Abstract
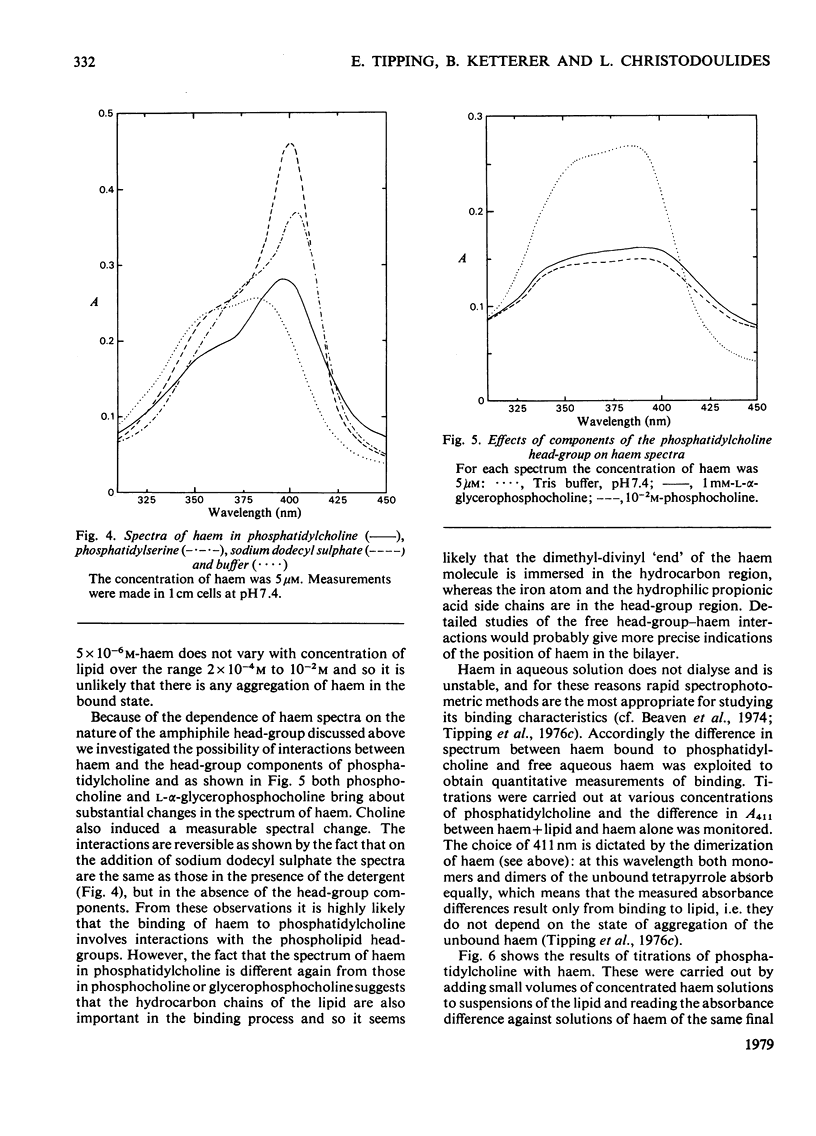
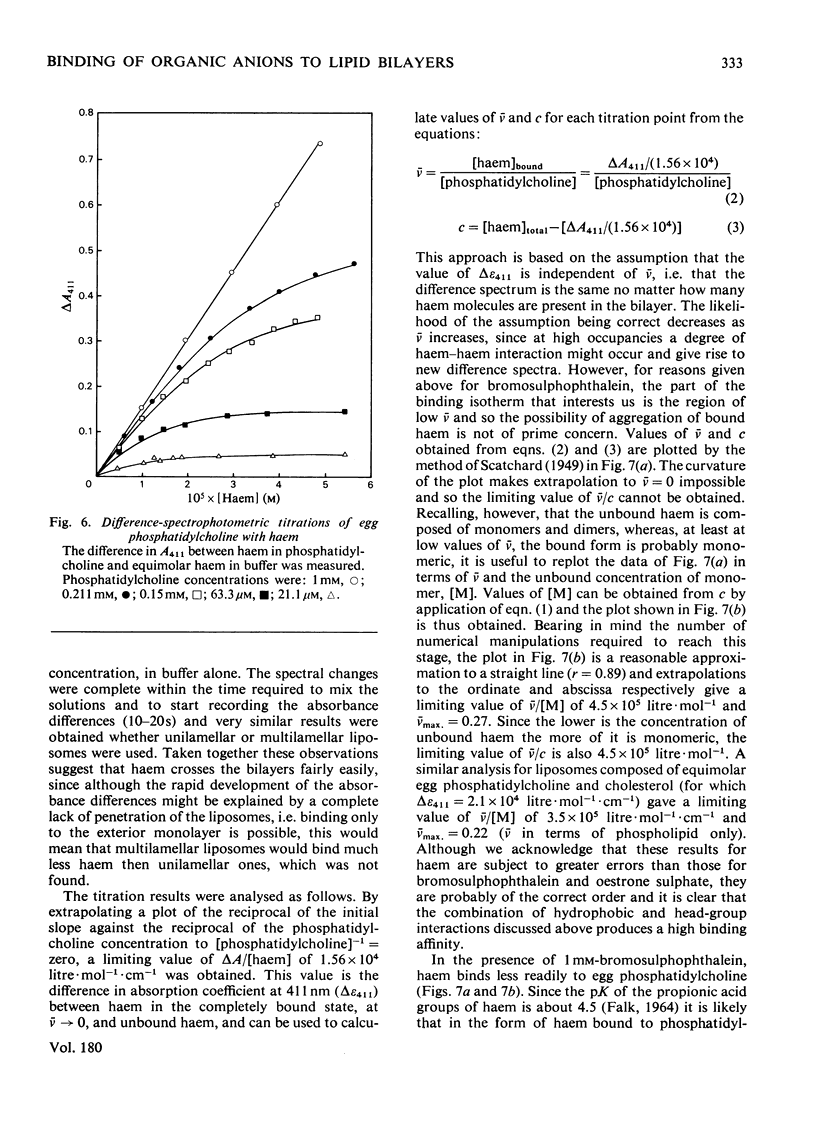
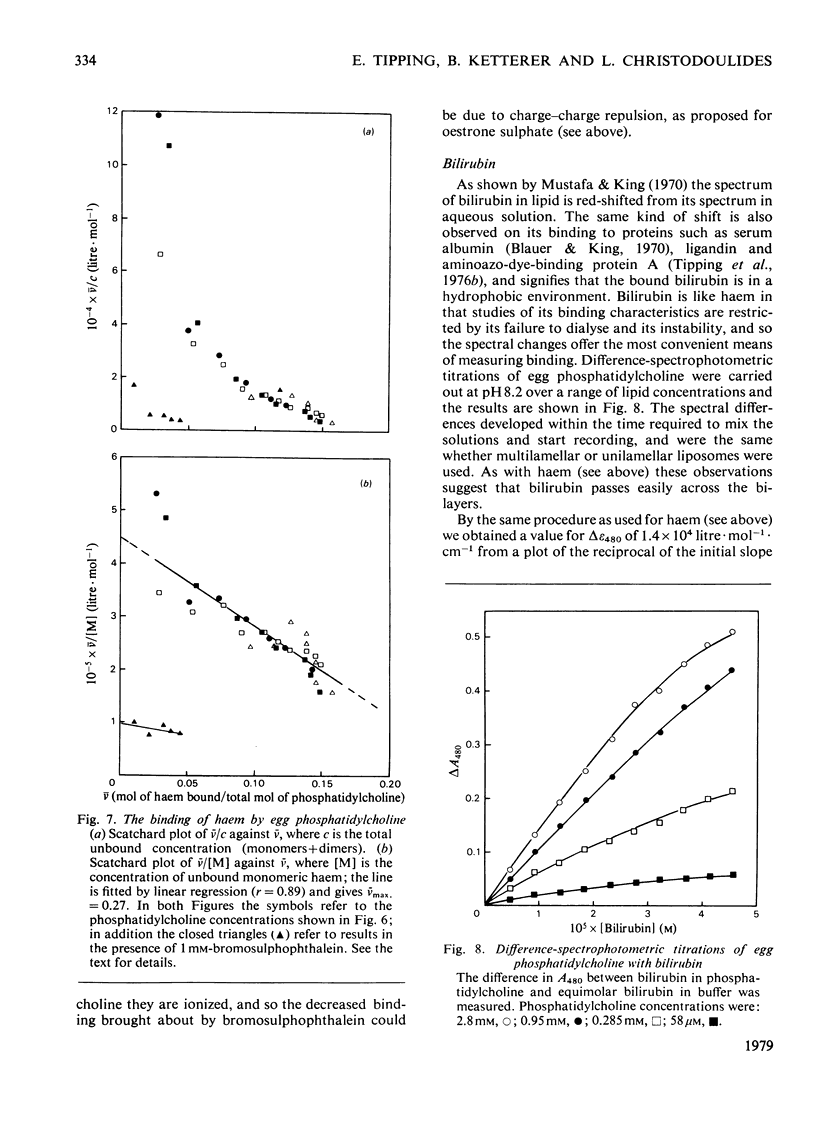
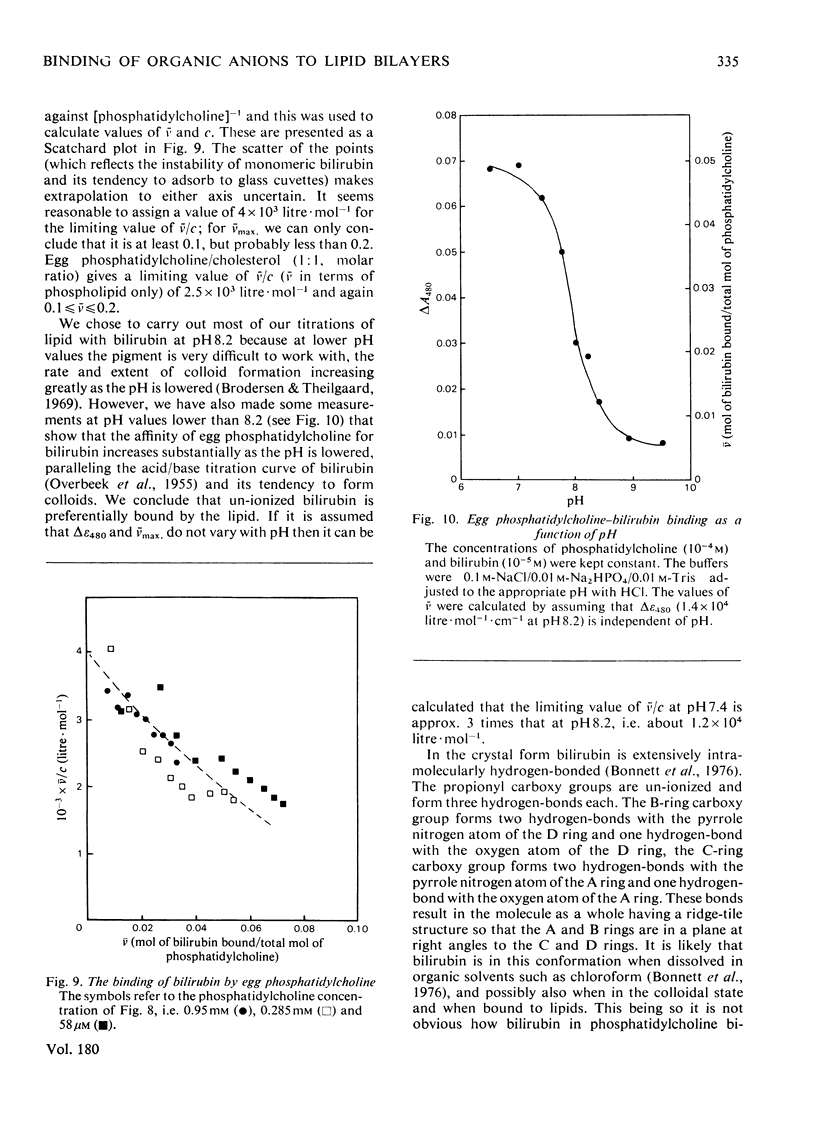
1. To assess the possible involvement of ligandin and aminoazo-dye-binding protein A in intracellular transport it is necessary to know how their ligands, most of which are molecules with hydrophobic moieties, interact with cellular membranes. To obtain such information we examined the interactions of bromosulphophthalein, oestrone sulphate, haem and bilirubin with aqueous dispersions of egg phosphatidylcholine and egg phosphatidylchone/cholesterol (1:1, molar ratio) by equilibrium dialysis and spectrophotometry. 2. In all four cases, saturation effects were observed. Values of Vmax (v = mol of compound bound/mol of lipid phosphorus) at 25 degrees C were: for bromosulphophthalein, approximately 0.1; for oestrone sulphate, approximately 0.25; for haem, approximately 0.25 (all at pH 7.4); and for bilirubin 0.1--0.2 (at pH 8.2). 3. Limiting values of v/c (c = unbound concentration) as v leads to 0 at 25 degrees C and pH 7.4 are: for bromosulphophthalein, 6.25 x 10(4) litre-mol-1; for oestrone sulphate, 7.8 x 10(2) litre-mol-1; for haem, 4.5 x 10(5) litre-mol-1; and for bilirubin, approximately 1.2 x 10(4) litre-mol-1. For haem the result depends on the assumption that only the monomeric form binds to the lipid. 4. The binding of each compound was decreased by cholesterol; bromosulphophthalein and oestrone sulphate were affected more than haem and bilirubin. 5. Bromosulphophthalein at saturating concentration decreased the limiting values of v/c of the other three compounds by approximately one order of magnitude. 6. By assuming that the interactions with egg phosphatidylcholine resemble those with the phospholipid components of mammalian intracellular membranes the binding data for phosphyatidylcholine, together with data for binding to the intracellular proteins ligandin and aminoazo-dye-binding protein A, enable the subcellular distributions of the four compounds to be estimated. For the rat hepatocyte up to 92, 51, 98 and 47% of the total bromosulphophthalein, oestrone sulphate, haem and bilirubin respectively may be membrane-bound.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaven G. H., Chen S. H., d' Albis A., Gratzer W. B. A spectroscopic study of the haemin--human-serum-albumin system. Eur J Biochem. 1974 Feb 1;41(3):539–546. doi: 10.1111/j.1432-1033.1974.tb03295.x. [DOI] [PubMed] [Google Scholar]
- Blauer G., King T. E. Interactions of bilirubin with bovine serum albumin in aqueous solution. J Biol Chem. 1970 Jan 25;245(2):372–381. [PubMed] [Google Scholar]
- Brodersen R., Theilgaard J. Bilirubin colloid formation in neutral aqueous solution. Scand J Clin Lab Invest. 1969 Dec;24(4):395–398. doi: 10.3109/00365516909080178. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Dean T. C., Jones P. Aggregation of ferrihaems. Dimerization and protolytic equilibria of protoferrihaem and deuteroferrihaem in aqueous solution. Biochem J. 1970 May;117(4):733–739. doi: 10.1042/bj1170733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner J., Skrabal P., Hauser H. Single bilayer vesicles prepared without sonication. Physico-chemical properties. Biochim Biophys Acta. 1976 Dec 2;455(2):322–331. doi: 10.1016/0005-2736(76)90308-4. [DOI] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Listowsky I., Gatmaitan Z., Arias I. M. Interactions of bilirubin and other ligands with ligandin. Biochemistry. 1975 May 20;14(10):2175–2180. doi: 10.1021/bi00681a021. [DOI] [PubMed] [Google Scholar]
- Ketley J. N., Habig W. H., Jakoby W. B. Binding of nonsubstrate ligands to the glutathione S-transferases. J Biol Chem. 1975 Nov 25;250(22):8670–8673. [PubMed] [Google Scholar]
- Ketterer B., Tipping E., Hackney J. F., Beale D. A low-molecular-weight protein from rat liver that resembles ligandin in its binding properties. Biochem J. 1976 Jun 1;155(3):511–521. doi: 10.1042/bj1550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterer B., Tipping E., Meuwissen J., Beale D. Ligandin. Biochem Soc Trans. 1975;3(5):626–630. doi: 10.1042/bst0030626. [DOI] [PubMed] [Google Scholar]
- Mustafa M. G., King T. E. Binding of bilirubin with lipid. A possible mechanism of its toxic reactions in mitochondria. J Biol Chem. 1970 Mar 10;245(5):1084–1089. [PubMed] [Google Scholar]
- Nagaoka S., Cowger M. L. Interaction of bilirubin with lipids studied by fluorescence quenching method. J Biol Chem. 1978 Mar 25;253(6):2005–2011. [PubMed] [Google Scholar]
- Simplicio J. Hemin monomers in micellar sodium lauryl sulfate. A spectral and equilibrium study with cyanide. Biochemistry. 1972 Jun 20;11(13):2525–2528. doi: 10.1021/bi00763a022. [DOI] [PubMed] [Google Scholar]
- Tipping E., Ketterer B., Christodoulides L., Enderby G. Spectroscopic studies of the binding of bilirubin by ligandin and aminoazo-dye-binding protein A. Biochem J. 1976 Jul 1;157(1):211–216. doi: 10.1042/bj1570211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping E., Ketterer B., Christodoulides L., Enderby G. The interactions of haem with ligandin and aminoazo-dye-binding protein A. Biochem J. 1976 Aug 1;157(2):461–467. doi: 10.1042/bj1570461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping E., Ketterer B., Christodoulides L., Enderby G. The non-convalent binding of small molecules by ligandin. Interactions with steroids and their conjugates, fatty acids, bromosulphophthalein carcinogens, glutathione and realted compounds. Eur J Biochem. 1976 Aug 16;67(2):583–590. doi: 10.1111/j.1432-1033.1976.tb10724.x. [DOI] [PubMed] [Google Scholar]
- Tipping E., Ketterer B., Christodoulides L. Interactions of small molecules with phospholipid bilayers. Binding to egg phosphatidylcholine of some uncharged molecules (2-acetylaminofluorene, 4-dimethylaminoazobenzene, oestrone and testosterone) that bind to ligandin and aminoazo-dye-binding protein A. Biochem J. 1979 May 15;180(2):319–326. doi: 10.1042/bj1800319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping E., Ketterer B., Koskelo P. The binding of porphyrins by ligandin. Biochem J. 1978 Mar 1;169(3):509–516. doi: 10.1042/bj1690509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkooi J. M., Callis J. B. Pyrene. A probe of lateral diffusion in the hydrophobic region of membranes. Biochemistry. 1974 Sep 10;13(19):4000–4006. doi: 10.1021/bi00716a028. [DOI] [PubMed] [Google Scholar]


