ABSTRACT
Although most superior vena cava (SVC) syndromes are due to intrathoracic malignancies, some are iatrogenic, such as those following the intravenous implantation of pacemaker wires. To date, the occurrence of this syndrome after epicardial pacemaker removal has not been described. The initial auricular laceration after removal can be complicated by the administration of anticoagulant and antiplatelet drugs, forming a hematoma that compresses the SVC cranially. Therefore, standardized practice may be necessary in these patients to address anticoagulant and antiplatelet therapy, perform serial echocardiography, and pay attention to underlying symptoms, which may be insidious and delayed.
Keywords: Cardiac surgery, superior vena cava syndrome, temporary epicardial pacing wires
INTRODUCTION
Superior vena cava (SVC) syndrome is an extremely rare but serious complication. In 90% of cases, it is caused by extrinsic compression by an intrathoracic malignancy, especially lung cancer; however, iatrogenic causes are increasingly recognized. Many of these causes are related to cardiac surgery, such as placement of retractors to improve surgical visualization,[1] SVC thrombus formation over catheters or pacemaker leads[2] or intrapericardial thrombi around aortic grafts,[3] surgeries that entail an increase in venous pressure (Glenn shunt or anastomosis between the SVC and right pulmonary artery in congenital patients),[4] or the appearance of an aortic pseudoaneurysm causing compression of the SVC.[5] Most patients with SVC obstruction remain asymptomatic and symptomatic cases are rare (0.5%).[2]
We present the case of a patient who required reintervention 13 days after cardiac surgery to remove a clot that was compressing the SVC, atrium, and right ventricle. Informed consent was obtained from the patient.
CASE REPORT
A 61-year-old patient was admitted for non-ST-segment elevation myocardial infarction. He presented with severe proximal lesions in the anterior descending artery and in the first and second obtuse marginal arteries.
Given the poor quality of the distal coronary arteries, it was decided to perform an incomplete coronary revascularization in the operating room (coronary bypass to the anterior descending artery and the second obtuse marginal artery), and subsequently perform a percutaneous approach; also, the mitral valve was replaced. Weaning from cardiopulmonary bypass (CPB) required milrinone, dobutamine and norepinephrine, and temporary epicardial pacing wires (TEPW) were inserted into the atrium and right ventricle due to bradycardia and biventricular failure. A right ventricular pacing wire (Myo/Wire M-25 style (A and E Medical)) was placed on the diaphragmatic surface of the right ventricle via a suture at the end of the pacing wire. An atrial straight wire (Myo/Wire M-20 style (A and E Medical)) was loosely sutured to the right atrium. Since the anticoagulant effect of acenocoumarol is delayed, acenocoumarol and low-molecular-weight heparin (LMWH) were administered for the first three days until an INR within the target range (2.5–3.5) was achieved. On the fourth day after surgery, antiplatelet therapy with acetylsalicylic acid (ASA) was added, and on the fifth day, the TEPW was removed with an INR of 3.5, which increased to 5.2 the following day. Acenocoumarol was withdrawn and treatment was continued with LMWH, ASA, and clopidogrel. Nine days after surgery a drug-eluting stent was implanted over the occlusion of the right coronary artery, and two stents were implanted in the circumflex and first obtuse marginal arteries. Subsequent echocardiography (poor quality) revealed no abnormalities. The day after coronary angiography, double anticoagulation and antiplatelet therapy were continued. The patient had a correct clinical evolution, and only presented an insidious clinical manifestation of cervical pain, headache, and some edema in the upper extremities. He was discharged two weeks after surgery and was admitted the next day because of a progressive increase in edema in the upper limbs, chest, face, and facial plethora, with dyspnea and palpitations. Echocardiography showed pleural effusion, pericardial effusion, and a heterogeneous mass in the right atrium [Figure 1]. Computed tomography is shown in Figure 2. Surgery was performed to remove clots. At the start of surgery, the patient had an invasive blood pressure of 85/54 mmHg, noradrenaline administration, central venous pressure of 30 mmHg, atrial fibrillation at 120 bpm, and SpO2 92%. Orotracheal intubation was performed using a fibrobronchoscope under intravenous sedation (fentanyl 0.10 mg and midazolam 1 mg) as neck and thorax edema could hinder ventilation and orotracheal intubation. After intubation, anesthesia was induced with midazolam (8 mg) and rocuronium (70 mg). A large clot (10 × 10 cm) that had developed at the level of the epicardial atrial insertion of the pacemaker, compressing the SVC and cranial portion of the right atrium, was evacuated. Also, 1.500 ml of blood was aspirated at the pleural level. The patient was discharged 24 days after the reintervention.
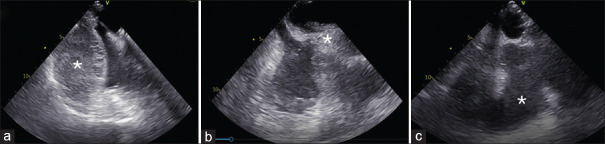
Figure 1.
Intraoperative transesophageal echocardiography images. (a) Mid-esophageal view focused on right heart chambers showing a mass (*) deforming and compressing the atrium and right ventricle. (b) Mid-esophageal bicaval view showing collapse of the superior vena cava (*). (c) Descending aortic short axis showing left pleural effusion (*).
Figure 2.
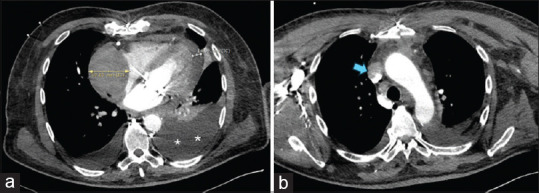
Computed tomography images. (a) Collection with a maximum thickness of 6 cm (yellow arrow) with a mass effect that deforms and compresses the posterior surface of the right atrium. Cardiac tamponade of 1.4 cm at the cardiac apex (white arrow). Severe left pleural effusion (**). (b) The collection also compresses the entrance to the superior vena cava (blue arrow)
DISCUSSION
To our knowledge, this is the first published case of SVC syndrome after the removal of TEPW. It is likely that the initial atrial laceration after epicardial removal of the pacemaker lead was progressively complicated by the administration of anticoagulant and antiplatelet therapy and became more severe after hospital discharge. In addition, changes in anticoagulant medication may also have favored the development of hematoma.
Placement of the TEPW is common before sternal closure in cardiac surgery because of the development of postoperative arrhythmias.[6] Although the occurrence of SVC syndrome after epicardial pacing lead extraction has not been described, its implantation, permanence, and extraction can cause perforation or rupture of the coronary grafts, mediastinal infections, arrhythmias, dyspnea, and death. Thus, although the overall incidence of complications is low (0.04%), it can increase the morbidity and length of hospital stay. Therefore, some authors have argued that the routine use of epicardial pacing leads after CABG may be unnecessary.[7] Some institutions monitor patients every 15 min for 1 h and then every hour for 4 h after TEPW removal.[8] In our center, the epicardial stimulation wires were removed on the fourth postoperative day, avoiding excessive extraction force and monitoring of vital signs, as in the present case. He only presented edema in the upper extremities, headache, and cervical pain, which, given their low magnitude and insidious course, did not raise suspicion of SVC syndrome during admission.
However, the administration of anticoagulant and antiplatelet medication probably favored the progression of extra-auricular hematoma and SVC syndrome. In any case, complete pre- or postoperative anticoagulation was an independent variable for the appearance of cardiac tamponade (CT), which our patient also presented with.[9] Kuvin et al.[10] observed that 84% of patients who developed CT had received anticoagulant treatment with warfarin or heparin in the first three days after cardiac surgery and that a prolonged partial thromboplastin time predicted its development. In our patient, several changes in anticoagulation were made, both to adjust clotting times and to achieve the best bleeding/ischemia balance for percutaneous coronary intervention. Other factors implicated in the development of CT, which were also present in our patient, included arterial hypertension, cardiac surgery other than CABG, prolonged extracorporeal circulation, and red blood cell transfusion.[9]
In addition, the SVC syndrome and CT presented in our patient were associated with severe pleural effusion. Increased systemic venous pressure causes increased leakage of parietal pleural capillaries and the development of pleural effusion, and elevation of intrapleural pressure transmitted to the heart could also contribute to the symptoms of cardiac tamponade.
In conclusion, the removal of TEPW can cause SVC syndrome days after its removal. The initial atrial laceration that occurs after the removal of the epicardial electrodes may progress in patients on anticoagulant and antiplatelet treatment and/or with changes in the dosage of these drugs. Therefore, close monitoring of coagulation with more frequent and strict INR controls should be performed. Standardized anticoagulant and antiplatelet therapies are also necessary before eliminating TEPW. Additionally, serial echocardiography should be considered in patients with risk factors even before hospital discharge. Finally, we must consider that CT and SVC obstruction have a clinical diagnosis, and echocardiography can provide false assurance in its early stages of appearance or patients with a poor acoustic window.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Adam WA, Juan NP, Geoffrey LH. An unusual cause of intraoperative acute superior vena cava syndrome. Ann Card Anaesth. 2013;16:133–6. doi: 10.4103/0971-9784.109770. [DOI] [PubMed] [Google Scholar]
- 2.Swetha MC, Sudheer OV, Murukesh S, Lakshmi K. Superior vena cava syndrome due to catheter related thrombus in a patient with a permanent pacemaker. Indian J Anaesth. 2015;59:758–60. doi: 10.4103/0019-5049.170042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wacker J, Djaiani G, Katznelson R, Karski J. External compression of superior vena cava after the replacement of ascending aorta. Eur J Echocardiogr. 2008;9:589–90. doi: 10.1093/ejechocard/jen116. [DOI] [PubMed] [Google Scholar]
- 4.Praveen KN, Manikandan S, Krishnamanohar SR, Ramesh CR. Superior vena cava syndrome after pulsatile bidirectional Glenn shunt procedure: Perioperative implications. Ann Card Anaesth. 2009;12:53–6. doi: 10.4103/0971-9784.45014. [DOI] [PubMed] [Google Scholar]
- 5.Panagiotis D, Ioannis N, Panagiotis H, Christos P, Paraskevi D, Efstratios A, et al. Case report superior vena cava syndrome in a patient with previous cardiac surgery: What else should we suspect? Diagn Pathol. 2010;5:43. doi: 10.1186/1746-1596-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001;135:1061–73. doi: 10.7326/0003-4819-135-12-200112180-00010. [DOI] [PubMed] [Google Scholar]
- 7.Puskas JD, Sharoni E, Williams WH, Petersen R, Duke P, Guyton RA. Is routine use of temporary epicardial pacing wires necessary after either OPCAB or conventional CABG/CPB? Heart Surg Forum. 2003;6:E103–6. [PubMed] [Google Scholar]
- 8.Cote CL, Baghaffar A, Tremblay P, Herman C. Incidence of tamponade following temporary epicadial pacing wire removal. J Cardíac Surg. 2020;35:1247–52. doi: 10.1111/jocs.14564. [DOI] [PubMed] [Google Scholar]
- 9.Hernández Leiva E, Carreño M, Rada Bucheli F, Cadena Bonfanti A, Umaña JP, Dennis RJ. Factors associated with delayed cardíac tamponade after cardíac surgery. Ann Card Anaesth. 2018;21:158–66. doi: 10.4103/aca.ACA_147_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuvin JT, Harati NA, Pandian NG, Bojar RM, Khabbaz KR. Postoperative cardiac tamponade in the modern surgical era. Ann Thorac Surg. 2002;74:1148–53. doi: 10.1016/s0003-4975(02)03837-7. [DOI] [PubMed] [Google Scholar]