Abstract
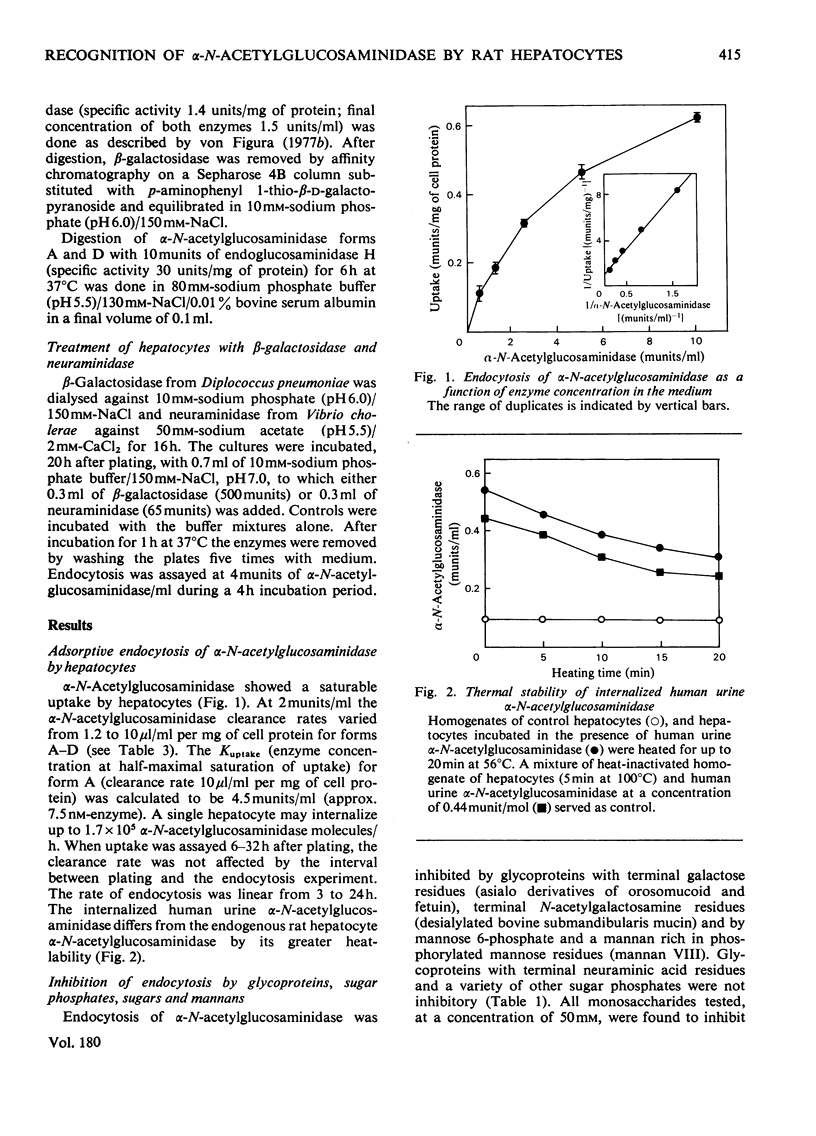
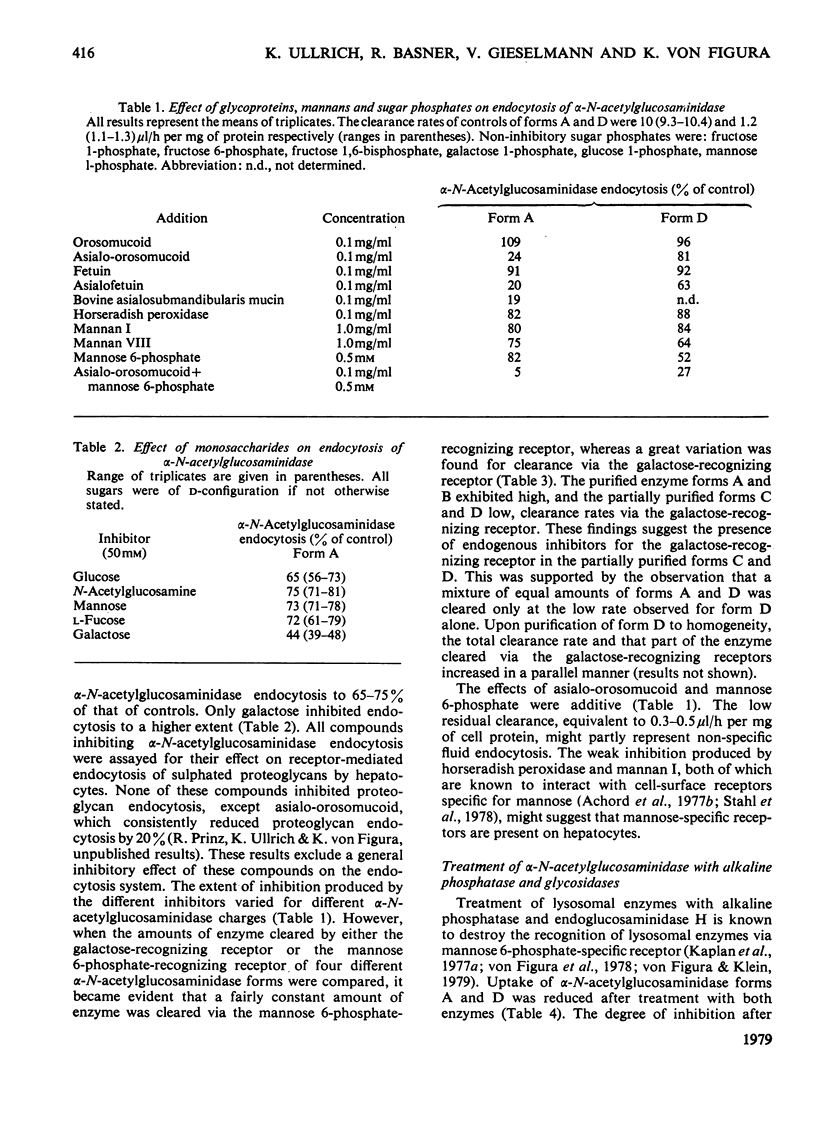
Adsorptive endocytosis of alpha-N-acetylglucosaminidase from human urine by isolated rat hepatocytes is inhibited by glycoproteins, polysaccharides and sugars that are known to bind to cell-surface receptors specific for either terminal galactose/N-acetylgalactosamine residues, terminal mannose residues or mannose 6-phosphate residues. Recognition of alpha-N-acetylglucosaminidase by a cell-surface receptor specific for terminal galactose/N-acetylgalactosamine residues is supported by the observations (a) that neuraminidase pretreatment of the enzyme enhances endocytosis, (b) that beta-galactosidase treatment decreases endocytosis and (c) that neuraminidase pretreatment of hepatocytes decreases alpha-N-acetylglucosaminidase endocytosis. Recognition of alpha-N-acetylglucosaminidase via receptors recognizing mannose 6-phosphate residues is lost after treatment of the enzyme with alkaline phosphatase and endoglucosaminidase H. The effect of endoglucosaminidase H supports the view that the mannose 6-phosphate residues reside in N-glycosidically linked oligosaccharide side chains of the high-mannose type. The weak inhibition of endocytosis produced by compounds known to interact with cell-surface receptors specific for mannose residues suggests that this recognition system plays only a minor role in the endocytosis of lysosomal alpha-N-acetylglucosaminidase by hepatocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D. T., Brot F. E., Bell C. E., Sly W. S. Human beta-glucuronidase: in vivo clearance and in vitro uptake by a glycoprotein recognition system on reticuloendothelial cells. Cell. 1978 Sep;15(1):269–278. doi: 10.1016/0092-8674(78)90102-2. [DOI] [PubMed] [Google Scholar]
- Achord D. T., Brot F. E., Sly W. S. Inhibition of the rat clearance system for agalacto-orosomucoid by yeast mannans and by mannose. Biochem Biophys Res Commun. 1977 Jul 11;77(1):409–415. doi: 10.1016/s0006-291x(77)80213-1. [DOI] [PubMed] [Google Scholar]
- Achord D., Brot F., Gonzalez-Noriega A., Sly W., Stahl P. Human beta-glucuronidase. II. Fate of infused human placental beta-glucuronidase in the rat. Pediatr Res. 1977 Jul;11(7):816–822. doi: 10.1203/00006450-197707000-00008. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Baynes J. W., Wold F. Effect of glycosylation on the in vivo circulating half-life of ribonuclease. J Biol Chem. 1976 Oct 10;251(19):6016–6024. [PubMed] [Google Scholar]
- Bearpark T., Stirling J. L. Clearance of human N-acetyl-beta-hexosaminidases from rat circulation. Biochem J. 1977 Dec 15;168(3):435–439. doi: 10.1042/bj1680435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. L., Henderson L. A., Thorpe S. R., Baynes J. W. The effect of alpha-mannose-terminal oligosaccharides on the survival of glycoproteins in the circulation. Rapid uptake and catabolism of bovine pancreatic ribonuclease B by nonparenchymal cells of rat liver. Arch Biochem Biophys. 1978 Jun;188(2):418–428. doi: 10.1016/s0003-9861(78)80026-5. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fiddler M. B., Desnick R. J. Enzyme therapy. Differential in vivo retention of bovine hepatic, renal, and splenic beta-glucuronidases and evidence for enzyme stabilization by intermolecular exchange. Arch Biochem Biophys. 1977 Mar;179(2):397–408. doi: 10.1016/0003-9861(77)90127-8. [DOI] [PubMed] [Google Scholar]
- Furbish F. S., Steer C. J., Barranger J. A., Jones E. A., Brady R. O. The uptake of native and desialylated glucocerebrosidase by rat hepatocytes and Kupffer cells. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1047–1053. doi: 10.1016/0006-291x(78)91456-0. [DOI] [PubMed] [Google Scholar]
- HUGHES R. C., JEANLOZ R. W. THE EXTRACELLULAR GLYCOSIDASES OF DIPLOCOCCUS PNEUMONIAE. I. PURIFICATION AND PROPERTIES OF A NEURAMINIDASE AND A BETA-GALACTOSIDASE. ACTION ON THE ALPHA-1-ACID GLYCOPROTEIN OF HUMAN PLASMA. Biochemistry. 1964 Oct;3:1535–1543. doi: 10.1021/bi00898a025. [DOI] [PubMed] [Google Scholar]
- HUGHES R. C., JEANLOZ R. W. THE EXTRACELLULAR GLYCOSIDASES OF DIPLOCOCCUS PNEUMONIAE. II. PURIFICATION AND PROPERTIES OF A BETA-N-ACETYLGLUCOSAMINIDASE. ACTION ON A DERIVATIVE ON THE ALPHA-1-ACID GLYCOPROTEIN OF HUMAN PLASMA. Biochemistry. 1964 Oct;3:1543–1548. doi: 10.1021/bi00898a026. [DOI] [PubMed] [Google Scholar]
- Iino N., Yoshida K. An improved method for the syntheses of p-aminophenyl 1-thio-beta-D-glycosides. Carbohydr Res. 1976 Nov;51(2):223–228. doi: 10.1016/s0008-6215(00)83329-7. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Isolation and characterization of an avian hepatic binding protein specific for N-acetylglucosamine-terminated glycoproteins. J Biol Chem. 1977 Sep 25;252(18):6536–6543. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marinkovic D. V., Petrovic S. L., Martinovic J. V., Marinkovic J. N. Uptake of iodinated human kidney alpha-D-mannosidase by rat liver- Association with membrane elements and stability in vivo and in vitro. Biochem J. 1976 Dec 1;159(3):729–736. doi: 10.1042/bj1590729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieels J. P., Pizzo S. V., Glasgow L. R., Paulson J. C., Hill R. L. Hepatic receptor that specifically binds oligosaccharides containing fucosyl alpha1 leads to 3 N-acetylglucosamine linkages. Proc Natl Acad Sci U S A. 1978 May;75(5):2215–2219. doi: 10.1073/pnas.75.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando G. N., Neufeld E. F. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- Schlesinger P., Rodman J. S., Frey M., Lang S., Stahl P. Clearance of lysosomal hydrolases following intravenous infusion. The role of liver in the clearance of beta-glucuronidase and N-acetyl-beta-D-glucosaminidase. Arch Biochem Biophys. 1976 Dec;177(2):606–614. doi: 10.1016/0003-9861(76)90472-0. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Rodman J. S., Doebber T. Recognition of lysosomal glycosidases in vivo inhibited by modified glycoproteins. Nature. 1976 Nov 4;264(5581):86–88. doi: 10.1038/264086a0. [DOI] [PubMed] [Google Scholar]
- Stahl P., Six H., Rodman J. S., Schlesinger P., Tulsiani D. R., Touster O. Evidence for specific recognition sites mediating clearance of lysosomal enzymes in vivo. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4045–4049. doi: 10.1073/pnas.73.11.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer C. J., Furbish F. S., Barranger J. A., Brady R. O., Jones E. A. The uptake of agalacto-glucocerebrosidase by rat hepatocytes and Kupffer cells. FEBS Lett. 1978 Jul 15;91(2):202–205. doi: 10.1016/0014-5793(78)81172-7. [DOI] [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. Hepatic binding protein: the protective role of its sialic acid residues. Science. 1977 Aug 12;197(4304):667–668. doi: 10.1126/science.877581. [DOI] [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. The existence of a second route for the transfer of certain glycoproteins from the circulation into the liver. Biochem Biophys Res Commun. 1976 Feb 9;68(3):988–993. doi: 10.1016/0006-291x(76)91243-2. [DOI] [PubMed] [Google Scholar]
- Thorpe S. R., Fiddler M. B., Desnick R. J. Enzyme therapy IV. A method for determining the in vivo fate of bovine beta-glucuronidase in beta-glucuronidase deficient mice. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1464–1470. doi: 10.1016/s0006-291x(74)80448-1. [DOI] [PubMed] [Google Scholar]
- Ullrich K., Mersmann G., Fleischer M., von Figura K. Epithelial rat liver cells have cell surface receptors recognizing a phosphorylated carbohydrate on lysosomal enzymes. Hoppe Seylers Z Physiol Chem. 1978 Nov;359(11):1591–1598. doi: 10.1515/bchm2.1978.359.2.1591. [DOI] [PubMed] [Google Scholar]
- Ullrich K., Mersmann G., Weber E., Von Figura K. Evidence for lysosomal enzyme recognition by human fibroblasts via a phosphorylated carbohydrate moiety. Biochem J. 1978 Mar 15;170(3):643–650. doi: 10.1042/bj1700643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelhake J. L., Nicolson G. L. Aglycosylantibody. Effects of exoglycosidase treatments on autochthonous antibody survival time in the circulation. J Biol Chem. 1976 Feb 25;251(4):1074–1080. [PubMed] [Google Scholar]
- Winkelhake J. L., Nicolson G. L. Preparation of protease-free neuraminidase by affinity adsorption on fetuin-derivatized cellulose. Anal Biochem. 1976 Mar;71(1):281–289. doi: 10.1016/0003-2697(76)90037-3. [DOI] [PubMed] [Google Scholar]
- von Figura K. Human alpha-N-acetylglucosaminidase. 1. Purification and properties. Eur J Biochem. 1977 Nov 1;80(2):523–533. [PubMed] [Google Scholar]
- von Figura K. Human alpha-n-acetylglucosaminidase. 2. Activity towards natural substrates and multiple recognition forms. Eur J Biochem. 1977 Nov 1;80(2):535–542. doi: 10.1111/j.1432-1033.1977.tb11909.x. [DOI] [PubMed] [Google Scholar]


