Abstract
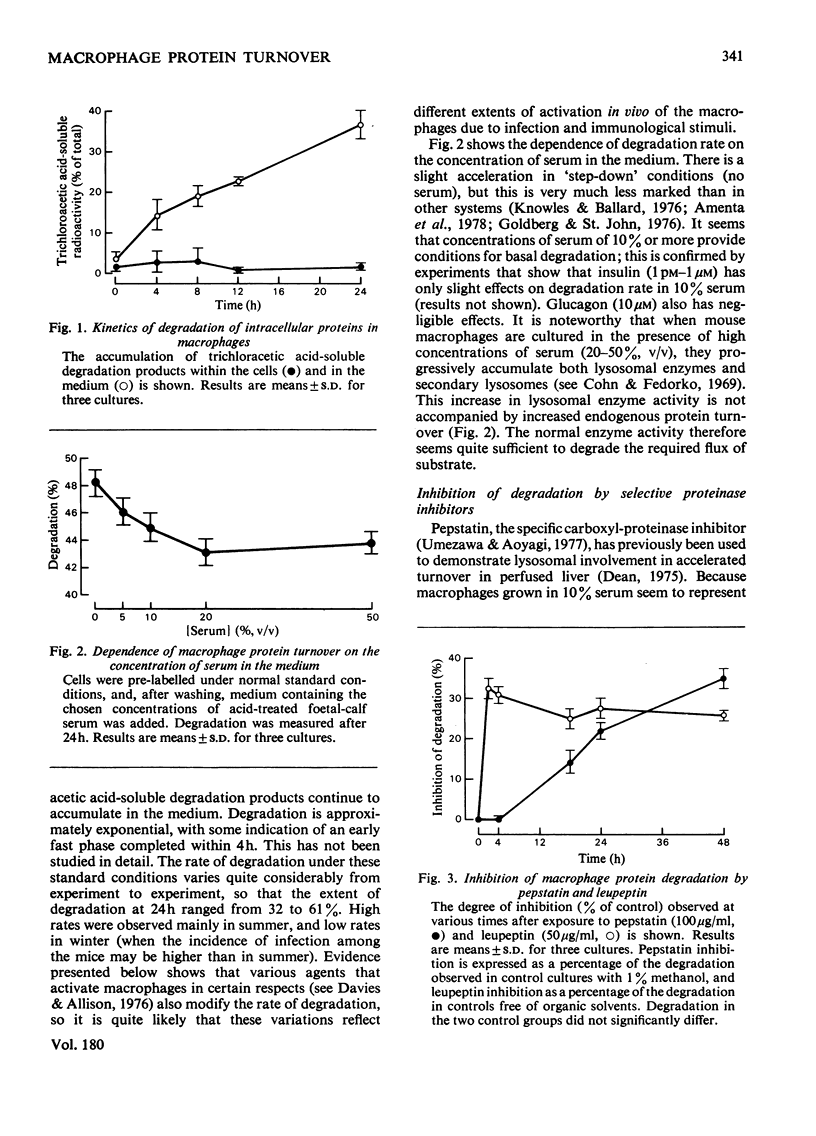
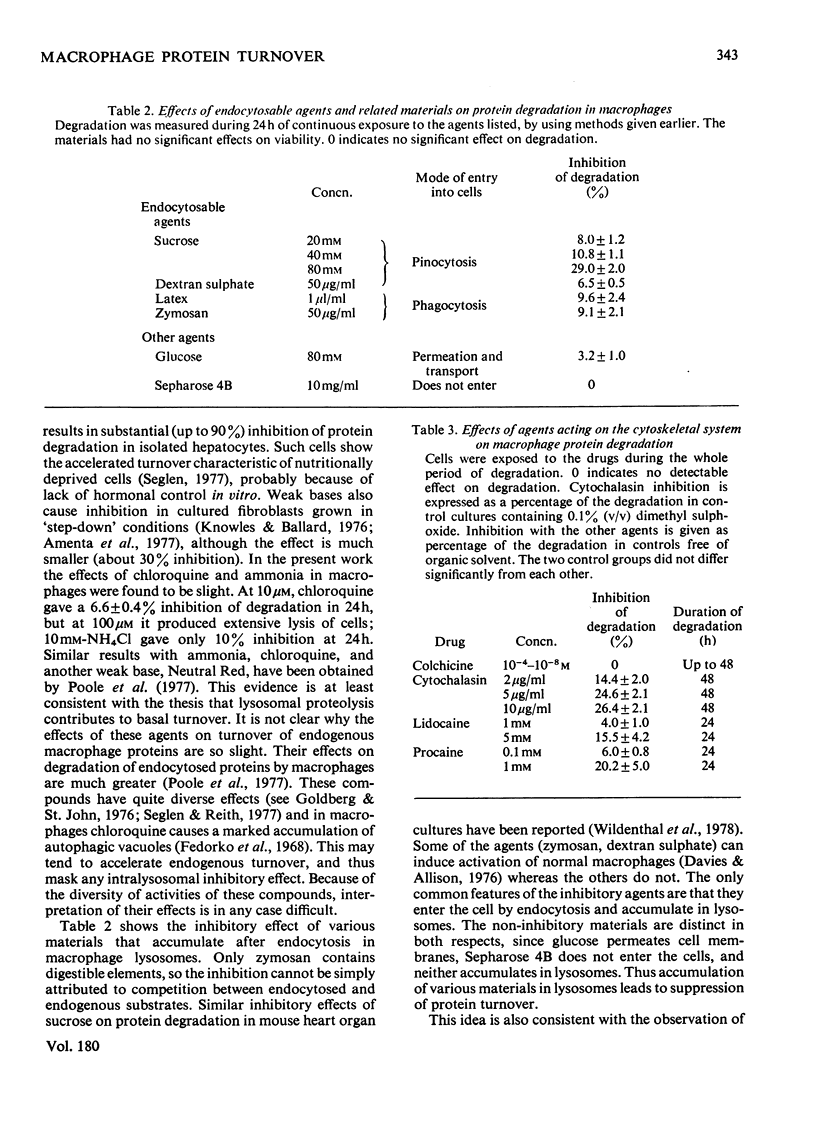
1. Turnover of intracellular proteins in cultured mouse macrophages was found to be slightly accelerated by the omission of serum from the culture medium. Media containing 10% (v/v) or more of serum established basal degradation rates in the cultures. 2. Basal degradation rates varied considerably between experiments, probably as a result of variable activation in vivo of the macrophages. 3. The selective carboxyl proteinase inhibitor pepstatin, which appeared to enter the lysosomes of the cells by pinocytosis, gave a progressive inhibition of basal proteolysis up to a maximum of about 40%. Cellular cathepsin D was largely inhibited after 48h of cultivation with pepstatin (100 micrograms/ml). 4. Leupeptin and 7-amino-1-chloro-3-tosylamidoheptan-2-one are less selective proteinase inhibitors. They also induced 25--35% inhibition of degradation, but their actions may not have been restricted to lysosomes. 5. Several solutes and particles that are endocytosed by macrophages and stored in lysosomes induce some inhibition of basal proteolysis, whether or not they themselves are substrates for proteolysis. 6. Colchicine was without effect on protein degradation, but cytochalasin B and the local anesthetics lidocaine and procaine, all of which have effects on microfilaments, were significantly inhibitory. This inhibition may result from a decrease in the rate of autophagy, and thus of lysosomal proteolysis, due to prevention of microfilament action.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amenta J. S., Sargus M. J., Baccino F. M. Effect of microtubular or translational inhibitors on general cell protein degradation. Evidence for a dual catabolic pathway. Biochem J. 1977 Nov 15;168(2):223–227. doi: 10.1042/bj1680223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta J. S., Sargus M. J., Venkatesan S., Shinozuka H. Role of the vacuolar apparatus in augmented protein degradation in cultured fibroblasts. J Cell Physiol. 1978 Jan;94(1):77–86. doi: 10.1002/jcp.1040940110. [DOI] [PubMed] [Google Scholar]
- Ballard F. J. Intracellular protein degradation. Essays Biochem. 1977;13:1–37. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Allison A. C., Haswell A. D. The quantitative estimation of pinocytosis using radioactive colloidal gold. Biochem Biophys Res Commun. 1973 May 15;52(2):627–634. doi: 10.1016/0006-291x(73)90759-6. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Barrett A. J. Lysosomes. Essays Biochem. 1976;12:1–40. [PubMed] [Google Scholar]
- Dean R. T. Direct evidence of importance of lysosomes in degradation of intracellular proteins. Nature. 1975 Oct 2;257(5525):414–416. doi: 10.1038/257414a0. [DOI] [PubMed] [Google Scholar]
- Dean R. T. Lysosomes and protein degradation. Acta Biol Med Ger. 1977;36(11-12):1815–1820. [PubMed] [Google Scholar]
- Dunn W. A., Aronson N. N., Jr Inhibition of glycoprotein catabolism in vivo and in the perfused rat liver. Acta Biol Med Ger. 1977;36(11-12):1917–1921. [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. 5'-Nucleotidase activity of mouse peritoneal macrophages. II. Cellular distribution and effects of endocytosis. J Exp Med. 1976 Dec 1;144(6):1596–1608. doi: 10.1084/jem.144.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson P. J., Zwiebel R., Cohn Z. A. The pinocytic rate of activated macrophages. J Exp Med. 1975 Nov 1;142(5):1150–1164. doi: 10.1084/jem.142.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorko M. E., Hirsch J. G., Cohn Z. A. Autophagic vacuoles produced in vitro. I. Studies on cultured macrophages exposed to chloroquine. J Cell Biol. 1968 Aug;38(2):377–391. doi: 10.1083/jcb.38.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leary R., Shaw E. Inactivation of cathepsin B1 by diazomethyl ketones. Biochem Biophys Res Commun. 1977 Dec 7;79(3):926–931. doi: 10.1016/0006-291x(77)91199-8. [DOI] [PubMed] [Google Scholar]
- Poole B., Ohkuma S., Warburton M. J. The accumulation of weakly basic substances in lysosomes and the inhibition of intracellular protein degradation. Acta Biol Med Ger. 1977;36(11-12):1777–1788. [PubMed] [Google Scholar]
- Poste G., Papahadjopoulos D., Nicolson G. L. Local anesthetics affect transmembrane cytoskeletal control of mobility and distribution of cell surface receptors. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4430–4434. doi: 10.1073/pnas.72.11.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Effects of amino acids, ammonia and leupeptin on protein synthesis and degradation in isolated rat hepatocytes. Biochem J. 1978 Aug 15;174(2):469–474. doi: 10.1042/bj1740469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Protein-catabolic stage of isolated rat hepatocytes. Biochim Biophys Acta. 1977 Jan 24;496(1):182–191. doi: 10.1016/0304-4165(77)90126-x. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Reith A. Ammonia inhibits protein secretion in isolated rat hepatocytes. Biochim Biophys Acta. 1977 Jan 24;496(1):29–35. doi: 10.1016/0304-4165(77)90112-x. [DOI] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenthal K., Wakeland J. R., Morton P. C., Griffin E. E. Inhibition of protein degradation in mouse hearts by agents that cause lysosomal dysfunction. Circ Res. 1978 Jun;42(6):787–792. doi: 10.1161/01.res.42.6.787. [DOI] [PubMed] [Google Scholar]


