2019) 167–196. [Google Scholar]
- [330].Pilz da Cunha M, Debije MG, Schenning APHJ, Bioinspired light-driven soft robots based on liquid crystal polymers, Chem. Soc. Rev 49 (2020) 6568–6578. [DOI] [PubMed] [Google Scholar]
- [331].Shahsavan H, Aghakhani A, Zeng H, Guo Y, Davidson ZS, Priimagi A, Sitti M, Bioinspired underwater locomotion of light-driven liquid crystal gels, Proceedings of the National Academy of Sciences 117 (2020) 5125–5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Ahn C, Liang X, Cai S, Bioinspired Design of Light-Powered Crawling, Squeezing, and Jumping Untethered Soft Robot, Advanced Materials Technologies 4 (2019) 1900185. [Google Scholar]
- [333].Jacques SL, Optical properties of biological tissues: a review, Physics in Medicine and Biology 58 (2013) R37–R61. [DOI] [PubMed] [Google Scholar]
- [334].Choi C-E, Chakraborty A, Coyle A, Shamiya Y, Paul A, Contact-Free Remote Manipulation of Hydrogel Properties Using Light-Triggerable Nanoparticles: A Materials Science Perspective for Biomedical Applications, n/a 2102088. [DOI] [PubMed] [Google Scholar]
- [335].Cao J, Zhu B, Zheng K, He S, Meng L, Song J, Yang H, Recent Progress in NIR-II Contrast Agent for Biological Imaging 7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


