ABSTRACT
Sulfate- and sulfite-reducing bacteria (SRB) are a group of strict anaerobes found within the human gut. Bilophila wadsworthia, a sulfite-reducing bacterium which produces hydrogen sulfide (H2S) from taurine and isethionate respiration, is a common member of the healthy commensal human gut microbiota but has been implicated in several disease states including inflammatory bowel disease and colorectal cancer. Bacteroides thetaiotaomicron, one of the most prominent gut bacteria, has sulfatases which release sulfate, serving as a potential substrate for sulfate-reducing bacteria. Here, we showed that when B. thetaiotaomicron and B. wadsworthia were in co-culture, there was a significant increase in B. thetaiotaomicron’s growth and in H2S production by B. wadsworthia. Differential gene expression analysis revealed increased expression of B. wadsworthia’s dsrMKJOP complex in co-culture, which delivers electrons for sulfite reduction to H2S. This was accompanied by a decreased expression of genes associated with taurine, sulfolactate, and thiosulfate respiration, indicating that B. thetaiotaomicron may provide an alternative source of sulfite to B. wadsworthia. We hypothesized adenosine 5’-phosphosulfate (APS) to be this intermediate. Indeed, B. wadsworthia was able to grow using APS or sulfite as electron acceptors. Endometabolomic and transcriptomic analyses revealed decreased production of indole by B. thetaiotaomicron in co-culture with B. wadsworthia due to enhanced tryptophan utilization by B. wadsworthia. The results of this microbe−microbe interaction could have significant pro-inflammatory effects in the human gut environment.
KEYWORDS: Sulfite-reducing bacteria, hydrogen sulfide, adenosine 5’-phosphosulfate, indole
Introduction
Sulfate- and sulfite-reducing bacteria (SRB) comprise a group of strict anaerobes and are found within the colonic mucosa as part of the commensal gut microbiota in at least 50% of humans1,2. SRB utilize inorganic sulfate (SO42–) or sulfite (SO32–) as a terminal electron acceptor during energy metabolism, a process which occurs concomitantly with oxidation of molecular hydrogen or organic compounds. Hydrogen sulfide (H2S) is the final product of dissimilatory sulfate reduction by SRB3 and can freely diffuse across cell membranes.4 H2S is recognized as a hazardous product as it is both corrosive and toxic to many organs even at low concentrations.5 Classical sulfate-reducing bacteria reduce sulfate to sulfite via sulfate adenylyltransferase (Sat) and adenylylsulfate reductase (AprAB)6 (Figure S1). Sulfite then enters the dissimilatory sulfite reduction pathway. Bilophila wadsworthia is a Gram-negative member of the SRB, first identified in 1989 from gangrenous and perforated appendicitis samples.7 Unlike other SRB, B. wadsworthia cannot utilize sulfate,7–9 and instead degrades organosulfate compounds such as taurine, isethionate, and sulfoquinovase, generating H2S as a by-product.10,11 In contrast, the majority of living organisms have the assimilatory sulfate reduction pathway.12 This involves the reduction of sulfate to APS via sulfate adenylyltransferase (Sat), which is then converted to phosphoadenosine phosphosulfate (PAPS) via adenylylsulfate kinase/APS kinase. PAPS is then reduced to sulfite by phosphoadenosine phosphosulfate (PAPS) reductase, and the sulfite is then converted to H2S by assimilatory sulfite reductase12 (Figure S1).
B. wadsworthia is present in the feces of 50–60% of healthy individuals,13 and can also be isolated from buccal and vaginal samples.14 B. wadsworthia is considered to be virulent, as it is the third most common anaerobic isolate from appendicitis samples and appears to be clinically important in a variety of anaerobic infections.15 Furthermore, it exhibits endotoxic activity,16 and is adherent to human embryonic intestinal cells in vitro.17 Association studies in humans have linked B. wadsworthia enrichment in the gut with many diseases including colorectal cancer,18 multiple sclerosis,19 Parkinson’s disease,20 dementia,21 nonalcoholic steatohepatitis,22 intrahepatic cholestasis in pregnancy,23 diabetic kidney disease,24 and schizophrenia.25 Additionally, it was demonstrated that a human stool-derived B. wadsworthia strain was able to induce systemic inflammation in specific-pathogen-free mice.26 Given B. wadsworthia‘s status as a potential pathobiont in the human gut, it is important to investigate the factors that influence its abundance and function.
Bacteroides thetaiotaomicron is a Gram-negative obligate anaerobe within the Bacteroidaceae family.27 Originally isolated from the feces of a healthy adult,27 B. thetaiotaomicron is highly abundant constituting 1–6% of the total bacteria28 with a 46% prevalence29 and is a key commensal member of the human gut microbiota. B. thetaiotaomicron plays a major role in the human gut, including modulation of the host mucosal immune system,30 and utilization of a wide range of polysaccharides.31 Some of these polysaccharides can be host-derived and include glycosaminoglycans such as chondroitin sulfate, mucin, hyaluronate, and heparan sulfate.32 The degradation of polysaccharides yields simple sugars for fermentation, resulting in the production of beneficial short-chain organic acids including acetate, lactate, succinate, and propionate33; B. thetaiotaomicron has also been shown to support the growth of butyrate-producing Anaerostipes caccae in vitro.34 Short-chain fatty acids (SCFAs) are a valuable source of energy for human colonocytes and aid in maintaining healthy barrier function.35 B. thetaiotaomicron encodes 28 sulfatases which can cleave sulfated residues of the host glycosaminoglycans to yield free sulfate in the mouse gut.36 By increasing the availability of free sulfate in vivo, B. thetaiotaomicron sulfatase activity could permit increased sulfate reduction and H2S production by SRB.37 Indeed, B. thetaiotaomicron and Desulfovibrio piger, a SRB, can co-colonize the gut, with B. thetaiotaomicron providing free sulfate to D. piger and promoting H2S production both in vitro and in vivo2. Additionally, B. thetaiotaomicron generates hydrogen during polysaccharide fermentation and molecular hydrogen, which is used by hydrogenotrophs, including SRB, as an electron donor.38,39 Given these established interactions with other SRBs, we aimed to further investigate the dynamics between B. thetaiotaomicron and B. wadsworthia. Here, we used transcriptomics and metabolomics to explore the mechanisms underpinning the interaction of B. wadsworthia and B. thetaiotaomicron during anaerobic co-culture.
Results and discussion
Co-culture of B. wadsworthia and B. thetaiotaomicron boosts H2S production
B. wadsworthia (QI0013) and three B. thetaiotaomicron strains (QI0072, DSM 108160 and DSM 108161) were grown anaerobically in monocultures and co-cultures on synthetic (BPM, see methods) media supplemented with 10 mM taurine at a 1:1 ratio (106 colony forming units (CFU)/mL inoculation density). At 8 hours post-inoculation, a significant increase in H2S concentration was observed when B. wadsworthia was in pairwise co-culture with the B. thetaiotaomicron strains compared to B. wadsworthia monoculture (Figure 1(a)) while B. wadsworthia abundance remained unaffected by the co-cultures (Figure 1(b)). The elevated H2S levels were attributed to a significantly increased H2S production per B. wadsworthia cell when in co-culture with each of the three B. thetaiotaomicron strains (Figure 1(c)). Interestingly, the interaction also appeared to benefit B. thetaiotaomicron, as all three strains grew to significantly higher abundance when in co-culture with B. wadsworthia (Figure 1(d)).
Figure 1.
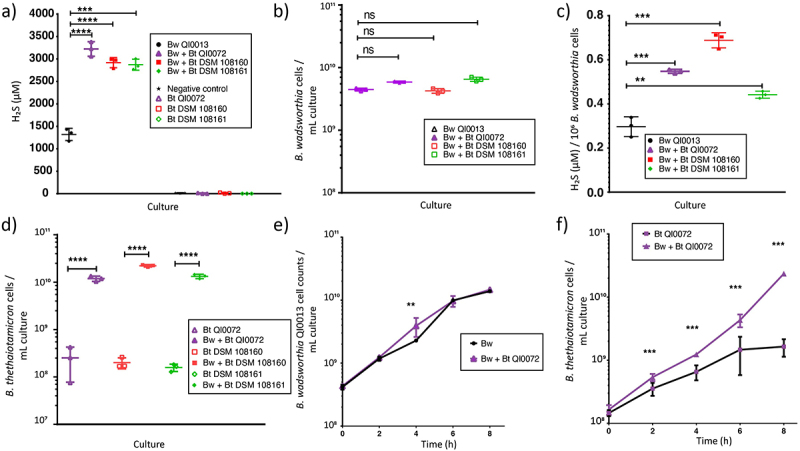
Co-culture of B. wadsworthia (Bw) QI0013 and B. thetaiotaomicron (Bt). a) H2S concentrations (µM) at 8 h of B. wadsworthia in monoculture or in co-culture with B. thetaiotaomicron QI0072, DSM 108160, and DSM 108161. b) Cell counts of B. wadsworthia at 8 h post-inoculation measured via qPCR. c) H2S concentration (µM) per 106 B. wadsworthia cells at 8 h. d) Cell counts of B. thetaiotaomicron at 8 h post-inoculation measured via qPCR. Each point represents a culture replicate (n = 7). In the negative control bacterial cells were not added. e-f) the cell numbers were tracked over time for the co-culture of B wadsworthia QI0013 and B. thetaiotaomicron QI0072. Horizontal lines represent average, and error bars represent SD. Results of unpaired t-tests are shown where **= p ≤ 0.01, ***= p ≤ 0.001, ****= p ≤ 0.0001.
To gain further insights into the potential mechanism that increases H2S production in B. wadsworthia, we selected B. thetaiotaomicron strain QI0072 (Figure 1(e-f)) and used transcriptomic and metabolomic analysis of the mono and co-cultures at 8 h to investigate their activity. The most impacted pathways are discussed below.
Respiration of sulfite via hydrogen or lactate by B. wadsworthia was increased in co-culture
In the co-culture, four genes associated with sulfur metabolism leading to H2S production in B. wadsworthia were overexpressed (Figure 2(a), Table S1). These genes are part of the dissimilatory sulfite reductase protein complex (dsrMKJOP), including dsrM (WCP94_001671), dsrK (WCP94_001670), dsrO (WCP94_001668), and dsrP (WCP94_001667) (Table S1). When B. wadsworthia respires taurine, sulfite (SO32-) generated enters the dissimilatory sulfite reduction pathway and is further reduced by DsrAB to produce H2S (Figure S1). Expression of dsrMKJOP can be increased under H2-rich growth conditions in D. vulgaris40; the observed up-regulation in co-culture may suggest a higher bioavailability of H2 to B. wadsworthia. B. thetaiotaomicron can generate hydrogen (H2) during polysaccharide fermentation41; therefore, B. wadsworthia could utilize this directly as an electron donor during dissimilatory sulfite reduction.38,42 The increased expression of dsrMKOP in co-culture alludes to sulfite utilization as an electron acceptor, potentially leading to increased H2S production.
Figure 2.
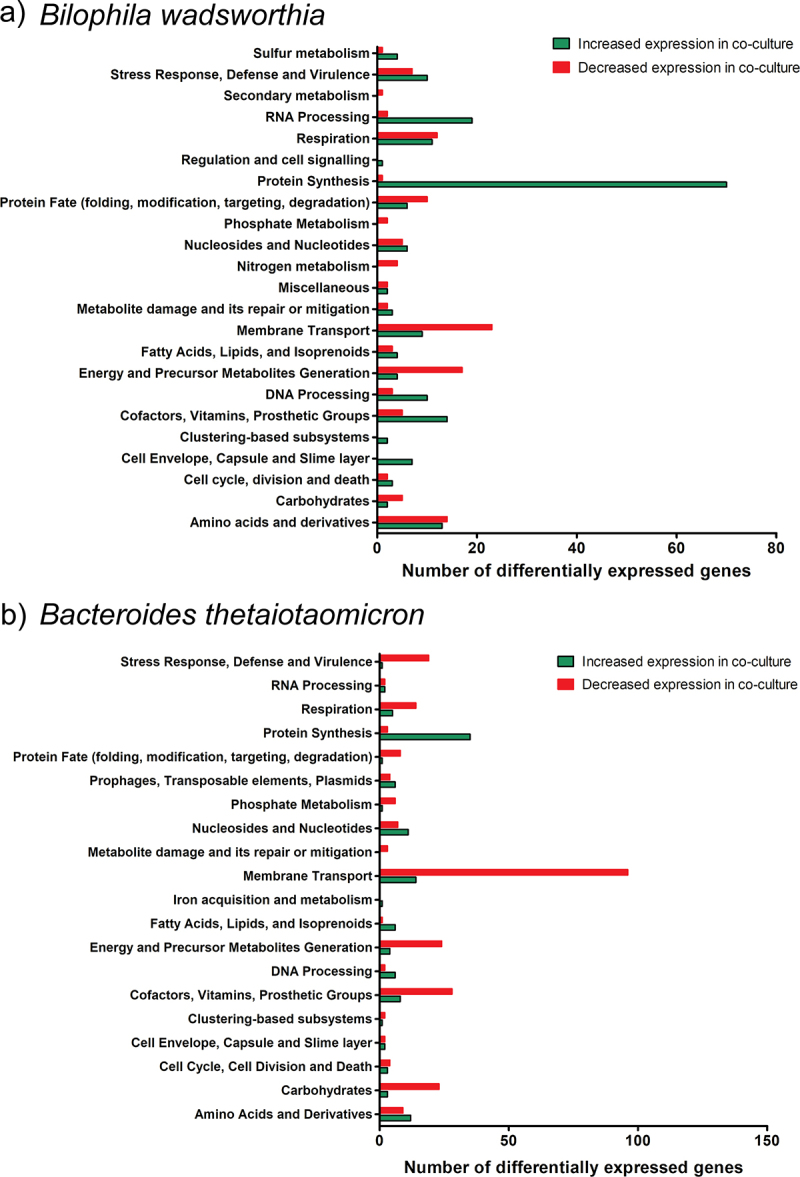
Differentially expressed genes (DEGs) of B. wadsworthia and B. thetaiotaomicron in co-culture versus monoculture. a) DEGs in B. wadsworthia. b) DEGs in B. thetaiotaomicron. Bar charts show numbers of DEGs increased (green) and decreased (red) in expression in co-culture relative to the respective monoculture in each functional gene class as annotated in BV-BRC.
In addition to hydrogen, lactate can be utilized as an electron donor for the respiration of sulfite10,43,44 (Figure 3). Interestingly, two genes encoding lactate permeases were overexpressed in co-culture (WCP94_002306, WCP94_000357) in B. wadsworthia (Table S1), suggesting enhanced utilization of lactate. The presence of different electron donors might contribute to the increased expression of dsrMKJOP in B. wadsworthia under co-culture conditions, leading to a higher H2Sproduction as a by-product. Taken together, the transcriptomic data revealed an increased capacity for uptake and utilization of hydrogen and lactate in B. wadsworthia in co-culture with B. thetaiotaomicron; both compounds are key electron donors for dissimilatory sulfite reduction. This is in line with increased H2S concentration by B. wadsworthia in co-culture (Figure 1(a)).
Figure 3.
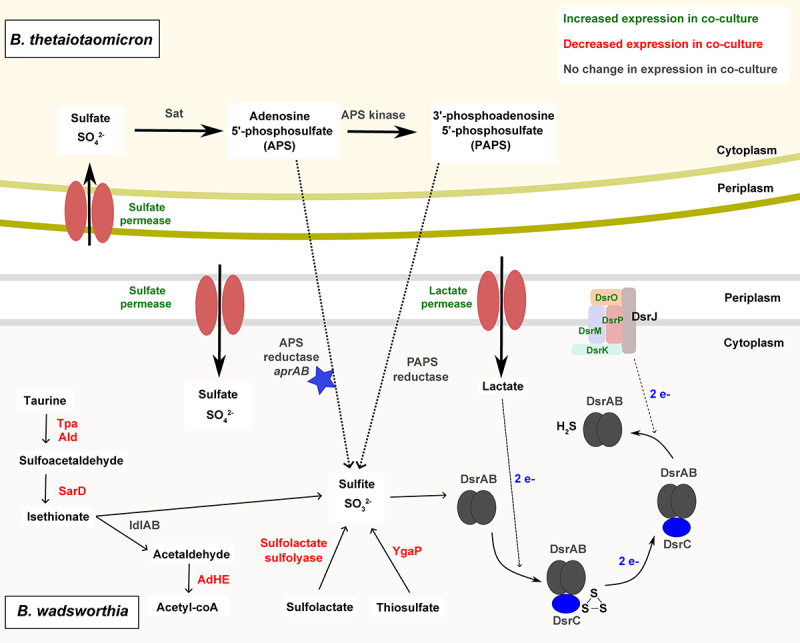
Model of cross-feeding interaction between B. wadsworthia and B. thetaiotaomicron. Enzymes are shown in colors corresponding to transcriptomic data: green showed increased gene expression in co-culture; red showed decreased expression in co-culture; gray showed no change in expression between co-culture and monoculture. The blue star represents a putative enzyme where functionality is unproven. The dotted line represents a putative cross-feeding mechanism. Dashed lines represent electron transfer.
Surprisingly, the gene cluster associated with taurine metabolism exhibited reduced expression in the co-culture, despite the observed increase in H2S concentration. This gene cluster includes taurine pyruvate dehydrogenase (tpa, WCP94_00949), alanine dehydrogenase (ald, WCP94_00950) and sulfoacetaldehyde reductase (sarD, WCP94_00947)10 (Figure S1, Figure 3, Table S1). It has been shown that expression of these enzymes is elevated in B. wadsworthia cells grown in taurine, but not in isethionate-grown cells.10 Isethionate sulfite-lyase islA and isethionate sulfite-lyase activating protein islB were not among the differentially expressed genes, but acetaldehyde dehydrogenase (adhE, WCP94_001941), which encodes an enzyme able to convert acetaldehyde (a product of isethionate degradation) to acetyl coA was decreased in expression in co-culture (Table S1). The downregulation of tpa and sarD, combined with the increased expression of dsrMKJOP suggests that B. wadsworthia can also utilize the respiration pathway from sulfite or a precursor molecule in the presence of B. thetaiotaomicron (Figure 3). Sulfite could be produced through different metabolic pathways in B. wadsworthia: i) from sulfolactate, via the activity of a sulfo-lyase (WCP94_000771-772) which converts sulfolactate to sulfite and pyruvate; this enzyme showed decreased expression in co-culture; or ii) through the inner membrane protein YgaP (WCP94_002882), a rhodanese-domain-containing protein that generates sulfite from thiosulfate, and which also showed decreased expression in co-culture (Table S1). Therefore, it is likely that in co-culture with B. thetaiotaomicron, B. wadsworthia utilizes an alternative source of sulfite (Figure 3).
Modulation of sulfur metabolism in B. wadsworthia and B. thetaiotaomicron co-culture
As taurine utilization by B. wadsworthia as a sulfite source in co-culture appeared to be decreased, alongside an increased expression of genes associated with the dissimilatory sulfite reductase pathway and elevated H2S concentration, we investigated other possible sources of sulfite for B. wadsworthia in the co-culture. Increased expression of sulfate permease (WCP94_001830) and sulfatase (WCP94_001848) genes was observed in B. wadsworthia in co-culture (Table S1), suggesting an increased bioavailability of sulfate; however, whether these transporters are promiscuous for other compounds such as sulfite is unknown.
Given that B. wadsworthia cannot utilize sulfate, it was previously assumed that the genes for sulfate utilization were absent from the genome.7,8,45 The B. wadsworthia QI0013 genome does not contain genes encoding the Sat enzyme necessary for sulfate reduction. However, our analysis revealed that B. wadsworthia QI0013 encodes two gene clusters of the alpha and beta subunits of the adenylylsulfate reductase (WCP94_000309-310 and WCP94_000741-742) (Table S2, Figure S1). Despite low amino acid sequence similarity to AprAB proteins from Desulfovibrio strains (Figure S2, Table S3), we identified conserved protein domains, including FAD-dependent oxidoreductase 2 and succinate dehydrogenase/fumarate reductase flavoprotein, supporting the annotation of AprAB (Table S4). Furthermore, we found that the putative AprAB genes were widely distributed in publicly available B. wadsworthia genomes (Table S5). This suggests a potential role for B. wadsworthia in APS reduction via adenylylsulfate reductase. The putative proteins encoded by these genes could facilitate the conversion of APS to sulfite, offering an alternative precursor molecule for the dissimilatory sulfite reduction pathway. Interestingly, the B. thetaiotaomicron QI0072 genome encodes some enzymes involved in assimilatory sulfite reduction, including sulfate adenylyltransferase (Sat) (QI0072_1554, QI0072_1555) and adenylylsulfate kinase/APS kinase (QI0072_1556) (Table S2), which would catalyze the conversion of sulfate to both APS and PAPS. However, B. thetaiotaomicron QI0072 does not encode the enzymes for further utilization of PAPS and APS. B. wadsworthia QI0013 expresses both phosphoadenosine phosphosulfate (PAPS) reductase (WCP94_00194), which reduces PAPS to sulfite, in addition to the putative AprAB which reduces APS to sulfite (Table S2).
We hypothesize that B. thetaiotaomicron QI0072 produces sulfate from sulfated saccharides via sulfatases,46 an activity which has been previously described to support the growth of D. piger via cross-feeding, and then reduces the sulfate to APS and/or PAPS. In B. thetaiotaomicron, genes encoding sulfate adenylyltransferase subunits 1 and 2 and adenylylsulfate kinase were consistently expressed at similar levels in both co-culture and monoculture (Table S2), suggesting the production of APS and PAPS. B. thetaiotaomicron-derived APS and PAPS could then be utilized directly by B. wadsworthia, where either AprAB or PAPS reductase yield sulfite, which then enters the dissimilatory sulfate reduction pathway (Figure 3). In this way, B. thetaiotaomicron may provide B. wadsworthia with an alternative source of sulfite, which may be energetically favorable over taurine degradation, or utilized when taurine or other organosulfur compounds are depleted. Indeed, we observed a decreased expression of genes associated with sulfite generation via taurine, thiosulfate, and sulfolactate under co-culture conditions, with the phenotypic observation of increased H2S concentration (Figure 1). The adenylylsulfate reductase and PAPS reductase genes were expressed in B. wadsworthia in both co-culture and monoculture, without significant differences in expression levels (Table S2).
Next, we investigated if B. wadsworthia would grow using sulfite or APS as electron acceptors instead of taurine. B. wadsworthia was able to show a significantly higher growth in media supplemented with 4 mM sulfite, 10 mM APS, or 10 mM taurine, compared to only anaerobe basal broth (ABB) (Figure 4).
Figure 4.
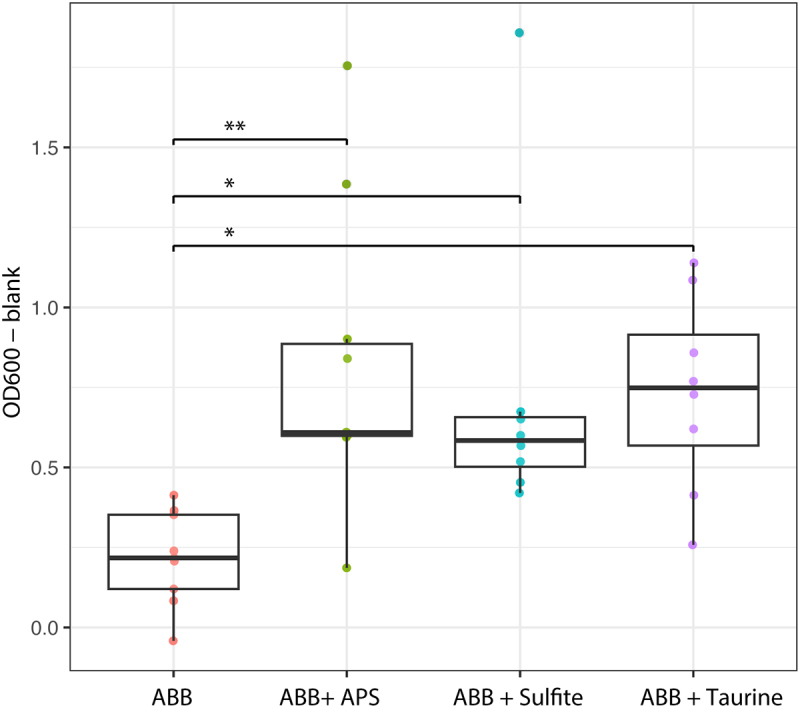
Growth (OD600) of B. wadsworthia in ABB media supplemented with 10 mM adenosine 5’-phosphosulfate (APS), 4 mM sulfite or 10 mM taurine. Growth was compared to B. wadsworthia grown in ABB media. Each box plot represents the median and interquartile range of the distribution of seven culture replicate. Results of mixed linear model analyses are shown where *= p ≤ 0.01, **= p ≤ 0.001.
Next, we tested the capacity of B. thetaiotaomicron to promote the sulfidogenic activity of other B. wadsworthia strains i.e B. wadsworthia QI0012, QI0014, and QI0015 strains. Co-culture with B. thetaiotaomicron resulted in significantly increased H2S concentration in all B. wadsworthia strains tested compared to their respective monocultures (Figure 5(a)), suggesting that the sulfidogenic potential of B. thetaiotaomicron is not strain specific. Indeed, QI0014 did not produce detectable H2S in monoculture at 8 h, whereas 195.3 ± 18.60 µM was produced in co-culture with B. thetaiotaomicron (Figure 5(a)). Interestingly, qPCR analysis of B. wadsworthia cell abundance revealed differences between strains with QI0012 and QI0013 showing slight increases in abundance in co-culture with B. thetaiotaomicron although this effect was not significant with QI0013 (Figure 5(b)), as shown previously for this strain (Figure 1). A decreased abundance was observed in co-culture of B. wadsworthia QI0014 and QI0015 strains with B. thetaiotaomicron (Figure 5(b)). Upon standardizing H₂S concentrations relative to B. wadsworthia cell numbers, it was observed that B. thetaiotaomicron consistently enhanced H₂S production across the various B. wadsworthia strains. Nonetheless, the specific mechanisms behind this increase may differ among strains (Figure 5(d)).
Figure 5.
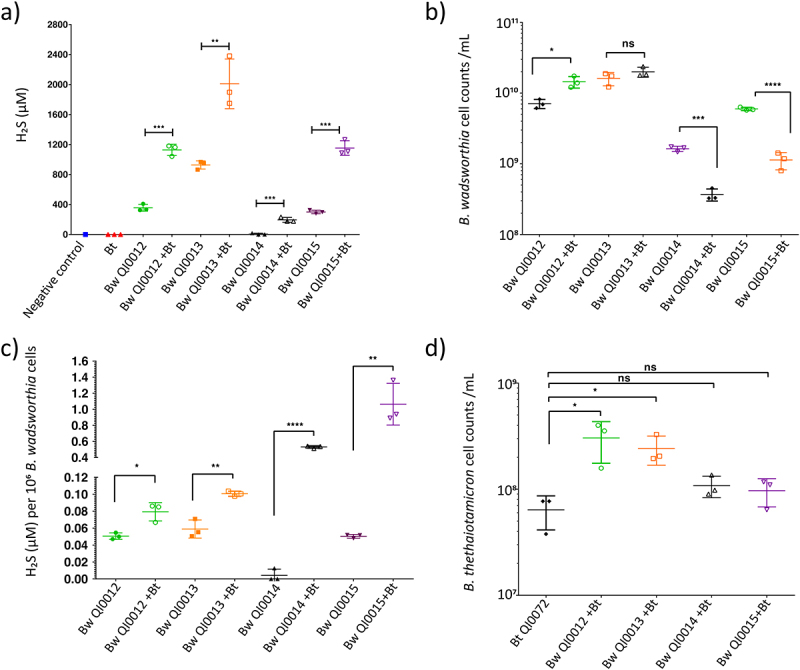
Pairwise co-culture of B. thetaiotaomicron (Bt) QI0072 with four B. wadsworthia (Bw) strains (QI0012, QI0013, QI0014, QI0015). a) H2S concentrations (µM) at 8 h. b) qPCR-determined B. wadsworthia cell counts at 8 h. c) H2S concentration (µM) per 106 B. wadsworthia cells. d) qPCR-determined B. thetaiotaomicron cell counts at 8 h. Each point represents a culture replicate (n = 3). Horizontal lines represent average, and error bars represent SD. Statistical significance between culture conditions was established using one-way analysis of variance (ANOVA) with Tukey’s multiple comparison tests with a significance level set at α = 0.05. Results show *= p ≤ 0.05, **= p ≤ 0.01, ***= p ≤ 0.001, ****= p ≤ 0.0001, ns = not significant (p > 0.05).
The increased H₂S levels in QI0012 and QI0013 co-cultures are attributable to a combination of higher B. wadsworthia abundance and elevated H₂S production per cell. In contrast, the rise in H₂S observed with QI0014 and QI0015 is predominantly due to a substantial increase in H₂S production per B. wadsworthia cell (Figure 5(c)). Additionally, B. thetaiotaomicron exhibited a slight increase in abundance in all co-cultures compared to its monoculture (Figure 5(d)). Overall, B. thetaiotaomicron showed an ability to increase H2S production in all four B. wadsworthia strains that were tested.
H2S-utilizing amino acid biosynthetic pathways were reduced in expression in B. thetaiotaomicron
Based on our genome analyses, B. thetaiotaomicron QI0072 does not encode known genes for H2S production, but it does have the capacity to utilize H2S during the biosynthesis of amino acids including cysteine and homocysteine. B. thetaiotaomicron QI0072 over-expressed 12 genes and under-expressed nine genes in co-culture associated with amino acid metabolism, compared to monoculture (Figure 2(b)). Interestingly, this strain under-expressed O-acetylserine sulfhydrolase (QI0072_3844) in co-culture (Table S6); this enzyme catalyses the conversion of O3-acetyl-L-serine and H2S to L-cysteine and acetate, utilizing pyridoxal-5’-phosphate (vitamin B6) as a co-factor.47 This is relevant as this indicates that although B. thetaiotaomicron can utilize B. wadsworthia-derived H2S, the gene encoding this enzyme is heavily decreased in expression in co-culture with B. wadsworthia. Similarly, two genes involved in H2S-utilizing homocysteine biosynthesis were also differentially expressed; one gene encoding O-acetylhomoserine sulfhydrylase (EC 2.5.1.49) was notably decreased in expression in co-culture (QI0072_3143, −5.12 logFC), whereas another gene encoding this enzyme was increased in expression in co-culture (QI0072_2658, 1.24 logFC) (Table S6). This enzyme catalyses the conversion of O-acylhomoserine and H2S to homocysteine.48,49 Overall, decreased homocysteine and cysteine biosynthesis by B. thetaiotaomicron in co-culture with B. wadsworthia results in lower H2S utilization by B. thetaiotaomicron.
Alterations to the endometabolome in B. wadsworthia and B. thetaiotaomicron co-culture
The endometabolome of the B. wadsworthia and B. thetaiotaomicron co-culture was analyzed at 8 hours post-inoculation, alongside monocultures. The B. thetaiotaomicron monoculture metabolite abundance clustered distinctly from the B. wadsworthia monoculture and co-culture conditions based on partial least squares discriminant analysis (PLS-DA) (Figure S3a). The heatmap displaying the top 50 differentially abundant metabolites showed clear differences between the conditions, most notably when the B. thetaiotaomicron monoculture is compared to the co-culture conditions (Figure S3b). The variable importance in projection (VIP) scores showed that the top 15 compounds contributing to differences between conditions were relatively high in abundance in the B. thetaiotaomicron monoculture and in relatively low abundance in the co-culture (Figure 6(a)). This suggests that these compounds are produced by B. thetaiotaomicron and either not produced by B. thetaiotaomicron in co-culture with B. wadsworthia or consumed by B. wadsworthia. The highest-scoring compound was Ala-Val, followed by the ester phenethyl acetate, sphinganine and nicotinic compound 1-Methylnicotinamide (Figure 6(a)). Kynurenic acid also had a significantly lower abundance in co-culture compared to B. thetaiotaomicron monoculture (Figure 6(a,b)), suggesting a reduction in tryptophan metabolism via this pathway. Kynurenic acid is a product of tryptophan degradation in mammals,50 however, Escherichia coli has been demonstrated to produce kynurenic acid from L-kynurenine in the rat small intestine51 and the human gut microbiota may also produce this compound, since kynurenic acid concentration is high in the distal colon relative to other body sites.52 Sphinganine can be produced by Bacteroides strains in the gut and has the potential to control the levels of bioactive lipids in the liver,53 underscoring its potential role in host−microbial interactions.
Figure 6.
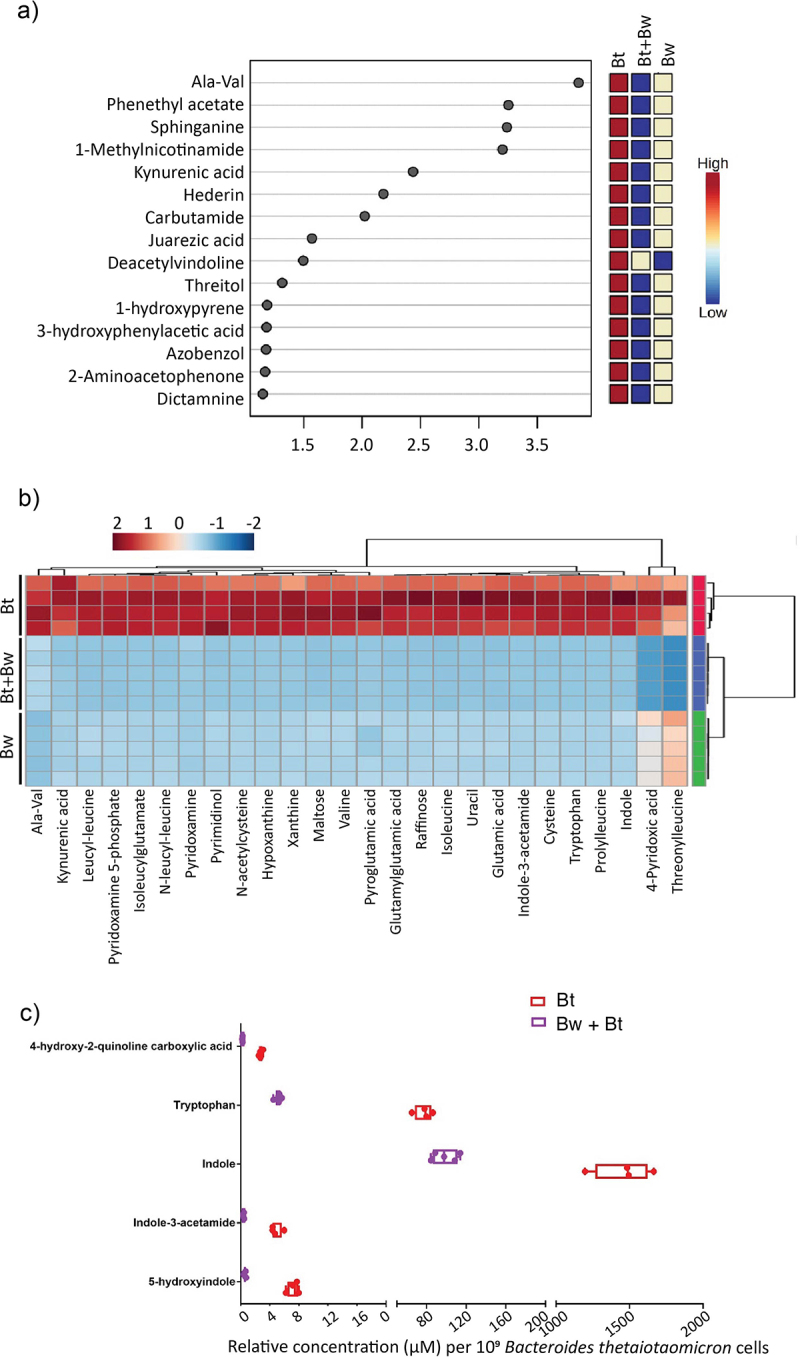
Comparisons of the endometabolome of B. wadsworthia and B. thetaiotaomicron in co-culture (Bw + Bt) with monocultures (Bw, Bt). a) the top compounds ranked based on the variable importance in projection (VIP) scores. The colored boxes on the right indicate the relative concentrations of the corresponding metabolite in each group. b) specific metabolites of interest manually curated from the compound list detected via untargeted LC-MS. c) relative intracellular concentrations of compounds related to tryptophan metabolism in B. thetaiotaomicron (Bt) in monoculture and co-culture with B. wadsworthia (Bw + Bt). Concentration is standardized to B. thetaiotaomicron cell counts. Each point represents a culture replicate. Box and whisker plots show line at mean, box at 25th −75th percentile, whiskers to minimum and maximum.
The transcriptomic data identified branched-chain amino acids (BCAAs) as significant metabolites of interest, showcasing differential expression in Liv system genes responsible for ABC transporters specific to BCAAs in B. wadsworthia (Figure 2(a), supplementary Table S1). This may signify a modified bioavailability of leucine, isoleucine, and valine for B. wadsworthia in the co-culture environment. Indeed, valine and isoleucine were detected in the endometabolome in all culture conditions, in addition to dipeptides of leucine including threonylleucine, prolylleucine, N-leucyl-leucine and leucyl-leucine (Figure 6(b)). In all cases, relative abundance was high in B. thetaiotaomicron monoculture, lower in B. wadsworthia monoculture and the lowest abundance was in the co-culture condition (Figure 6(b)). Taken together, this suggests lower bioavailability of BCAAs in the endometabolome of B. wadsworthia and B. thetaiotaomicron in co-culture at 8 h compared to monocultures. While further research is needed, this interplay may have potential implications for human health, as increased concentrations of aromatic and BCAAs by the gut microbiome have been associated with markers of insulin resistance and type 2 diabetes mellitus.54
Impact on tryptophan metabolism between B. thetaiotaomicron and B. wadsworthia: insights from combined metabolomic and transcriptomic analyses
The endometabolome revealed differences in the relative concentrations of tryptophan, indole, and indole-3-acetamide between B. thetaiotaomicron and B. wadsworthia monocultures and their co-culture; the abundance of these metabolites was higher in the B. thetaiotaomicron monoculture compared to the other culture conditions (Figure 6(b,c)). Bacterially produced indole has been shown to modulate intestinal inflammation in animals, and inhibit quorum sensing and virulence factor production.55 Indole-producing members of the human gut microbiota include E. coli, Proteus vulgaris, Paracolobactrum coliforme, Achromobacter liquefaciens, and Bacteroides spp.56 These strains can degrade tryptophan to indole, pyruvate and ammonia via the enzyme tryptophanase (TnaA), the activity of which is induced by tryptophan57 and inhibited by glucose.56 Interestingly, indole production from tryptophan is commonly observed in B. thetaiotaomicron,58 and B. thetaiotaomicron-derived indole has been shown to inhibit virulence in enteropathogenic E. coli (EPEC) and Vibrio cholerae by inhibiting T3SS expression.58 Given that B. wadsworthia cannot produce indole,59 it is likely that indole and its by-products are derived from B. thetaiotaomicron. In co-culture, the relative abundance of these metabolites decreased compared to the B. thetaiotaomicron monoculture (Figure 6(b)). To confirm that this effect was not due to the concentrations being standardized to the total cell count, the indole and tryptophan relative abundances were determined in the B. thetaiotaomicron monoculture and co-culture, normalized to 109 B. thetaiotaomicron cells only as determined by qPCR. This showed a decreased relative abundance of indole and tryptophan in the endometabolome of the co-culture compared to B. thetaiotaomicron monoculture (Figure 6(c)). This suggests that B. wadsworthia may either utilize indole derived from B. thetaiotaomicron or directly use tryptophan, thereby limiting the availability of tryptophan for B. thetaiotaomicron. Transcriptomic data supported this observation, showing increased expression of a tryptophan-specific transport protein (WCP94_002980) and tryptophanyl-tRNA synthetase gene (WCP94_002967) in B. wadsworthia during co-culture, indicating enhanced tryptophan uptake and incorporation into new proteins (Table S1). These combined metabolomic and transcriptomic findings suggest that, under co-culture conditions, B. wadsworthia intensifies its tryptophan uptake and protein incorporation, potentially diminishing tryptophan availability for B. thetaiotaomicron‘s indole and kynurenic acid production. These observations underscore a dynamic interplay between the two bacteria in the utilization of tryptophan and its downstream metabolites in the co-culture environment.
Conclusions
The increased H2S production by B. wadsworthia when co-cultured with B. thetaiotaomicron could have implications for human gut health. Both H2S and B. wadsworthia have been implicated in gut inflammation and pathogenesis, making it crucial to understand the change in H₂S production. Our co-culture experiments demonstrated enhanced growth of B. thetaiotaomicron, indicating a potentially beneficial syntrophic relationship. Transcriptomic analysis of B. wadsworthia revealed reduced expression of genes involved in sulfite production from taurine, thiosulfate, and sulfolactate, suggesting an alternative sulfite source in the co-culture. Our integrated transcriptomic and genomic data indicate a putative cross-feeding mechanism, with B. thetaiotaomicron potentially providing APS to B. wadsworthia for sulfite production; we next demonstrated that B. wadsworthia can grow to high densities using APS as an electron acceptor, substantiating this proposed cross-feeding interaction. Gene expression data suggested an increased uptake of lactate in B. wadsworthia, which could be used as an electron donor in dissimilatory sulfite reduction. Beyond sulfur metabolism, we observed alterations in tryptophan metabolism in co-culture. B. wadsworthia‘s increased expression of genes associated with tryptophan uptake suggests a competition for tryptophan, potentially impacting B. thetaiotaomicron‘s indole production. Reduced indole production by B. thetaiotaomicron in co-culture aligns with increased tryptophan utilization by B. wadsworthia, hinting at potential implications for gut health and inflammation.60,61 In conclusion, the increased H2S production by B. wadsworthia in co-culture, coupled with the reduced H2S utilization by B. thetaiotaomicron, underscores a complex microbial interaction. This proposed APS-mediated cross-feeding mechanism opens avenues for further exploration into sulfate metabolism in the human gut. The overall shift toward decreased indole and increased H2S production in co-culture suggests potential pro-inflammatory effects in the human gut environment, warranting further investigation.
It is important to note that the presence of B. wadsworthia or of microbially-derived colonic H2S is not always detrimental; indeed, it has been shown that B. wadsworthia-derived H2S may enhance colonization resistance against pathogens.62 It seems likely that an optimum H2S concentration range exists at which adaptive benefits may be provided to the host, likely in the µM range, whereas H2S in the mM range could be pro-inflammatory.63 In our study, we observed significantly increased H₂S production in the mM range in vitro when B. wadsworthia was in the presence of B. thetaiotaomicron but further work is needed to confirm the biological consequence of these findings in vivo. Interestingly, a study investigating B. wadsworthia colonization in a germ-free mouse model in the context of a high-fat diet, either with or without a simplified humanized microbial consortium (SIHUMI) which included B. thetaiotaomicron DSM 2079, observed the highest H2S concentrations in the SIHUMI + B. wadsworthia group at day 2 of colonization, peaking at ~4 mM before declining over time.64 This may reflect the sulfidogenic interaction between these strains in vivo; nonetheless, further exploration of the in vivo interaction between these strains is required.
Materials and methods
B. thetaiotaomicron and B. wadsworthia isolation and growth
B. thetaiotaomicron QI0072 was isolated from a fecal sample donated by a healthy adult between 50–80 years old recruited via the COMBAT study (ClinicalTrials.gov Identifier: NCT03679533). An initial enrichment was obtained in anaerobic anaerobe basal broth (ABB) liquid broth media (Oxoid, Thermo Fisher Scientific) supplemented with 10 mM taurine, and the isolate was purified on Brain Heart Infusion agar (BHI, Oxoid) prepared according to the manufacturer’s instructions then supplemented with 0.5% v/v vitamin K solution in ethanol (10 µL/L), resazurin (1 mg/L), hemin (5 mg/L) and L-cysteine hydrochloride (0.5 g/L) (further referred as BHI+C) with 1.5% (w/v) agar. Additional chemicals were from Merck. B. wadsworthia strains QI0012, QI0013, QI0014 and QI0015 were isolated from stool samples from healthy human donors recruited via the QIB Colon Model study (ClinicalTrials.gov Identifier: NCT02653001) using anaerobic ABB supplemented with 10 mM taurine as described by Sayavedra et al.64 B. thetaiotaomicron strains DSM 108160 and 108161 were sourced from the DSMZ culture collection and grown in BHI + supplements (BHI+S). Before autoclaving, BHI+S was prepared by adding hemin (10 mg/L) and yeast extract (5 g/L) to BHI broth. Cell pellets were cryopreserved at −80°C using Protect Select Anaerobe Cryopreservation tubes (Technical Service Consultants, UK).
Bacterial co-culture experiments
BHI, de Man, Rogosa and Sharpe (MRS, Oxoid) and ABB liquid broth media were prepared according to the manufacturer’s instructions. Where required, solid media was made by the addition of 1.5% (w/v) agar to the media before autoclaving. All media and culture vessels were maintained under anaerobic conditions using an anaerobic cabinet (Don Whitley, UK) with materials pre-reduced before use for at least 18 hours in an atmosphere of 5% CO2, 10% H2 in N2 at 37°C. For co-culture experiments, B. thetaiotaomicron was grown on BHI+C, and B. wadsworthia was grown on ABB supplemented with 10 mM taurine. After overnight incubation, the second passage cultures were diluted to ~ 108 CFU/mL using estimated cell count per optical density at 600 nm (OD600) factor. OD600 was quantified using a SPECTROstar Nano instrument (BMG Labtech). Co-culture experiments used anaerobic synthetic media containing a mix of 1:1:1 (v/v) of BHI+C, MRS and Postgate C, supplemented with 10 mM taurine (further referred to as BPM media). Postgate C contained per liter of distilled water: sodium lactate (6 g), sodium sulfate (4.5 g), ammonium chloride (1 g), yeast extract (1 g) potassium dihydrogen phosphate (0.5 g), sodium citrate tri basic (0.3 g), magnesium sulfate 7-hydrate (0.06 g), iron sulfate 7-hydrate (4 mg), calcium chloride (0.04 g), L-cysteine hydrochloride (0.5 g), and resazurin (0.8 mg).65
Co-cultures were prepared by inoculating ~ 106 CFU/mL anaerobic BPM. The CFU used for inoculum were confirmed by plating on ABB supplemented with 10 mM taurine for B. wadsworthia, and BHI+C media for B. thetaiotaomicron. Experimental conditions included negative control (no inoculum), monocultures and co-culture with final culture volumes of 10 mL. All cultures were performed in at least triplicate. Sub-samples were taken at 0, 2, 4, 6 and 8 h post-inoculation.
Growth of B. wadsworthia with different electron acceptors
The growth of B. wadsworthia under various conditions was assessed by monitoring its OD600 after 48 hours of incubation on ABB media enriched with different electron acceptors at a 1:100 dilution. These included 4 mM sodium sulfite, 10 mM adenosine 5′-phosphosulfate (APS) sodium salt (Merck, UK), and 10 mM taurine, alongside a control containing ABB only. We used at least seven technical replicates. Differences in growth were tested using a linear model with the glmmTMB package.
Colourimetric determination of H2S concentration
Determination of H2S concentration in samples was performed using the methylene blue assay modified from Cline.66 Briefly, 500 µL of bacterial cultures were taken and immediately fixed 1:1 with 5% zinc acetate and stored at −20°C. For calibration, zinc sulfide solutions were prepared in media diluted 1:200 within concentration range 0–40 µM. For analysis, fixed samples were diluted 1:100 in water to a final volume of 1 mL, producing a final sample dilution of 1:200. 80 µL of diamine reagent (250 mL 6 M HCl, 1 g N,N-dimethyl-1,4-phenylendiamine sulfate, 1.5 g FeCl3.6 H20) was added to all samples and standards which were then stored in the dark to allow methylene blue color development. After 30 min, the samples were centrifuged at 13,000 × g for 5 min to pellet biomass. 300 µL of supernatant was taken for spectrophotometric absorbance measurement at 670 nm. H2Sconcentration in µM was determined using the calibration curve as a standard. Statistical significance between culture conditions was established using unpaired t-test pair-wise comparisons and displayed graphically using GraphPad Prism 7 (GraphPad Software, Boston, USA). Results of unpaired t tests are shown, where * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, **** = p ≤ 0.0001, ns = not significant (p > 0.05).
Absolute quantification of bacterial cells via qPCR
For quantification of bacterial cells in experimental cultures, DNA extraction was performed using the Maxwell® RSC Blood DNA kit (Promega) according to the manufacturer’s protocol. Prior to extraction, 200 µL samples were boiled at 90°C for 10 min and 150 µL was transferred to a sterile microcentrifuge tube containing 30 µL of proteinase K solution and 300 µL lysis buffer. Each sample was vortexed for 10 s and incubated at 56°C for 20 min. Quantification of B. wadsworthia and B. thetaiotaomicron was performed via qPCR using KiCqStart® SYBR® Green qPCR ReadyMix™ with ROX (Sigma-Aldrich) on a StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific). Absolute quantification of gene copy numbers was performed by comparing samples to calibration standards prepared with known gene copy numbers in the range of 10–1 × 109 copies. The number of gene copies per cell was used to calculate the absolute cell counts per mL of culture. All samples and standards were assayed in triplicate. The reaction conditions and primers used are shown in Table 1. Statistical significance between culture conditions was established using unpaired t-test pair-wise comparisons and displayed graphically using GraphPad Prism 7.
Table 1.
Primers and reaction conditions used for absolute quantification of B. wadsworthia and B. thetaiotaomicron via qPCR.
| Target bacterium | Primer name | Product size (bp) | Primer sequence (5’ to 3’) (W = A + T) |
Primer conc. (nM) | Reaction conditions | Ref |
|---|---|---|---|---|---|---|
| B. wadsworthia | TPA-F | 150 | CAACGTCCCCACCATCAAGTTCTCTG | 100 | 95°C 2 min, 40 cycles of 95°C 15 s, 62°C 30 s. | 67 |
| TPA-R | TGAATTCGCGGAAGGAGCGAGAGGTC | 100 | ||||
| B. thetaiotao-micron | F_Bacter 11 | 131 | CCTWCGATGGATAGGGGTT | 200 | 95°C 10 min, 40 cycles of 95°C 30 s, 60°C 1 min. | 68 |
| R_Bacter 08 | CACGCTACTTGGCTGGTTCAG | 200 |
Transcriptomic sequencing and analysis
5 mL of cultures were taken at 8 h post-inoculation and cells were immediately pelleted at 15,000 × g for 2 min. The supernatant was removed, cell pellets were snap frozen on dry ice, and stored at −80°C. RNA was extracted using the RNeasy® Mini Kit (Qiagen, Germany) according to manufacturer’s protocol with on-column DNase digestion. RNA concentration was determined via Qubit RNA High Sensitivity kit (Thermo Fisher Scientific, Massachusetts, USA) and Qubit 3.0 fluorometer (Life Technologies, Massachusetts, USA). Total RNA was sequenced at Azenta Genewiz. rRNA was depleted using NEBNext rRNA depletion kits for human/mouse/rat and bacteria species (E6310 and E7850). Samples were sequenced using an Illumina NovaSeq 6000 instrument with 2 × 150bp configuration using a sequencing depth of 20 M paired-end reads per sample.
For differential expression analysis, raw data were analyzed as previously described.69 Briefly, reads were cleaned to remove sequencing adapters and residual ribosomal RNA sequences using BBDuk (v 38.06, Bushnell B. - sourceforge.net/projects/bbmap/). Cleaned reads were mapped to the QI0013 and QI0072 reference genomes using BBSplit (v 38.06). The number of transcripts per gene was estimated using featureCounts (v.2.0).70 Reference genomes were annotated with the BV-BRC comprehensive genome analysis tool.71 Differentially expressed genes were identified using edgeR with TMM normalization72 using the Trinity RNASeq package,73 with a p value cutoff of 0.01 and a log2FC of 1 equating to a 2-fold change in gene expression. In the case of specific genes of interest where there was no functional annotation, InterProScan74 was used to assign predicted protein functions based on domains where possible using the predicted amino acid sequence. Additionally, Pathway Tools v23.075 was used for preliminary pathway enrichment analysis and pathway predictions using the reference genomes.
Endometabolome analysis via LC-MS
5 mL of cultures were taken at 8 h post-inoculation and cells were immediately pelleted at 4,000 × g for 10 min. All supernatant was removed, and cell pellets were snap-frozen on dry ice. All LC-MS analysis was performed by Creative Proteomics, New York, USA. Prior to analysis, bacterial pellets were thawed and 240 µL methanol added for metabolite extraction. Samples were vortexed for 60 s, sonicated for 30 min at 4°C and stored at −20°C for 1 h. Samples were pelleted at 12,000 × g for 15 min at 4°C. Finally, 200 μL of supernatant and 5 μL of DL-o-Chlorophenylalanine (0.2 mg/mL) was transferred to a vial for LC-MS analysis. QC samples were prepared by pooling all the samples in triplicate. All samples were injected in triplicate. Separation was performed by ACQUITY UPLC (Waters) combined with Q Exactive MS (Thermo) and screened with ESI-MS. The LC system was comprised of ACQUITY UPLC HSS T3 (100 × 2.1 mm × 1.8 μm) with ACQUITY UPLC (Waters). The mobile phase was composed of solvent A (0.05% formic acid water) and solvent B (acetonitrile) with a gradient elution (0–1 min, 5% B; 1–12.5 min, 5%-95% B; 12.5–13.5 min, 95% B; 13.5–13.6 min, 95%-5% B; 13.6–16 min, 5% B). The flow rate of the mobile phase was 0.3 mL/min. The column temperature was maintained at 40°C, and the sample manager temperature set at 4°C. Mass spectrometry parameters in ESI+ and ESI- mode were as follows: ESI+: Heater Temp 300°C; Sheath Gas Flow rate, 45 arb; Aux Gas Flow Rate, 15 arb; Sweep Gas Flow Rate, 1 arb; spray voltage, 3.0 KV; Capillary Temp, 350°C; S-Lens RF Level, 30%. ESI-: Heater Temp 300°C, Sheath Gas Flow rate, 45 arb; Aux Gas Flow Rate, 15 arb; Sweep Gas Flow Rate, 1 arb; spray voltage, 3.2 KV; Capillary Temp, 350°C; S-Lens RF Level, 60%.
Metabolites were identified using Compound Discoverer 3.0 (Thermo Fisher Scientific). Progenesis QI v 2.1 (Waters) was used for manual screening of the identified compounds, in order to minimize false positive identification results. Data was normalized using the Total Ion Count (TIC) method where the peak area of each metabolite was divided by the SUM of all metabolites area and then multiplied by one million.
To remove compounds with high analytical variability, compounds with RSDQC >20% were discarded.76 Endometabolomic data was standardized by calculating the concentration (µM) per 109 bacterial cells present as measured via qPCR, in order to account for cell density differences in monocultures and co-cultures.77 Compounds at low concentrations across all samples (<2 µM per 109 cells) were removed. In positive ion mode, 65% of the identified metabolites remained after quality control (320 left from 490), and in negative mode 70% remained (267 left from 377). Given the higher peak intensity and number of reported compounds, positive mode was used for further analysis. The metabolomic data was auto-scaled and analyzed using Metaboanalyst 5.0,78 to obtain the PLS-DA for the global profile changes and Variable Importance in Projection (VIP) compounds that contribute highly to inter-condition differences, and the heatmaps showing feature clustering and inter-condition differences in relative abundance of compounds.
DNA extraction from isolates for whole genome sequencing
For whole genome sequencing, 1 mL of overnight culture was taken and centrifuged at 13,000 g for 2 min. Pellets were stored at −20°C until extraction. For extraction of high molecular weight DNA, Fire Monkey High Molecular Weight DNA (HMW-DNA) extraction kit was used according to manufacturer’s protocol (Revolugen, UK). The additional overnight elution step recommended by the manufacturer was used to maximize DNA yield. DNA was quantified using Qubit High Sensitivity DNA Quantification Kit (Thermo Scientific, UK) and Qubit 3.0 fluorometer.
Whole genome sequencing
For whole genome sequencing of B. thetaiotaomicron, a hybrid approach using both short- and long-read sequencing was performed in-house at Quadram Institute Bioscience. For short-read sequencing, genomic DNA was normalized to 5 ng/µL with EB buffer (10 mM Tris-HCl) and sequencing was performed using an Illumina NextSeq 500 system with 2 × 150bp paired-end reads. Libraries were prepared using Bead Linked Transposomes (BLT) (Illumina) and P7 and P5 Illumina 9 bp barcodes. Paired-end sequencing reads were received as fastq files. For long-read sequencing, a minION was used (Oxford Nanopore Technologies, Oxford, UK).
For genome assembly, short reads were cleaned using BBDuk (v 38.79) to trim the reads and remove sequencing adaptors. Long reads were trimmed using Porechop (v 0.2.3)79 and a hybrid assembly was reconstructed using Unicycler (v 0.4.9).80 The completeness and contamination of the assembly was checked using CheckM (v 1.0.18).81 Genomes were annotated using Prokka (v 1.14.6) and BV-BRC.71,82
Sequence comparison of sulfur metabolism genes
For genes of interest, amino acid sequences were obtained from B. wadsworthia (QI0013) and B. thetaiotaomicron strain 1 (QI0072) genomes in addition to those from reference genomes D. desulfuricans subsp. desulfuricans DSM 642, D. gigas DSM 1382 and D. alaskensis G20 via BV-BRC.71 InterProScan74 was used to assign predicted protein function based on domains. Protein−protein comparison was performed using blastp on NCBI-BLAST using default settings.83 Amino acid alignments were performed using Clustal omega v1.2.2 in Geneious prime v 2022.1.1 (Biomatters Ltd) and visualized with GeneDoc v 2.7.000 (National Resource for Biomedical Supercomputing).
Supplementary Material
Acknowledgments
The authors thank David Baker (Quadram Institute Bioscience) for library preparation and whole genome sequencing.
Funding Statement
This work was supported by UKRI-BBSRC via the Norwich Research Park Doctoral Training Partnership [grant no. BB/M011216/1] and institute strategic programme grants Gut Microbes and Health [BB/R012490/1 theme BBS/E/F/000PR10356] and Food, Microbiome and Health (BB/X011054/1 theme BBS/E/F/000PR13633). UKRI-BBSRC had no role in the manner of conduct or outcome of the research project. Transcriptomic sequencing was funded through a Quadram Institute Bioscience Institute Development Grant [20414000X]. LS was supported by a BBSRC Discovery Fellowship [BB/Z514445/10].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors’ contributions
JD, LS and AN conceived and designed the project. JD and LS designed the experiments. JD performed experiments and analyzed the data. LS supervised transcriptomic analyses. JD and LS wrote the manuscript with contributions from all coauthors.
Data availability statement
Transcriptomic sequencing data was submitted to NCBI under PRJNA1115121. The genome of Bilophila wadsworthia QI0013 is available under project PRJNA1085689. The genome of Bacteroides thetaiotaomicron was submitted under project PRJNA1151643.
Ethics statement
The colon model study was approved by the Quadram Institute Bioscience Human Research Governance Committee (IFR01/2015), and London–Westminster Research Ethics Committee (15/LO/2169). The COMBAT study was approved by the University of East Anglia’s Faculty of Medicine and Health Sciences Ethical Review Committee (Reference: 201819–039) and the Health Research Authority (IRAS number: 237251). The colon model study was registered under the ClinicalTrials.gov Identifier NCT02653001 and the COMBAT was registered under the NCT03679533.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2024.2431644
References
- 1.Stewart J, Chadwick V, Murray A.. Carriage, quantification, and predominance of methanogens and sulfate-reducing bacteria in faecal samples. Lett Appl Microbiol. 2006;43(1):58–19. doi: 10.1111/j.1472-765X.2006.01906.x. [DOI] [PubMed] [Google Scholar]
- 2.Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern P, Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium Proceedings of the National Academy of Sciences; Vol. 110 (33). 2013. p. 13582–13587. doi: 10.1073/pnas.1312524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos A, Venceslau S, Grein F, Leavitt W, Dahl C, Johnston D, Pereira IAC. A protein trisulfide couples dissimilatory sulfate reduction to energy conservation. Science. 2015;350(6267):1541. doi: 10.1126/science.aad3558. [DOI] [PubMed] [Google Scholar]
- 4.Mathai JC, Missner A, Kügler P, Saparov SM, Zeidel ML, Lee JK, Pohl, P. No facilitator required for membrane transport of hydrogen sulfide Proceedings of the National Academy of Sciences; Vol. 106 (39). 2009. p. 16633–16638. doi: 10.1073/pnas.0902952106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guidotti T. Hydrogen sulphide. Occup Med. 1996;46(5):367–371. doi: 10.1093/occmed/46.5.367. [DOI] [PubMed] [Google Scholar]
- 6.Parey K, Demmer U, Warkentin E, Wynen A, Ermler U, Dahl C, Pereira IAC. Structural, biochemical and genetic characterization of dissimilatory ATP sulfurylase from Allochromatium vinosum. PLoS One. 2013;8(9):e74707. doi: 10.1371/journal.pone.0074707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron E, Summanen P, Downes J, Roberts M, Wexler H, Finegold S. Bilophila wadsworthia, gen. nov. and sp. nov. a unique gram-negative anaerobic rod recovered from appendicitis specimens and human faeces. Microbiology. 1989;135(12):3405–3411. doi: 10.1099/00221287-135-12-3405. [DOI] [PubMed] [Google Scholar]
- 8.Denger K, Laue H, Cook A. Anaerobic taurine oxidation: a novel reaction by a nitrate-reducing Alcaligenes sp. Microbiology. 1997;143(6):1919–1924. doi: 10.1099/00221287-143-6-1919. [DOI] [PubMed] [Google Scholar]
- 9.Baron E. Bilophila wadsworthia: a unique gram-negative anaerobic rod. Anaerobe. 1997;3(2–3):83–86. doi: 10.1006/anae.1997.0075. [DOI] [PubMed] [Google Scholar]
- 10.Peck S, Denger K, Burrichter A, Irwin S, Balskus E, Schleheck D. A glycyl radical enzyme enables hydrogen sulfide production by the human intestinal bacterium Bilophila wadsworthia. Proceedings of the National Academy of Sciences; Vol. 116. 2019. p. 3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson B, Kits K, Löffler J, Burrichter A, Fiedler A, Denger K, Frommeyer B, Herbold CW, Rattei T,Karcher N, Segata N, Schleheck D, Loy, A. Sulfoquinovose is a select nutrient of prominent bacteria and a source of hydrogen sulfide in the human gut. The ISME J 15 2779–2791. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kushkevych I, Cejnar J, Treml J, Dordević D, Kollar P, Vítězová M. Recent advances in metabolic pathways of sulfate reduction in intestinal bacteria. Cells. 2020;9(3):698. doi: 10.3390/cells9030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scanlan P, Shanahan F, Marchesi J. Culture-independent analysis of Desulfovibrios in the human distal colon of healthy, colorectal cancer and polypectomized individuals. FEMS Microbiol Ecol. 2009;69(2):213–221. doi: 10.1111/j.1574-6941.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 14.Baron E, Curren M, Henderson G, Jousimies-Somer H, Lee K, Lechowitz K, Strong CA, Summanen P, Tunér K, Finegold SM, et al. Bilophila wadsworthia isolates from clinical specimens. J Clin Microbiol. 1992;30(7):1882–1884. doi: 10.1128/jcm.30.7.1882-1884.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finegold S, P S, Hunt GS, B E. Clinical importance of Bilophila wadsworthia. Eur J Clin Microbiol Infect Dis. 1992;11(11):1058–1063. doi: 10.1007/BF01967799. [DOI] [PubMed] [Google Scholar]
- 16.Mosca A, D’Alagni M, Del Prete R, de Michele G, Summanen P, Finegold S, Miragliotta G. Preliminary evidence of endotoxic activity of Bilophila wadsworthia. Anaerobe. 1995;1(1):21–24. doi: 10.1016/S1075-9964(95)80379-3. [DOI] [PubMed] [Google Scholar]
- 17.Hunt Gerardo S, Garcia M, Wexler H, Finegold S. Adherence of Bilophila wadsworthia to cultured human embryonic intestinal cells. Anaerobe. 1998;4(1):19–27. doi: 10.1006/anae.1997.0134. [DOI] [PubMed] [Google Scholar]
- 18.Yazici C, Wolf P, Carroll T, Mutlu E, Xicola R, Llor X, Jung BH, Ellis NA, Gaskins HR. Bilophila wadsworthia is more abundant in the colonic microbiome of colorectal cancer cases compared to healthy controls. Gastroenterology. 2015;148(4):S–100. doi: 10.1016/S0016-5085(15)30343-7. [DOI] [Google Scholar]
- 19.Tremlett H, Fadrosh D, Faruqi A, Zhu F, Hart J, Roalstad S, Graves J, Lynch S, Waubant E. Gut microbiota in early pediatric multiple sclerosis: a case−control study. Eur J Neurol. 2016;23(8):1308–1321. doi: 10.1111/ene.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldini F, Hertel J, Sandt E, Thinnes CC, Neuberger-Castillo L, Pavelka L, Betsou F, Krüger R, Thiele I, Aguayo G, et al. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 2020;18(1):62. doi: 10.1186/s12915-020-00775-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheng C, Yang K, He B, Du W, Cai Y, Han Y. Combination of gut microbiota and plasma amyloid-β as a potential index for identifying preclinical Alzheimer’s disease: a cross-sectional analysis from the SILCODE study. Alz Res Ther. 2022;14(1):35. doi: 10.1186/s13195-022-00977-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67(10):1881. doi: 10.1136/gutjnl-2017-314307. [DOI] [PubMed] [Google Scholar]
- 23.Li G-H, Huang S-J, Li X, Liu X-S, Du Q-L. Response of gut microbiota to serum metabolome changes in intrahepatic cholestasis of pregnant patients. World J Gastroenterol. 2020;26(46):7338–7351. doi: 10.3748/wjg.v26.i46.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He X, Sun J, Liu C, Yu X, Li H, Zhang W, Li Y, Geng Y, Wang Z. Compositional alterations of gut microbiota in patients with diabetic kidney disease and type 2 diabetes mellitus. Diabetes, Metabolic Syndr Obes. 2022;15:755–765. doi: 10.2147/DMSO.S347805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thirion F, Speyer H, Hansen TH, Nielsen T, Fan Y, Le Chatelier E, Fromentin S, Berland M, Plaza Oñate F, Pons N, et al. Alteration of gut microbiome in patients with schizophrenia indicates links between bacterial tyrosine biosynthesis and cognitive dysfunction. Biol Psychiatry Global Open Sci. 2022;3(2):283–291. doi: 10.1016/j.bpsgos.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Z, Long W, Hao B, Ding D, Ma X, Zhao L, Pang X. A human stool-derived Bilophila wadsworthia strain caused systemic inflammation in specific-pathogen-free mice. Gut Pathog. 2017;9(1):59–. doi: 10.1186/s13099-017-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Bjursell Magnus K, Himrod J, Deng S, Carmichael Lynn K, Chiang Herbert C, Hooper LV, Gordon JI. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299(5615):2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 28.Hong P-Y, Wu J-H, Liu W-T. Relative abundance of Bacteroides spp. In stools and wastewaters as determined by hierarchical oligonucleotide primer extension. Appl Environ Microbiol. 2008;74(9):2882–2893. doi: 10.1128/AEM.02568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garber JM, Hennet T, Szymanski CM. Significance of fucose in intestinal health and disease. Mol Microbiol. 2021;115(6):1086–1093. doi: 10.1111/mmi.14681. [DOI] [PubMed] [Google Scholar]
- 31.Freitas M, Axelsson L-G, Cayuela C, Midtvedt T, Trugnan G. Indigenous microbes and their soluble factors differentially modulate intestinal glycosylation steps in vivo. Histochem Cell Biol. 2005;124(5):423–433. doi: 10.1007/s00418-005-0004-1. [DOI] [PubMed] [Google Scholar]
- 32.Hooper LV, Gordon JI. Glycans as legislators of host–microbial interactions: spanning the spectrum from symbiosis to pathogenicity. Glycobiology. 2001;11(2):1R–10R. doi: 10.1093/glycob/11.2.1R. [DOI] [PubMed] [Google Scholar]
- 33.Chia LW, Mank M, Blijenberg B, Aalvink S, Bongers RS, Stahl B, Knol J, Belzer C. Bacteroides thetaiotaomicron fosters the growth of butyrate-producing Anaerostipes caccae in the presence of lactose and total human milk carbohydrates. Microorganisms. 2020;8(10):8. doi: 10.3390/microorganisms8101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chia LW, Mank M, Blijenberg B, Aalvink S, Bongers RS, Stahl B, Knol J, Belzer C. Bacteroides thetaiotaomicron fosters the growth of butyrate-producing Anaerostipes caccae in the presence of lactose and total human milk carbohydrates. Microorganisms. 2020;8(10):1513. doi: 10.3390/microorganisms8101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porter Nathan T, Larsbrink J, Avci Fikri Y, Avci FY. Investigation and alteration of organic acid synthesis pathways in the mammalian gut symbiont Bacteroides thetaiotaomicron. Microbiol Spectr. 2022;10(1):e02312–21. doi: 10.1128/spectrum.02312-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye M, Yu J, Shi X, Zhu J, Gao X, Liu W. Polysaccharides catabolism by the human gut bacterium - Bacteroides thetaiotaomicron: advances and perspectives. Crit Rev In Food Sci Nutr. 2021;61(21):3569–3588. doi: 10.1080/10408398.2020.1803198. [DOI] [PubMed] [Google Scholar]
- 37.Dordević D, Jančíková S, Vítězová M, Kushkevych I. Hydrogen sulfide toxicity in the gut environment: meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J Adv Res. 2021;27:55–69. doi: 10.1016/j.jare.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Silva S, Venceslau S, Fernandes C, Valente F, Pereira I. Hydrogen as an energy source for the human pathogen Bilophila wadsworthia. Antonie van Leeuwenhoek. 2008;93(4):381–390. doi: 10.1007/s10482-007-9215-x. [DOI] [PubMed] [Google Scholar]
- 39.Khelaifia S, Lagier JC, Nkamga VD, Guilhot E, Drancourt M, Raoult D. Aerobic culture of methanogenic archaea without an external source of hydrogen. Eur J Clin Microbiol Infect Dis. 2016;35(6):985–991. doi: 10.1007/s10096-016-2627-7. [DOI] [PubMed] [Google Scholar]
- 40.Keon RG, Fu R, Voordouw G. Deletion of two downstream genes alters expression of the hmc operon of Desulfovibrio vulgaris subsp. vulgaris Hildenborough. Archiv Microbiol. 1997;167(6):376–383. doi: 10.1007/s002030050458. [DOI] [PubMed] [Google Scholar]
- 41.Ichikawa Y, Yamamoto H, S-I H, Sato B, Takefuji Y, Satoh F. The overlooked benefits of hydrogen-producing bacteria. Med Gas Res. 2023;13(3):108–111. doi: 10.4103/2045-9912.344977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muyzer G, Stams A. The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol. 2008;6(6):441–454. doi: 10.1038/nrmicro1892. [DOI] [PubMed] [Google Scholar]
- 43.Qian Z, Tianwei H, Mackey HR, van Loosdrecht Mcm, Guanghao C, van Loosdrecht MCM. Recent advances in dissimilatory sulfate reduction: from metabolic study to application. Water Res. 2019;150:162–181. doi: 10.1016/j.watres.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Duarte AG, Barbosa ACC, Ferreira D, Manteigas G, Domingos RM, Pereira IAC. Redox loops in anaerobic respiration - the role of the widespread NrfD protein family and associated dimeric redox module. Biochim et Biophys Acta (BBA) - Bioenerg. 2021;1862(7):148416. doi: 10.1016/j.bbabio.2021.148416. [DOI] [PubMed] [Google Scholar]
- 45.Burrichter AG, Dörr S, Bergmann P, Haiß S, Keller A, Fournier C, Franchini P, Isono E, Schleheck D. Bacterial microcompartments for isethionate desulfonation in the taurine-degrading human-gut bacterium Bilophila wadsworthia. Vol. 21. ; 2021. p. 340 doi: 10.1186/s12866-021-02386-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luis AS, Jin C, Pereira GV, Glowacki RWP, Gugel SR, Singh S, Byrne DP, Pudlo NA, London JA, Baslé A, et al. A single sulfatase is required to access colonic mucin by a gut bacterium. Nature. 2021;598(7880):332–337. doi: 10.1038/s41586-021-03967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benoni R, De Bei O, Paredi G, Hayes CS, Franko N, Mozzarelli A, Bettati S, Campanini B. Modulation of Escherichia coli serine acetyltransferase catalytic activity in the cysteine synthase complex. FEBS Lett. 2017;591(9):1212–1224. doi: 10.1002/1873-3468.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guédon E, Martin-Verstraete I. Cysteine metabolism and its regulation in bacteria. Amino acid biosynthesis~ pathways, regulation and metabolic engineering. 5 Microbiology Monographs. Berlin, Heidelberg: Springer; 2006. p. 195–218 doi: 10.1007/7171_2006_060. [DOI] [Google Scholar]
- 49.Kulikova R VV, Bazhulina SV, Anufrieva NP, Kotlov NV, Koval MI, Koval, Vs VS, Morozova EA, Hayashi H, Belyi YF, et al. Identification of O-acetylhomoserine sulfhydrylase, a putative enzyme responsible for methionine biosynthesis in Clostridioides difficile: gene cloning and biochemical characterizations. IUBMB Life. 2019;71(11):1815–1823. doi: 10.1002/iub.2139. [DOI] [PubMed] [Google Scholar]
- 50.Dalgliesh C. Biological degradation of tryptophan. Q Rev Chem Soc. 1951;5(3):227–244. doi: 10.1039/qr9510500227. [DOI] [Google Scholar]
- 51.Kuc D, Zgrajka W, Parada-Turska J, Urbanik-Sypniewska T, Turski WA. Micromolar concentration of kynurenic acid in rat small intestine. Amino Acids. 2008;35(2):503–505. doi: 10.1007/s00726-007-0631-z. [DOI] [PubMed] [Google Scholar]
- 52.Turski MP, Turska M, Paluszkiewicz P, Parada-Turska J, Oxenkrug GF. Kynurenic acid in the digestive system–-new facts, new challenges. Int J Tryptophan Res. 2013;6:IJTR.S12536. doi: 10.4137/IJTR.S12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson EL, Heaver SL, Waters JL, Kim BI, Bretin A, Goodman AL, Gewirtz AT, Worgall TS, Ley RE. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat Commun. 2020;11(1):2471. doi: 10.1038/s41467-020-16274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neis EP, Dejong CH, Rensen SS. The role of microbial amino acid metabolism in host metabolism. Nutrients. 2015;7(4):2930–2946. doi: 10.3390/nu7042930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J-H, Wood TK, Lee J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends In Microbiol. 2015;23(11):707–718. doi: 10.1016/j.tim.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Smith EA, Macfarlane GT. Formation of phenolic and indolic compounds by anaerobic bacteria in the human large intestine. Microb Ecol. 1997;33(3):180–188. doi: 10.1007/s002489900020. [DOI] [PubMed] [Google Scholar]
- 57.Li G, Young KD. Indole production by the tryptophanase TnaA in Escherichia coli is determined by the amount of exogenous tryptophan. Microbiology. 2013;159(Pt_2):402–410. doi: 10.1099/mic.0.064139-0. [DOI] [PubMed] [Google Scholar]
- 58.Gorelik O, Rogad A, Holoidovsky L, Meijler MM, Sal-Man N. Indole intercepts the communication between enteropathogenic E. coli and Vibrio cholerae. Gut Microbes. 2022;14(1):2138677. doi: 10.1080/19490976.2022.2138677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Claros MC, Schumacher U, Jacob M, Hunt Gerardo S, Kleinkauf N, Goldstein EJC, Finegold SM, Rodloff AC. Characterization of Bilophila wadsworthia isolates using PCR fingerprinting. Anaerobe. 1999;5(6):589–593. doi: 10.1006/anae.1999.0307. [DOI] [Google Scholar]
- 60.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proceedings of the national academy of sciences; Vol. 107. 2010. p. 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whitfield-Cargile CM, Cohen ND, Chapkin RS, Weeks BR, Davidson LA, Goldsby JS, Hunt CL, Steinmeyer SH, Menon R, Suchodolski JS, et al. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes. 2016;7(3):246–261. doi: 10.1080/19490976.2016.1156827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stacy A, Andrade-Oliveira V, McCulloch JA, Hild B, Oh JH, Perez-Chaparro PJ, Sim CK, Lim AI, Link VM, Enamorado M, et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell. 2021;184(3):615–27.e17. doi: 10.1016/j.cell.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo FF, Yu TC, Hong J, Fang JY. Emerging roles of hydrogen sulfide in inflammatory and neoplastic colonic diseases. Front Physiol. 2016;7:156. doi: 10.3389/fphys.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sayavedra L, Yasir M, Goldson A, Brion A, Le Gall G, Moreno-Gonzalez M, Altera, A, Paxhia, MD, Warren, M, Turner, A, Beraza, N, Narbad, A. Energy generation drives gut colonization by Bilophila wadsworthia researchsquare.com/article/rs-4502164/v1. 2024. doi: 10.21203/rs.3.rs-4502164/v1. [DOI] [Google Scholar]
- 65.Postgate John R. Versatile medium for the enumeration of sulfate-reducing bacteria. Appl Microbiol. 1963;11(3):265–267. doi: 10.1128/am.11.3.265-267.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cline J. Spectrophotometric determination of hydrogen sulphide in natural waters. Limnology And Oceanogr. 1969;14(3):454–458. doi: 10.4319/lo.1969.14.3.0454. [DOI] [Google Scholar]
- 67.Natividad J, Lamas B, Pham H, Michel M, Rainteau D, Bridonneau C, da Costa G, van Hylckama Vlieg J, Sovran B, Chamignon C, et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat Commun. 2018;9(1):2802–. doi: 10.1038/s41467-018-05249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Furet J-P, Firmesse O, Gourmelon M, Bridonneau C, Tap J, Mondot S, Doré J, Corthier G. Comparative assessment of human and farm animal faecal microbiota using real-time quantitative PCR. FEMS Microbiol Ecol. 2009;68(3):351–362. doi: 10.1111/j.1574-6941.2009.00671.x. [DOI] [PubMed] [Google Scholar]
- 69.Sayavedra L, Li T, Bueno Batista M, Seah BKB, Booth C, Zhai Q, Chen W, Narbad A. Desulfovibrio diazotrophicus sp. nov. a sulfate-reducing bacterium from the human gut capable of nitrogen fixation. Environ Microbiol. 2021;23(6):3164–3181. doi: 10.1111/1462-2920.15538. [DOI] [PubMed] [Google Scholar]
- 70.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 71.Olson RD, Assaf R, Brettin T, Conrad N, Cucinell C, Davis JJ, Dempsey D, Dickerman A, Dietrich E, Kenyon R, et al. Introducing the bacterial and viral bioinformatics resource center (BV-BRC): a resource combining PATRIC, IRD and ViPR. Nucleic Acids Res. 2023;51(D1):D678–D89. doi: 10.1093/nar/gkac1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of rna-seq data. Genome Biol. 2010;11(3):R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blum M, Chang H-Y, Chuguransky S, Grego T, Kandasaamy S, Mitchell A, Nuka G, Paysan-Lafosse T, Qureshi M, Raj S, et al. The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 2021;49(D1):D344–D54. doi: 10.1093/nar/gkaa977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karp PD, Midford PE, Billington R, Kothari A, Krummenacker M, Latendresse M, Ong WK, Subhraveti P, Caspi R, Fulcher C, et al. Pathway tools version 23.0 update: software for pathway/genome informatics and systems biology. Briefings In Bioinf. 2021;22(1):109–126. doi: 10.1093/bib/bbz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sánchez-Illana Á, Piñeiro-Ramos JD, Sanjuan-Herráez JD, Vento M, Quintás G, Kuligowski J. Evaluation of batch effect elimination using quality control replicates in LC-MS metabolite profiling. Analytica (Rome) Acta. 2018;1019:38–48. doi: 10.1016/j.aca.2018.02.053. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J, Luo W, Wang Z, Chen X, Lv P, Xu J. A novel strategy for D-psicose and lipase co-production using a co-culture system of engineered Bacillus subtilis and Escherichia coli and bioprocess analysis using metabolomics. Bioresour And Bioprocess. 2021;8(1):77. doi: 10.1186/s40643-021-00429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, Gauthier C, Jacques P-É, Li S, Xia J, et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49(W1):W388–W96. doi: 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wick RR, Judd LM, Gorrie CL, Holt KE. Completing bacterial genome assemblies with multiplex MinION sequencing. Microb Genomics. 2017;3(10):3. doi: 10.1099/mgen.0.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13(6):e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25(7):1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 83.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman, DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Transcriptomic sequencing data was submitted to NCBI under PRJNA1115121. The genome of Bilophila wadsworthia QI0013 is available under project PRJNA1085689. The genome of Bacteroides thetaiotaomicron was submitted under project PRJNA1151643.


