Abstract
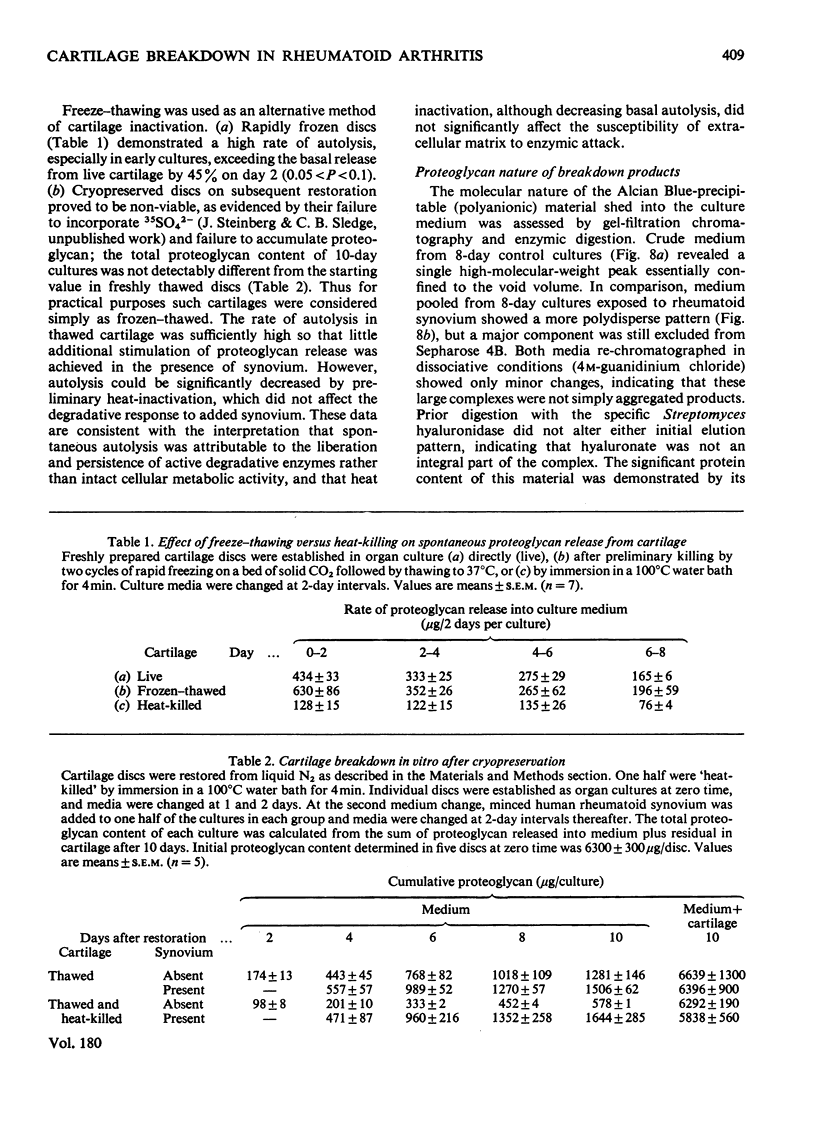
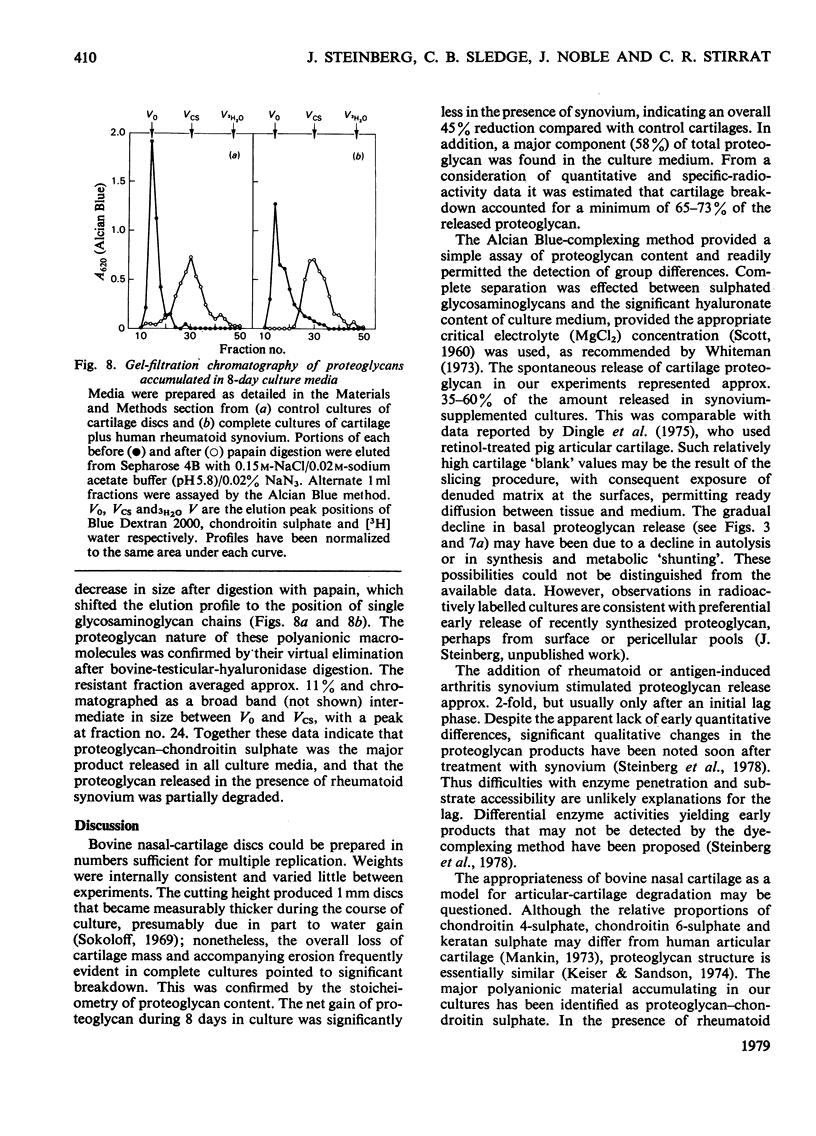
1. The destruction of articular cartilage in human rheumatoid and other arthritides is the result of diverse mechanical, inflammatory and local cellular factors. A tissue-culture model for studying cartilage–synovial interactions that may be involved in the final common pathway of joint destruction is described. 2. Matrix breakdown was studied in vitro by using bovine nasal-cartilage discs cultivated in contact with synovium. Synovia were obtained from human and animal sources. Human tissue came from patients with `classical' rheumatoid arthritis, and animal tissue from rabbits with antigen-induced arthritis. 3. Cartilage discs increased their proteoglycan content 2–3-fold during 8 days in culture. Proteoglycan was also released into culture medium, approx. 70% arising from cartilage breakdown. 4. Synovial explants from human rheumatoid and rabbit antigen-induced arthritis produced equivalent stimulation of proteoglycan release. After an initial lag phase, the breakdown rate rose abruptly to a maximum, resulting in a 2-fold increase of proteoglycan accumulation in culture medium after 8–10 days. 5. High-molecular-weight products shed into culture media were characterized chromatographically and by differential enzymic digestion. Proteoglycan–chondroitin sulphate accounted for 90% of the released polyanion, and its partial degradation in the presence of synovial explants was consistent with limited proteolytic cleavage. 6. Rheumatoid synovium applied to dead cartilage increased the basal rate of proteoglycan release. Living cartilage was capable of more extensive autolysis, even in the absence of synovium. However, optimal proteoglycan release required the interaction of living synovium with live cartilage. These findings support the view that a significant component of cartilage breakdown may be chondrocyte-mediated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y., Evans L. Enzymic degradation of cartilage in osteoarthritis. Fed Proc. 1973 Apr;32(4):1494–1498. [PubMed] [Google Scholar]
- Barratt M. E. The role of soft connective tissue in the response of pig articular cartilage in organ culture to excess of retinol. J Cell Sci. 1973 Jul;13(1):205–219. doi: 10.1242/jcs.13.1.205. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Horsfield P., Fell H. B., Barratt M. E. Breakdown of proteoglycan and collagen induced in pig articular cartilage in organ culture. Ann Rheum Dis. 1975 Aug;34(4):303–311. doi: 10.1136/ard.34.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M. G., Mankin H. J., Jones H., Wright R., Crispen C., Vigliani G. Collagenase and collagenase inhibitors in osteoarthritic and normal cartilage. J Clin Invest. 1977 Feb;59(2):226–233. doi: 10.1172/JCI108632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell H. B., Barratt M. E. The role of soft connective tissue in the breakdown of pig articular cartilage cultivated in the presence of complement-sufficient antiserum to pig erythrocytes. I. Histological changes. Int Arch Allergy Appl Immunol. 1973;44(3):441–468. doi: 10.1159/000230951. [DOI] [PubMed] [Google Scholar]
- Fell H. B., Jubb R. W. The effect of synovial tissue on the breakdown of articular cartilage in organ culture. Arthritis Rheum. 1977 Sep-Oct;20(7):1359–1371. doi: 10.1002/art.1780200710. [DOI] [PubMed] [Google Scholar]
- HOLLANDER J. L., MCCARTY D. J., Jr, ASTORGA G., CASTRO-MURILLO E. STUDIES ON THE PATHOGENESIS OF RHEUMATOID JOINT INFLAMMATION. I. THE "R.A. CELL" AND A WORKING HYPOTHESIS. Ann Intern Med. 1965 Feb;62:271–280. doi: 10.7326/0003-4819-62-2-271. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Glauert A. M., Murley A. H. Intracellular collagen fibers at the pannus-cartilage junction in rheumatoid arthritis. Arthritis Rheum. 1977 Mar;20(2):657–665. doi: 10.1002/art.1780200204. [DOI] [PubMed] [Google Scholar]
- Janoff A., Feinstein G., Malemud C. J., Elias J. M. Degradation of cartilage proteoglycan by human leukocyte granule neutral proteases--a model of joint injury. I. Penetration of enzyme into rabbit articular cartilage and release of 35SO4-labeled material from the tissue. J Clin Invest. 1976 Mar;57(3):615–624. doi: 10.1172/JCI108317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser H., Greenwald R. A., Feinstein G., Janoff A. Degradation of cartilage proteoglycan by human leukocyte granule neutral proteases--a model of joint injury. II. Degradation of isolated bovine nasal cartilage proteoglycan. J Clin Invest. 1976 Mar;57(3):625–632. doi: 10.1172/JCI108318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser H., Sandson J. I. Immunodiffusion and gel-electrophoretic studies of human articular cartilage proteoglycan. Arthritis Rheum. 1974 May-Jun;17(3):219–228. doi: 10.1002/art.1780170304. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 1975 Sep-Oct;18(5):475–483. doi: 10.1002/art.1780180507. [DOI] [PubMed] [Google Scholar]
- Mankin H. J. Biochemical and metabolic abnormalities in osteoarthritic human cartilage. Fed Proc. 1973 Apr;32(4):1478–1480. [PubMed] [Google Scholar]
- Marshall J. L., Fraser J. R., Muirden K. D. Lysosomal activation by neutral saccharides in cell cultures of synovium. Ann Rheum Dis. 1977 Apr;36(2):130–138. doi: 10.1136/ard.36.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirden K. D. Lysosomal enzymes in synovial membrane in rheumatoid arthritis. Relationship to joint damage. Ann Rheum Dis. 1972 Jul;31(4):265–271. doi: 10.1136/ard.31.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya T., Kaneko Y. Novel hyaluronidase from streptomyces. Biochim Biophys Acta. 1970 Mar 18;198(3):607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Sapolsky A. I., Howell D. S., Woessner J. F., Jr Neutral proteases and cathepsin D in human articular cartilage. J Clin Invest. 1974 Apr;53(4):1044–1053. doi: 10.1172/JCI107641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky A. I., Keiser H., Howell D. S., Woessner J. F., Jr Metalloproteases of human articular cartilage that digest cartilage proteoglycan at neutral and acid pH. J Clin Invest. 1976 Oct;58(4):1030–1041. doi: 10.1172/JCI108526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Dorling J. Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochemie. 1965 Oct 1;5(3):221–233. doi: 10.1007/BF00306130. [DOI] [PubMed] [Google Scholar]
- Sledge C. B., Noble J., Hnatowich D. J., Kramer R., Shortkroff S. Experimental radiation synovectomy by 165Dy ferric hydroxide macroaggregate. Arthritis Rheum. 1977 Sep-Oct;20(7):1334–1342. doi: 10.1002/art.1780200706. [DOI] [PubMed] [Google Scholar]
- Waxman B. A., Sledge C. B. Correlation of histochemical, histologic and biochemical evaluations of human synovium with clinical activity. Arthritis Rheum. 1973 May-Jun;16(3):376–382. doi: 10.1002/art.1780160313. [DOI] [PubMed] [Google Scholar]
- Whiteman P. The quantitative measurement of Alcian Blue-glycosaminoglycan complexes. Biochem J. 1973 Feb;131(2):343–350. doi: 10.1042/bj1310343. [DOI] [PMC free article] [PubMed] [Google Scholar]





