Abstract
Background.
Clopidogrel is an inactive pro-drug; it is converted to its active metabolite via the cytochrome P450 (CYP3A4) pathway, which also metabolizes calcium channel blocker (CCBs). Several studies have reported that CCBs reduce clopidogrel’s ability to inhibit platelet aggregability; one suggested that CCBs reduce the efficacy of clopidogrel.
Methods and Results.
We performed a post hoc analysis of the Clopidogrel for the Reduction of Events During Observation (CREDO) study to compare the treatment effect of clopidogrel in patients on CCBs versus not on CCBs. In CREDO, 2,116 patients were randomized to pretreatment with clopidogrel 300 mg 3-24 hours before a planned percutaneous coronary intervention followed by one year of clopidogrel 75 mg per day, vs. clopidogrel 75 mg at the time of the procedure and continued for just 28 days. The primary endpoints were a combined endpoint of death, MI, stroke at 28 days and one year. Among the 580 patients (27%) on CCBs at enrollment, at 28 days, the combined endpoint was reached in 17 patients (6%) on clopidogrel vs. 28 (9%) on placebo (hazard ratio [HR] 0.71, 95% confidence intervals [CI] 0.39-1.29). At 1 year, the combined endpoint was reached in 27 patients (10%) on clopidogrel vs. 46 (15%) on placebo (HR 0.68, 95% CI 0.42-1.09). Clopidogrel’s treatment effect in patients on CCBs was actually greater than in patients not on CCBs at one year (HR 0.78, 95% CI 0.56-1.09). After adjustment for differences between patients on and not on CCB, there was still no evidence of an interaction between clopidogrel treatment and CCB (HR for patients not on CCB 0.87, 95% CI, 0.62 to 1.23; HR for patients on CCB 0.74, 95% CI, 0.45 to 1.21).
Conclusion.
In CREDO, there was no evidence that CCBs decrease the efficacy of clopidogrel.
Keywords: angioplasty, drugs, platelets, stents, thrombosis
Introduction
In patients with cardiovascular disease, antiplatelet medications are generally administered in addition to disease modifying agents, most commonly antihypertensive and lipid-lowering agents. Thus, the possibility of drug-drug interactions exist.
Clopidogrel has become widely utilized due to its beneficial effects in patients receiving coronary, carotid or peripheral arterial stents, acute coronary syndromes and stable vascular disease. It is an inactive pro-drug which requires conversion to its active metabolite in the liver. It is believed this occurs in part through the cytochrome P450 3A4 (CYP3A4) pathway. The active metabolite inhibits platelet activation and recruitment by blocking the adenosine diphosphate (ADP) P2Y12 receptor (1).
Calcium channel blockers (CCBs) are often also used in patients with cardiovascular disease for the treatment of hypertension, angina, atrial fibrillation, other arrhythmia, and other indications as well. All CCBs are believed to be primarily metabolized by the same cytochrome P450 3A system. Ex vivo studies of platelet function suggest that CCBs reduce the conversion of the clopidogrel pro-drug to its active metabolite, therefore reducing clopidogrel’s ability to inhibit platelet aggregation (2,3). The clinical significance of this laboratory assessment of platelet activity, however, remains unclear. In the only study performed to determine whether there existed a clinically significant interaction between clopidogrel and CCBs, patients on both agents had a higher frequency of the combined endpoint of death, myocardial infarction, and need for a revascularization procedure in the 6 months after a percutaneous coronary intervention (PCI) as compared to patients on clopidogrel alone, even after adjustment for differences between the two agents (adjusted hazard ratio 3.5; 95% confidence intervals 1.4 to 8.6, p=0.005) (2).
Methods.
The full details of the design, methods and findings for the CREDO trial have previously been published (4). Briefly, CREDO was a prospective, multicenter, double-blind, randomized, placebo-controlled trial comparing two dosage regimens of clopidogrel among patient planned for PCI: a 300 mg loading dose administered 3 to 24 hours prior to PCI followed by 75 mg daily for 1 year, versus 75 mg daily for 28 days without a loading dose. Eligible patients were ≥ 21 years of age with symptomatic coronary artery disease referred for PCI or thought to be at high likelihood for requiring PCI. Exclusion criteria included contraindications to antiplatelet therapy; left main disease; a recent failed PCI; coronary anatomy not amendable to stent placement; persistent ST elevation; a planned staged PCI; receipt of a glycoprotein (GP) IIb/IIIa inhibitor within the past 7 days; clopidogrel within the past 10 days, or thrombolytic therapy within the prior 24 hours. The primary endpoint of the trial was the composite of death, myocardial infarction and a revascularization procedure through one year. Follow up was assessed on days 2, 28, 60, 180, 270 and 365 after randomization.
There were 2,116 patients enrolled in the CREDO trial between June 1999 and April 2001. For the current analysis, we stratified these patients according to whether or not they were on CCB at study entry. We analyzed the combined endpoint of death, myocardial infarction and stroke at 28 days and one year for this study to determine the risk reduction associated with clopidogrel vs. placebo in patients on and not on CCB. We then tested for an interaction between CCBs and clopidogrel.
Statistical Analysis.
Baseline clinical characteristics in patients on CCB and not on CCB at study entry were summarized using percents and chi-square tests. The composite endpoint of death, MI and stroke at 28 days was calculated using percents and compared with chi-square tests. To assess the treatment effect of clopidogrel in patients on and not on CCB, hazard ratios (HR) were calculated.
The composite endpoint of death, MI and stroke through one year was estimated using Kaplan-Meier methods, and log-rank tests were conducted to test for differences. Hazard ratios (HR) and their associated 95% confidence intervals (CI) were calculated using Cox proportional hazards models.
Interactions for treatment of clopidogrel and baseline CCB were assessed in multivariable models. The product term of treatment with clopidogrel and CCB at baseline was created and entered in the model along with the main effects of these variables. Estimates of the treatment effect of clopidogrel (hazard ratios and 95% confidence intervals) were calculated for patients on a CCB and not on a CCB. All p-values < 0.05 are considered statistically significant. No adjustments were made for multiple comparisons. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
Results
Baseline characteristics.
Of the 2,116 patients enrolled in CREDO, 580 patients (27%) were on CCBs and 1536 (73%) patients were not on CCB at the time of enrollment in the trial. Baseline characteristics based on CCB use at study entry are shown in Table 1. Patients on CCB tended to be older, were more commonly female, more frequently had a BMI >30, diabetes mellitus, hypertension, hyperlipidemia, cerebrovascular disease, and peripheral vascular disease. They also had more commonly undergone previous CABG and a prior PCI.
Table 1.
Baseline clinical characteristics of the study population.
| CCB 580 | No CCB 1536 | |||
|---|---|---|---|---|
|
| ||||
| Placebo N (%) | Clopidogrel N (%) | Placebo N (%) | Clopidogrel N (%) | |
| Total number | 312 | 268 | 751 | 785 |
| Age ≥65 years | 151 (48) | 132 (49) | 304 (41) | 305 (39) |
| Male | 212 (68) | 181 (68) | 554 (74) | 563 (72) |
| Body mass index >30 | 151 (49) | 125 (47) | 317 (42) | 329 (42) |
| Smoking within one year | 86 (28) | 74 (28) | 226 (31) | 261 (34) |
| Stable angina | 84 (27) | 82 (31) | 211 (28) | 200 (26) |
| Unstable angina | 182 (59) | 139 (52) | 382 (51) | 414 (53) |
| History of: | ||||
| Hypertension | 272 (87) | 234 (88) | 468 (63) | 476 (61) |
| Hyperlipidemia | 243 (81) | 214 (81) | 555 (76) | 563 (74) |
| CHF | 31 (10) | 23 (9) | 69 (9) | 62 (8) |
| MI | 117 (40) | 82 (32) | 248 (34) | 271 (35) |
| Atrial fibrillation | 14 (5) | 7 (3) | 34 (5) | 41 (5) |
| Diabetes mellitus | 91 (29) | 85 (32) | 179 (24) | 205 (26) |
| Cerebrovascular disease | 34 (11) | 20 (8) | 35 (5) | 37 (5) |
| Peripheral vascular disease | 40 (13) | 34 (13) | 48 (7) | 54 (7) |
| CABG | 61 (20) | 49 (19) | 110 (15) | 115 (15) |
| PCI | 111 (37) | 88 (34) | 205 (28) | 186 (25) |
| Baseline ACE inhibitor | 200 (35) | 92 (34) | 256 (34) | 255 (33) |
| Baseline aspirin | 192 (33) | 88 (33) | 211 (28) | 227 (29) |
| Baseline beta blocker | 331 (57) | 141 (53) | 506 (67) | 523 (67) |
| Baseline statin | 367 (63) | 169 (63) | 411 (55) | 394 (50) |
Clinical Outcome.
Among the 580 patients on CCBs upon enrollment in the trial, 268 (46%) were randomly assigned to pretreatment with a loading dose of clopidogrel and treatment for one year and 312 (54%) received no pretreatment and received clopidogrel for only 28 days. In patients on CCB at study entry, the one year combined endpoint of death, myocardial infarction and stroke was reached in 27 patients (10%) on one year of clopidogrel vs. 46 patients (15%) on placebo (HR 0.68, 95% confidence intervals [CI], 0.42 to1.09) (Figure 1). Among patients not on CCB at study entry, the one year combined endpoint was reached in 62 patients (8%) on clopidogrel vs. 76 (10%) on placebo HR 0.78, 95% CI, 0.56 to 1.09). This primary combined endpoint in patients on CCB at study entry was driven by a large reduction in myocardial infarction (Figure 2). Analysis to determine whether there was an interaction between clopidogrel treatment effect and CCB revealed that there was not a significant interaction (Wald chi-square p=0.64). To eliminate potential confounders, the model was adjusted for the differences between patients on CCB and not on CCB found in Table 1. After adjustments for these variables, the lack of an interaction between clopidogrel treatment and CCB remained (Wald chi-square p=0.58; HR for patients not on CCB 0.87, 95% CI, 0.62 to 1.23; HR for patients on CCB, 0.74, 95% CI, 0.45 to 1.21).
Figure 1.
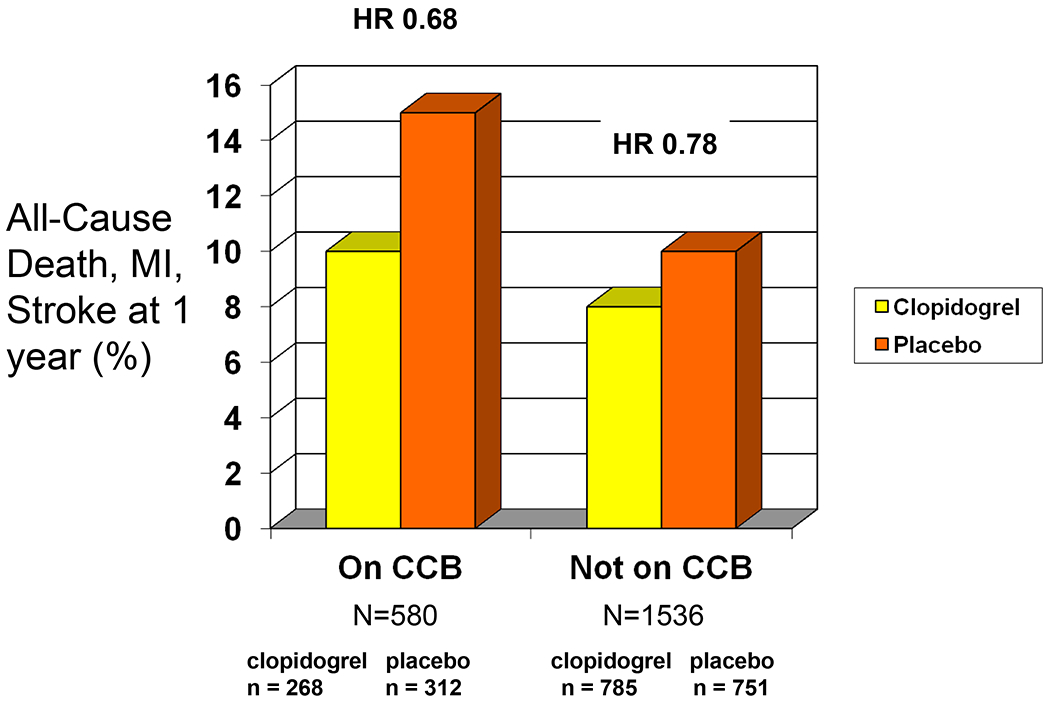
The frequency of death, myocardial infarction and stroke at one year with clopidogrel vs. aspirin in patients on, and not on, a calcium channel blocker at study entry. It can be seen that patients on calcium channel blockers had a higher event rate, but the risk reduction associated with clopidogrel was actually greater in such patients.
Figure 2.
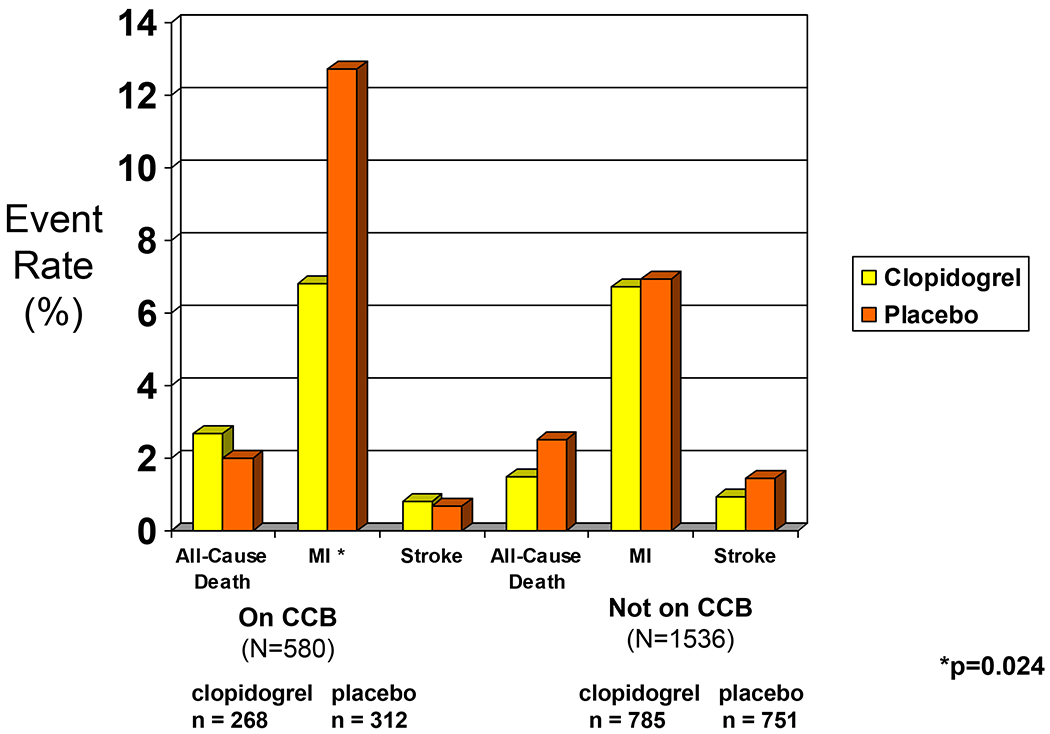
The frequency of the individual components of the combined primary endpoint of the study in patients on clopidogrel vs. aspirin, in patients on, and not on, a calcium channel blocker at study entry.
Among patients on a CCB, the 28 day combined endpoint was reached in 17 patients (6%) on clopidogrel vs. 28 patients (9%) on placebo (HR 0.71, 95% CI, 0.39 to 1.29) (Figure 3). Among those patients not on CCBs, the 28 day combined endpoint was reached in 41 patients (5%) on clopidogrel vs. 45 patients (6%) on placebo (HR 0.87, 95% CI, 0.57 to 1.33).
Figure 3.
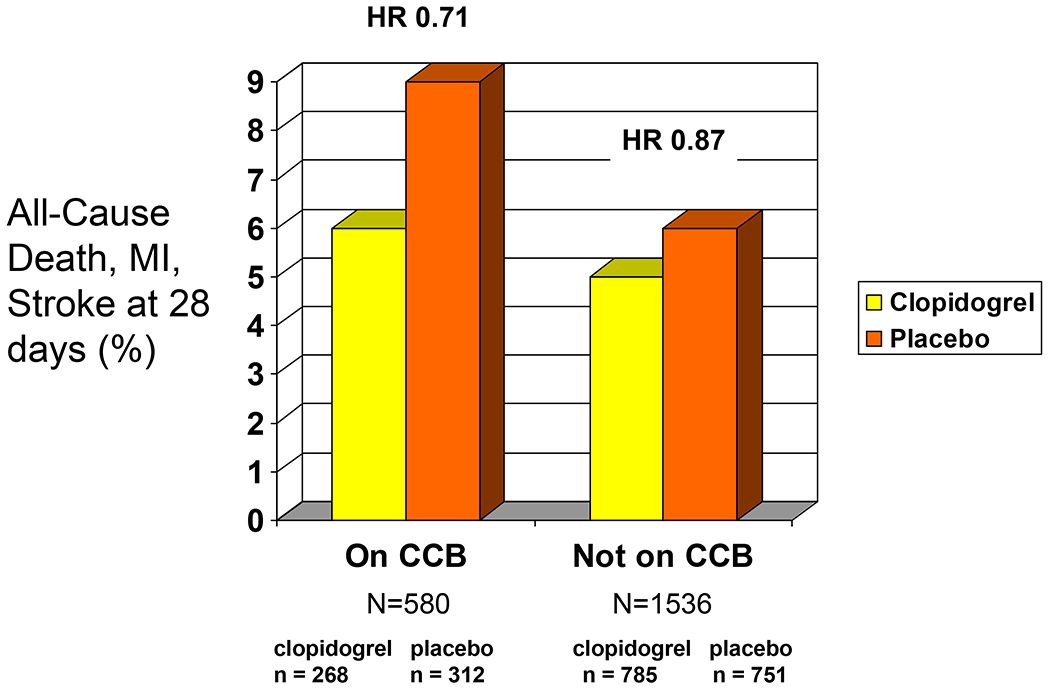
The frequency of death, myocardial infarction and stroke at 28 days with clopidogrel vs. aspirin in patients on, and not on, a calcium channel blocker at study entry.
Discussion
In this post-hoc analysis of the CREDO trial, we found no evidence that patients on CCB derive less benefit from clopidogrel. In fact, the relative risk reduction associated with pre-treatment with a loading dose and long-term clopidogrel was numerically greater among those patients on a CCB than among those who were not.
Background.
The possibility that other drugs that require the CYP 3A4 metabolic pathway might interfere with the conversion of clopidogrel pro-drug to its active metabolite first surfaced with atorvastatin. Many preclinical studies suggested that atorvastatin reduced the inhibition of platelet aggregation by clopidogrel using ADP-stimulated expression of P-selectin by flow cytometry, platelet aggregation measured by the point-of-care MICROS cell counter, and optical aggregometry. (5–7). Subsequently, several confounded registry analyses suggested that indeed there might be a negative interaction. However, retrospective analyses of unbiased clinical trials subsequently proved strong evidence against such an interaction. (8–10).
Prior studies of calcium channel blockers.
The concern that an interaction between clopidogrel and CCBs might exist also stems from their common metabolism via the CYP 3A4 pathway. A study by Siller-Matula and colleagues utilized flow cytometry to assess VASP phosphorylation, which is believed to be an excellent measure of P2Y12 inhibition (2). They found the patients on both CCB and clopidogrel had a higher platelet reactivity index, suggesting less inhibition of aggregation from clopidogrel, than patients not on CCBs. They also found that ADP-induced platelet aggregation was greater in patients not on CCBs than those on it. In the only study evaluating a possible interaction between CCBs and clopidogrel utilizing clinical endpoints, these same investigators evaluated the 6-month clinical outcomes of 200 patients who underwent a PCI and were treated with clopidogrel. Using a composite endpoint of cardiovascular death, myocardial infarction and revascularization they found that the 45 patients (23%) on a CCB had a higher event rate than those not on a CCB; the adjusted hazard ratio was 3.5 (95% CI 1.4 to 8.6, p =0.005) for CCB use. The study, however, was limited by its small sample size, the inability of even complex statistical methods to adjust for many important differences between groups, and that the endpoint was driven by a difference in revascularization between days 100 and 200 which was likely due to restenosis, a phenomenon not believed to be reduced by clopidogrel. A more recent study by Gremmel and colleagues utilized light transmission aggregometry and the VerifyNow P2Y12 assay and also identified a negative interaction between CCBs and clopidogrel. (3). They found higher on-treatment platelet reactivity in patients who were on concomitant CCB therapy as opposed to those who were not by both lab tests. They, however, did not assess the impact of the interaction on clinical outcomes.
Proton pump inhibitors.
Currently, similar concerns have been raised about proton pump inhibitors (PPIs) interfering with the metabolic activation of clopidogrel (11, 12). Registries have suggested that patients who are on clopidogrel and PPIs have worse outcomes than patients on clopidogrel but not on PPIs. (13). A preliminary report from the CREDO trial suggested that that too may be due to confounding (14). Further studies are ongoing.
Implications.
The current study raises questions about the appropriateness of clinical decision making based on ex vivo measures of platelet function. Though several ex vivo platelet function tests have correlated with clinical outcome in clinical studies (15–17) they have been misleading in terms of the ability to predict benefit from clopidogrel in patients treated with medications that reportedly reduce the ability of the liver to convert clopidogrel to its active metabolite. Until prospective randomized studies, like The Gauging Responsiveness with A VerifyNow assay-Impact on Thrombosis And Safety (GRAVITAS) trial are completed, we would recommend that ex vivo platelet function tests not be used to guide clinical practice (18).
Limitations.
Our analysis has some important limitations. This was a post hoc analysis of a randomized trial. The fact that patients on CCBs have more adverse events than patients not on CCBs supports the hypothesis that confounding may contribute to the prior clinical study that did suggest an interaction between CCBs and clopidogrel. The type of CCB was not recorded in the CREDO database; however, all are believed to be primarily metabolized by the same CYP3A4 pathway (19).
Two other limitations are potentially important, however. In CREDO, it was known if patients were on CCB at the time of enrollment into the trial, but subsequent prescription of and adherence to CCB therapy was not evaluated. Therefore, it is possible that some patients stopped their CCB after enrollment and others began treatment with them after enrollment during the course of the one year follow up period. However, approximately 87% of patients on CCBs had hypertension, and 30% had diabetes mellitus; these conditions would not have been influenced by the index PCI procedure, so that nearly all patients on CCBs had continued indications for the drug and were likely continued on it. Additionally, the 28 day combined endpoint was primarily affected by the difference in treatment between the two arms within the 3 to 24 hours after enrollment, before any changes in medication were likely to have occurred. In that analysis, patients on CCBs continued to have greater risk reduction with clopidogrel than patients not on calcium channel blockers. The other potentially important limitation is that the study included a relatively small number of patients (n=2,116), limiting its power to detect a difference in therapeutic effect of clopidogrel in patients on and not on CCBs. However, the risk reduction with clopidogrel in patients on CCBs for both the one year and 28 day endpoints was paradoxically greater than for patients not on CCBs. Given this observation, the likelihood that CCBs actually do reduce the therapeutic benefits of clopidogrel is remote. Furthermore, the current study is more than ten times the size of the only prior study evaluating the clinical impact of CCBs potential interaction with clopidogrel (2).
Conclusion.
In the CREDO trial, there was no evidence that CCB decrease the efficacy of clopidogrel in the year after a percutaneous coronary intervention. These data raise questions about the appropriateness of clinical decision making based on ex vivo measures of platelet function.
What is known:
Clopidogrel is an inactive pro-drug that is converted to its active metabolite via the cytochrome P450 pathway.
Calcium channel blockers are also metabolized by the cytochrome P450 system.
Prior studies have reported that calcium channel blockers decrease the efficacy of clopidogrel by limiting the ability of clopidogrel to inhibit platelet aggregation.
What this article adds:
This article provides evidence to refute the findings from earlier studies by demonstrating that patients taking calcium channel blockers do not derive any less clinical benefit from clopidogrel compared to patients not taking these agents.
Conflict of Interest Disclosures
There are no conflicts to disclose for Christopher W. Good, DO and Danielle M. Brennan, MS.
Conflicts for the remaining authors are as follows: Steven R. Steinhubl, MD is currently an employee of The Medicines Company. Michael Lincoff, MD has research funding with BMS, Sanofi, Schering-Plough, Medicines Company, Takeda, Roche, and Kai therapeutics. Eric J. Topol, MD serves as a consultant to Sanofi-Aventis and Daiichi-Sankyo. Peter B. Berger, MD serves as a consultant to Accumetrics, Boehringer Ingelheim, Eli Lilly/Daiichi Sankyo, Novartis/Portola, and AstraZeneca. He has research funding from Thrombovision, Helena, Accumetrics, AstraZeneca, Haemoscope, The Medicines Company, Corgenix/Aspirinworks, and Eli Lilly/Daiichi-Sankyo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher W. Good, Geisinger Clinic, Danville PA, USA.
Steven R. Steinhubl, Geisinger Clinic, Danville PA, USA.
Peter B. Berger, Geisinger Clinic, Danville PA, USA.
References
- 1.Savi P, Pereillo JM, Uzabiaga MF, Combalbart J, Picard C, Maffrand JP, Pascal M, Herbert JM. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost 2000;84:891–896. [PubMed] [Google Scholar]
- 2.Siller-Matula JM, Lang I, Christ G, Jilma B. Calcium-channel blockers reduce the antiplatelet effect of clopidogrel. J Am Coll Cardiol 2008;52:1557–1563. [DOI] [PubMed] [Google Scholar]
- 3.Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. Calcium-channel blockers decrease clopidogrel-mediated platelet inhibition. Heart 2009; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, DeLago A, Wilmer C, Topol EJ, CREDO Investigators. Clopidogrel for the Reduction of Events During Observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;288:2411–2420. [DOI] [PubMed] [Google Scholar]
- 5.Müller I, Besta F, Schulz C, Li Z, Massberg S, Gawaz M. Effects of statins on platelet inhibition by a high loading dose of clopidogrel. Circulation 2003;108:2195–2197. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer H, Günesdogan B, Hanefeld C, Spiecker M, Mügge A. Lipophilic statins interfere with the inhibitory effects of clopidogrel on platelet function--a flow cytometry study. Eur Heart J 2003;24:1744–1749. [DOI] [PubMed] [Google Scholar]
- 7.Lau WC, Waskell LA, Watkins PB, Neer CJ, Horowitz K, Hop AS, Tait AR, Carville DG, Guyer KE, Bates ER. Atorvastatin reduces thee ability of clopidogrel to inhibit platelet aggregation: a new drug-drug interaction. Circulation 2003;107:32–37. [DOI] [PubMed] [Google Scholar]
- 8.Saw J, Brennan DM, Steinhubl SR, Bhatt DL, Mak KH, Fox K, Topol EJ, CHARISMA Investigators. Lack of evidence of a clopidogrel-statin interaction in the CHARISMA trial. J Am Coll Cardiol 2007;50:291–295. [DOI] [PubMed] [Google Scholar]
- 9.Steinhubl SR, Akers WS. Clopidogrel-statin interaction: a mountain or a mole hill? Am Heart J 2006;152:200–203. [DOI] [PubMed] [Google Scholar]
- 10.Lotfi A, Schweiger MJ, Giugliano GR, Murphy SA, Cannon CP. High-dose atorvastatin does not negatively influence clinical outcomes among clopidogrel treated acute coronary syndrome patients--a Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) analysis. Am Heart J 2008;155:954–958. [DOI] [PubMed] [Google Scholar]
- 11.Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, Mansourati J, Mottier D, Abgrail JF, Boschat J. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008;51:256–260. [DOI] [PubMed] [Google Scholar]
- 12.Siller-Matula JM, Spiel AO, Lang IM, Kreiner G, Christ G, Jilma B. Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J 2009;157:148.e1–148.e5. [DOI] [PubMed] [Google Scholar]
- 13.Pezalla E, Day D, Pulliadath I. Initial assessment of clinical impact of a drug interaction between clopidogrel and proton pump inhibitors. J Am Coll Cardiol 2008;52:1038–1039 [DOI] [PubMed] [Google Scholar]
- 14.Dunn SP, Macaulay TE, Brennan DM, Campbell CL, Charnigo RJ, Smyth SS, Berger PB, Steinhubl SR, Topol EJ. Understanding variability in antiplatelet drug effects in acute coronary syndromes. Baseline proton pump inhibitor use is associated with increased cardiovascular events with and without the use of clopidogrel in the CREDO trial. Circulation 2008;118:S815. (Abstract). [Google Scholar]
- 15.Malinin A, Pokov A, Swaim L, Kotob M, Serebruany V. Validation of a VerifyNow-P2Y12 cartridge for monitoring platelet inhibition with clopidogrel. Methods Find Exp Clin Pharmacol 2006;28:315–322. [DOI] [PubMed] [Google Scholar]
- 16.Marcucci R, Gori AM, Paniccia R, Giusti B, Valente S, Giglioli C, Buonamici P, Antoniucci D, Abbate R, Gensini GF. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: A 12-month follow-up. Circulation 2009;119:237–242. [DOI] [PubMed] [Google Scholar]
- 17.Price MJ, Endemann S, Gollapudi RR, Valencia R, Stinis CT, Levisay JP, Ernst A, Sawhney NS, Schatz RA, Teirstein PS. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J 2008;29:992–1000. [DOI] [PubMed] [Google Scholar]
- 18.Price MJ, Berger PB, Angiolillo DJ, Teirstein PS, Tanguay JF, Kandzari DE, Cannon CP, Topol EJ. Evaluation of individualized clopidogrel therapy after drug-eluting stent implantation in patients with high residual platelet reactivity: design and rationale of the GRAVITAS trial. Am Heart J 2009;157:818–824. [DOI] [PubMed] [Google Scholar]
- 19.Kleiman NS. Clopidogrel and calcium-channel antagonists. Another drug-drug interaction for the ever-wary clinician? J Am Coll Cardiol 2008;52:1564–1566. [DOI] [PubMed] [Google Scholar]


