ABSTRACT
Background:
Due to affluent lifestyles, primary care physicians are concerned about metabolic syndrome (MetS). Inflammation and insulin resistance are caused by extra adipose tissue. Our study seeks to evaluate, taking into account numerous variables, the relationship between high-sensitivity C-reactive protein (hsCRP) levels and MetS in adults aged 50+ in Northern Kashmir, India.
Materials and Methods:
In Northern Kashmir, India, a hospital-based cross-sectional study looked into the relationship between hsCRP and MetS in people over 50. The recruitment process included people looking for basic healthcare services. Utilising modified NCEP-ATP III criteria, MetS was established. The association between MetS and hsCRP levels was evaluated statistically while taking into account various variables.
Results:
The waist circumference, body mass index, systolic and diastolic blood pressure, as well as the prevalence of hypertension, diabetes mellitus (DM), and dyslipidemia were all greater in those with MetS. While demonstrating decreased levels of high-density lipoprotein cholesterol (HDL-C), they also showed higher levels of high-sensitive C-reactive protein (hsCRP) and fasting plasma glucose. A study of correlations revealed a substantial inverse relationship between hsCRP and HDL-C. Elevated hsCRP levels were found to be substantially linked with MetS by the use of logistic regression, along with obesity, uric acid levels, hypertension, DM, and dyslipidemia. These results underline how crucial it is to keep an eye on these variables in order to recognize and treat MetS as soon as possible.
Conclusion:
Among this investigation, we found strong evidence that high- hsCRP, an independent risk factor for MetS, was present among middle-aged and elderly residents of the northern Kashmir region of India.
Keywords: C-reactive protein, elderly, metabolic syndrome, middle-aged, risk factors
Introduction
Due to the predominance of affluent lifestyles and an increase in lifespan, metabolic syndrome (MetS) has become a prominent concern in family medicine and primary care. The term “MetS” refers to a collection of metabolic disorders, such as hypertension (BP), dyslipidemia, glucose intolerance, and obesity.[1] These elements raise the chance of acquiring cardiovascular disease and diabetes mellitus (DM).[2] The existence of excessive adipose tissue, which causes an excessive release of free fatty acids, is one of the underlying mechanisms in people with MetS. As a result, insulin resistance (IR) is caused, which lowers peripheral insulin sensitivity.[3] Additionally, enlarged adipose tissue causes monocyte-derived macrophages in the adipose tissue to overproduce proinflammatory cytokines such C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha.[4]
Along with IR, inflammation appears to be a key pathophysiologic component in MetS. High-sensitivity CRP (hsCRP) levels have been shown to be positively correlated with fasting insulin, IR, and MetS levels in numerous investigations.[5,6] Children and adults alike have both seen these associations throughout a range of age groups.[7,8] High-sensitivity C-reactive Protein (hsCRP) has also been linked favorably to a number of chronic diseases, including dyslipidemia, DM, and hypertension, according to earlier studies.[9,10] Lifestyle decisions have been shown to have an impact on hsCRP levels.[11] On the other hand, chronic diseases and MetS tend to become more common as people age.[12] Additionally, certain lifestyle choices like smoking and binge drinking alcohol have been linked to higher hsCRP levels.[11] There are several variables that can affect high hsCRP levels, but there are few studies that fully take into account these variables in relation to MetS. Given the rising prevalence of MetS and its implications for primary care providers, it is crucial to explore the association between hsCRP levels and MetS in the context of family medicine and primary care. With this aim, we have conducted this study to examine the correlation between hsCRP levels and MetS among individuals aged 50 years and older in the Northern Kashmir region (India). We have collected a comprehensive range of factors, such as laboratory data and anthropometric measurements, to facilitate this assessment. By investigating this relationship, primary care providers can enhance their understanding of the underlying mechanisms and develop more targeted interventions for patients at risk of developing MetS-related complications.
Materials and Methods
Study design and study population
This hospital-based cross-sectional study examined the relationship between hsCRP and MetS in an elderly population aged 50 years and older. The study was conducted in a setting where residents had access to basic medical care. Participant recruitment took place at the specified hospital from August 2021 to December 2022. The sample consisted of patients who visited the hospital for primary care during the study period and were 50 years of age or older. Potential volunteers were identified using a variety of recruitment strategies, including posters, hospital announcements, and referrals from medical professionals.
The inclusion criteria for the study were: age 50 years or older, community-dwelling patients, receiving primary healthcare services at the hospital, and capacity to give informed consent, while the exclusion criteria were: persons under the age of 50, those unable to give informed consent, those who live outside the target population, and those who have serious medical illnesses or disabilities that would make participation in the study difficult.
Interested and eligible participants were invited to participate in the study. Those who expressed interest were given a comprehensive explanation of the study’s goals, methods, potential risks, and benefits. Each participant had a face-to-face interview and completed a detailed questionnaire about their personal information and medical history. Anthropometric measurements and blood samples were collected by trained research assistants or nurses under the supervision of a doctor. All participants provided written informed consent before being enrolled in the study. The Institutional Review Board (IRB) of Government Medical College, Baramulla approved the research protocol to ensure ethical compliance and participant protection. The study strictly adhered to data confidentiality, and all data were anonymized to preserve privacy.
Measurements
Face-to-face interviews were conducted with participants to gather personal data such as age, gender, and smoking status (self-reported as current smoker or non-smoker). A thorough medical history was also collected. Blood pressure (BP) and anthropometric measurements such as height, weight, waist circumference (WC), and height were obtained. Participants were asked to stand with their feet spaced 25-30 cm apart, while their WC was measured at the midpoint between the iliac crest and the lower border of the 12th rib. After a 10-min break in a seated position, their BP was measured using an automated sphygmomanometer on their right arm, and the lowest value was recorded.
Body mass index (BMI) was calculated by dividing a person’s weight in kilograms by the square of their height in meters. Participants were instructed to fast for at least 12 h and abstain from high-fat foods and alcohol for at least 24 h before providing a blood sample. Venous blood samples were collected between 7 and 11 am and stored in a refrigerator at 4°C until they could be analyzed in the hospital laboratory. The clinical biochemical tests performed included hsCRP, fasting plasma glucose (FPG), total cholesterol (Total-C), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and uric acid (UA). The blood samples were analyzed by a highly qualified clinical laboratory that had undergone rigorous quality control and ongoing monitoring. The laboratory maintained all of the data that were collected.
Definition of metabolic syndrome and other diseases
MetS was defined as having three or more of the following risk factors: WC ≥ 90 cm for men and ≥ 80 cm for women, TG ≥ 150 mg/dL, HDL-C < 40 mg/dL for men and < 50 mg/dL for women, BP ≥ 130/85 mm Hg or current use of antihypertensive medications, and FPG ≥ 100 mg/dL. Participants meeting the MetS criteria were categorized as the metabolic group, while those not meeting the criteria were classified as the non-metabolic group.[13] Hypertension (HTN) was defined as having a systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or using medications for hypertension. DM was defined as having FPG ≥ 126 mg/dL or using oral hypoglycemic agents or insulin. Dyslipidemia was defined as having LDL-C ≥ 130 mg/dL, HDL-C < 40 mg/dL in men, HDL-C < 50 mg/dL in women, TG ≥ 150 mg/dL, total cholesterol ≥ 200 mg/dL, or using lipid-lowering medications. Obesity, according to the Ministry of Health and Welfare of Taiwan, was defined as having a BMI ≥ 27 kg/m2.[14] Elevated hsCRP was defined as plasma hsCRP levels ≥ 1 mg/L, which signifies a higher risk of cardiovascular disease.[15,16]
Statistical analysis
The participants were divided into two groups based on whether or not they had MetS. Categorical variables were presented as counts and percentages and were analyzed using the Chi-square test. The normality of continuous variables was assessed using the Shapiro-Wilk normality test. For normally distributed variables, the data were reported as mean ± standard deviation (SD). For variables that deviated from a normal distribution, the data were presented as median with the first and third quartiles.
To compare continuous variables, independent sample t-tests were conducted for normally distributed data, while the Mann–Whitney U-test was used for non-normally distributed data. Pearson’s correlation coefficient was calculated to analyze the associations between age, WC, BMI, SBP, DBP, FPG, HDL-C, TG, LDL-C, total cholesterol, and UA.
In the multivariate analysis, binary logistic regression was used to assess the relationship between MetS and hsCRP levels while adjusting for BMI, UA levels, age, sex, smoking status, hypertension, DM, and dyslipidemia. All statistical tests were two-sided, and a P value less than 0.05 was considered statistically significant. The data were analyzed using IBM SPSS Statistics software, version 22.
Results
After convenience sampling, we recruited a total of 489 participants through posters and notifications from the community office, announcements in the hospital, and referrals from healthcare professionals. From this initial group, 189 participants were excluded due to incomplete data, disabilities, refusal to participate, or acute illnesses at enrollment or recently. The final sample size for our study was determined to be 300 participants. The comparison of participant traits between the MetS and non-MetS (Non-MetS) groups is shown in Table 1. The participants in the entire sample had an average age of 63.48 ± 7.44 years. Between the non-MetS group (62.95 ± 7.32 years) and the MetS group (64.41 ± 8.62 years), there was no discernible difference in age [P = 0.11; Table 1]. Between the two groups, there were substantial differences in WC. WC was lower in the non-MetS group (81.00 ± 8.13 cm) than in the MetS group (91.72 ± 8.82 cm) (p < 0.01). Similar to this, the BMI of the MetS group was substantially higher (27.62 ± 3.62 kg/m2) than the non-MetS group (24.54 ± 3.23 kg/m2) (p < 0.01).
Table 1.
Comparison of participant characteristics between non-metabolic syndrome (Non-MetS) and metabolic syndrome (MetS) groups
| Variables | Total (n=300) | Non-MetS (n=195) | MetS (n=105) | P |
|---|---|---|---|---|
| Age (year) | 63.48±7.44 | 62.95±7.32 | 64.41±8.62 | 0.11 |
| WC (cm) | 84.06±9.67 | 81.00±8.13 | 91.72±8.82 | <0.01 |
| BMI (kg/m) | 24.64±3.62 | 24.54±3.23 | 27.62±3.62 | <0.01 |
| SBP (mmHg) | 128.60±15.61 | 125.12±14.20 | 136.76±18.58 | <0.01 |
| DBP (mmHg) | 77.83±12.46 | 74.79±12.34 | 77.78±11.28 | 0.01 |
| Gender, male (%) | 113 (37.66%) | 72 (37.00%) | 41 (39.04%) | 0.74 |
| Smoking, n (%) | 36 (12.00%) | 20 (10.25%) | 16 (15.23%) | 0.18 |
| HTN, n (%) | 148 (49.33%) | 75 (38.46%) | 73 (69.52%) | <0.01 |
| DM, n (%) | 56 (18.66%) | 18 (9.23%) | 38 (36.19%) | <0.01 |
| Dyslipidemia, n (%) | 202 (67.33%) | 109 (55.89%) | 93 (88.57%) | <0.01 |
| hsCRP (mg/L) | 1.30 [0.68, 2.43] | 1.07 [0.61, 2.08] | 1.75 [1.03, 3.78] | <0.01 |
| FPG (mg/dL) | 91.10 [82.09, 101.08] | 88.03 [82.01, 94.10] | 101.10 [88.09, 114.00] | <0.01 |
| HDL-C (mg/dL) | 54.42±13.83 | 58.95±13.81 | 46.23±11.23 | <0.01 |
| TG (mg/dL) | 107.10 [77.35, 144.75] | 92.08 [71.00, 114.50] | 150.00 [111.00, 184.00] | <0.01 |
| LDL-C (mg/dL) | 114.32±31.10 | 118.83±32.12 | 117.50±32.19 | 0.69 |
| Total-C (mg/dL) | 163.11±34.70 | 197.31±35.78 | 193.63±34.67 | 0.90 |
| Uric Acid (mg/dL) | 5.73±1.31 | 5.37±1.37 | 6.07±1.34 | <0.01 |
WC=Waist circumference, BMI=Body mass index, SBP=Systolic blood pressure, DBP=Diastolic blood pressure, HTN=Hypertension, DM=Diabetes mellitus, hsCRP=High-sensitivity C-reactive protein, FPG=Fasting plasma glucose, HDL-C=High-density lipoprotein cholesterol, TG=Triglyceride, LDL-C=Low-density lipoprotein cholesterol, Total-C=Total cholesterol Categorical variables are presented as counts and percentages (n (%)) and were analyzed using the Chi-square test. Continuous variables are reported as mean±standard deviation ([SD]) if they follow a normal distribution, and as median [Q1, Q3] if they significantly deviate from normal distribution. Independent sample t-tests were used to calculate p-values for normally distributed variables, while the Mann–Whitney U-test was employed for variables that were non-normally distributed
SBP and DBP were substantially higher in the MetS group than in the non-MetS group (p < 0.01 and P = 0.01) (136.76 ± 18.58 mmHg and 77.78 ± 11.28 mmHg, respectively). Between the two groups, there were no appreciable variations in the distribution of gender and smoking (P > 0.05). However, there were substantially more people with hypertension (HTN) in the MetS group (69.52%) than in the non-MetS group (38.46%) (p < 0.01). Similar to the prevalence of DM and dyslipidemia, the MetS group had a substantially greater rate of both conditions (36.19% and 88.57%, respectively) than the non-MetS group (9.23% and 55.89%) (p < 0.01). The MetS group had significantly higher levels of high-sensitive C-reactive protein (hsCRP) (1.75 [1.03, 3.78] mg/L) than the non-MetS group (1.07 [0.61, 2.08] mg/L) (p < 0.01). Additionally, the MetS group’s FPG levels were substantially higher (101.10 [88.09, 114.00] mg/dL) than the non-MetS group’s (88.03 [82.01, 94.10] mg/dL) (p < 0.01). HDL-C levels were also lower in the MetS group (46.23 ± 11.23 mg/dL) compared to the non-MetS group (58.95 ± 13.81 mg/dL) (p < 0.01). Triglyceride (TG) levels were substantially higher in the MetS group than in the non-MetS group (p < 0.01) (150.00 [111.00, 184.00] mg/dL vs. 92.08 [71.00, 114.50] mg/dL). LDL-C and total cholesterol (Total-C) levels did not significantly differ between the two groups.
hs-CRP and numerous cardiometabolic risk variables are shown to be correlated in Table 2 results. hs-CRP revealed modest and non-significant associations with age (years), waist circumference (WC in cm), body mass index (BMI in kg/m2), systolic blood pressure (SBP in mmHg), diastolic blood pressure (DBP in mmHg), fasting plasma glucose (FPG in mg/dL), and UA (mg/dL) (P > 0.05).
Table 2.
Correlation analysis of cardiometabolic risk factors with high-sensitivity C-reactive protein (hsCRP)
| Variables | hs-CRP (n=300) Pearson’s coefficient | hs-CRP (n=300) p |
|---|---|---|
| Age (year) | 0.05 | 0.90 |
| WC (cm) | 0.08 | 0.07 |
| BMI (kg/m2) | 0.07 | 0.12 |
| SBP (mmHg) | 0.05 | 0.44 |
| DBP (mmHg) | −0.02 | 0.91 |
| FPG (mg/dL) | 0.04 | 0.34 |
| HDL-C (mg/dL) | −0.17 | <0.001 |
| TG (mg/dL) | 0.08 | 0.07 |
| LDL-C (mg/dL) | −0.08 | 0.13 |
| Total-C (mg/dL) | −0.12 | 0.03 |
| Uric Acid (mg/dL) | 0.07 | 0.12 |
hsCRP=High sensitive C-reactive protein, WC=Waist circumference, BMI=Body mass index, SBP=Systolic blood pressure, DBP=Diastolic blood pressure, FPG=Fasting plasma glucose, HDL-C=High-density lipoprotein cholesterol, TG=Triglyceride, LDL-C=Low-density lipoprotein cholesterol, Total-C=Total cholesterol
However, there was a strong negative connection between HDL-C in mg/dL and hs-CRP (Pearson’s coefficient = −0.17, p < 0.001), indicating that greater levels of HDL-C are linked to lower levels of hs-CRP. Triglycerides (TG in mg/dL) and hs-CRP had a marginally positive connection (Pearson’s coefficient = 0.08, P = 0.07), suggesting that greater TG concentrations may be linked to marginally raised hs-CRP levels. Although these relationships were not statistically significant (P > 0.05), low-density lipoprotein cholesterol (LDL-C in mg/dL) and total cholesterol (Total-C in mg/dL) showed mild negative correlations with hs-CRP. These findings imply that HDL-C had the highest connection with hs-CRP of the cardiometabolic risk factors investigated, suggesting a potential inverse relationship between HDL-C levels and inflammation as determined by hs-CRP.
Figure 1 shows that people with hsCRP values of 1 mg/L or more were considered to have increased hsCRP. In contrast to the group with normal hsCRP, we observed a higher incidence of MetS in the group with raised hsCRP. Statistical significance was less than 0.001, and the prevalence of MetS was 21.7% in the normal hsCRP group and 43.2% in the increased hsCRP group Table 3 displays the findings of the logistic regression analyses. With an odds ratio (OR) of 2.69 (95% confidence interval [CI]: 1.72-4.33, p < 0.01), hsCRP levels (1 mg/L vs 1 mg/L) demonstrated a significant connection with MetS in the univariate logistic regression model. Similarly, there was a significant correlation between obesity and MetS, as determined by BMI (obesity vs. non-obesity), with an OR of 2.39 (95% CI: 1.51–3.82, p < 0.01). In the univariate analysis, UA levels, hypertension, DM, and dyslipidemia all demonstrated a significant association with MetS (OR: 1.3, 95% CI: 1.13–1.50, p < 0.01), as did hypertension (OR: 5.10, 95% CI: 3.18–6.01, p < 0.01) and hypertension (HTN) (OR: 6.01, 95% CI: 3.51–9.10).
Figure 1.
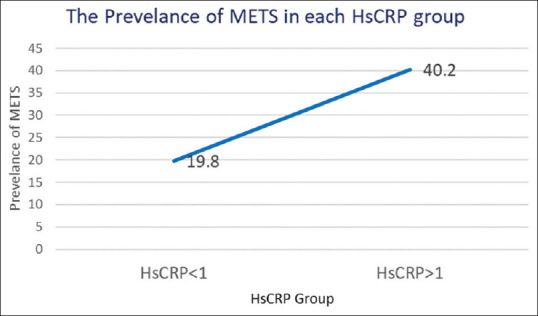
Prevalence of metabolic syndrome across different hsCRP groups in the elderly population. hsCRP = High-sensitivity C-reactive protein, MetS = Metabolic syndrome
Table 3.
Relationship between cardiometabolic risk factors and metabolic syndrome (MetS) using logistic regression analysis
| Variables | Univariate logistic regression | Multivariate logistic regression | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | (95% CI) | P | OR | (95% CI) | P | |
| hsCRP level (≥1 mg/L versus <1 mg/L) | 2.69 | (1.72-4.33) | <0.01 | 2.27 | (1.14-4.45) | 0.01 |
| BMI (Obesity versus non-obesity) | 2.39 | (1.51-3.82) | <0.01 | 1.18 | (0.45-2.56) | 0.65 |
| Uric Acid (mg/dL) | 1.3 | (1.13-1.50) | <0.01 | 1.24 | (1.00-1.88) | 0.04 |
| Age (year) | 1.03 | (1.00-1.20) | 0.21 | 1.04 | (0.98-2.04) | 0.43 |
| Gender (men versus women) | 1.2 | (0.59-1.50) | 0.54 | 1.40 | (0.72-2.94) | 0.40 |
| Smoking (yes versus no) | 1.49 | (0.82-3.97) | 0.19 | 1.69 | (0.73-3.98) | 0.25 |
| HTN (yes versus no) | 5.10 | (3.18-6.01) | <0.01 | 4.89 | (2.90-7.34) | <0.01 |
| DM (yes versus no) | 6.01 | (3.51-9.10) | <0.01 | 5.70 | (3.13-9.05) | <0.01 |
| Dyslipidemia (yes versus no) | 3.41 | (2.06-5.45) | <0.01 | 3.30 | (1.83-6.81) | <0.01 |
MetS=Metabolic syndrome, hsCRP=High-sensitive C-reactive protein, BMI=Body mass index, HTN=Hypertension, DM=Diabetes mellitus. Obesity: BMI ≥27 kg/m2. Non-obesity: BMI <27 kg/m2.
After taking into account potential confounders, the multivariate logistic regression model showed that hsCRP levels (1 mg/L vs 1 mg/L) were still substantially linked with MetS, with an OR of 2.27 (95% CI: 1.14–4.45, P = 0.01) and an OR of 2.27. The multivariate analysis, however, rendered the link between BMI and MetS non-significant (OR: 1.18, 95% CI: 0.45–2.56, P = 0.65). In either the univariate or multivariate analysis, the variables age, gender, and smoking failed to demonstrate a significant connection with MetS. Overall, these findings imply that the occurrence of MetS in the examined group is significantly influenced by high hsCRP levels, obesity, UA levels, hypertension, diabetes, and dyslipidemia.
Discussion
Based on measurements of the WC, BP, FPG, HDL-C, and TG, we evaluated the presence of the MetS in our study.[17] Between the group without MetS and the group with MetS, we found a substantial difference in these criteria. Our findings in Table 1 are consistent with earlier studies that found a favorable connection between serum UA and MetS.[18,19] According to past studies, the MetS group had a high prevalence of DM, hypertension (HTN), and dyslipidemia.[2] Furthermore, we discovered a strong and favorable association between hsCRP and MetS, which led us to think about the connection between hsCRP and MetS.
HDL-C acts as a protective factor against MetS, whereas WC, SBP, DBP, FPG, and TG are identified as risk factors in MetS. Significant correlations between hsCRP and the various MetS components have been shown in numerous investigations.[20,21] We noticed a similar pattern in the association between hsCRP and MetS as well. While hsCRP and HDL-C showed a negative association in Table 2, Pearson’s correlation analysis found substantial relationships between hsCRP and the MetS criteria. This finding begs the question of whether hsCRP could independently operate as a risk factor for MetS given that hsCRP shares the same risk and protective characteristics as MetS in our study. According to the findings shown in Figure 1, the prevalence of MetS was considerably higher in the group with raised hsCRP levels than in the group with low hsCRP levels.[22] Table 3 representation of the results of the logistic regression analysis used to further analyze the association. The univariate logistic regression analysis of Model 1 demonstrated significant positive correlations between MetS and hsCRP levels, obesity, UA, hypertension (HTN), DM, and dyslipidemia. Only hsCRP levels, UA HTN, DM, and dyslipidemia remained positively linked with MetS in Model 2, which used multivariate logistic regression. The results of our investigation revealed that hsCRP levels are a distinct risk factor for MetS. It is important to note that obesity and MetS both share a number of risk variables, and our univariate logistic regression study also found a favorable correlation between the two conditions. In comparison to the general population, obese people tend to consume more fructose. Additionally, it is well-known that the metabolism of fructose raises serum UA levels,[23] and MetS is closely linked to obesity.[24] In our study, both univariate and multivariate logistic regression analysis showed a substantial positive connection between UA and MetS. It is significant to remember that illnesses like DM, dyslipidemia, and hypertension, respectively, are characterized by high levels of plasma glucose, TG, and BP.[25,26] The definition of MetS also includes these elements.[27] It is, therefore, not surprising that MetS showed substantial correlations with HTN, DM, and dyslipidemia in both univariate and multivariate logistic regression models, which is consistent with prior findings.[2]
Obesity, hypertension, IR, DM, and dyslipidemia have all been linked to MetS, which has been identified as a risk factor for these illnesses. The healthcare systems in contemporary society are heavily taxed by these disorders linked to MetS. It would be helpful to have a predictive risk factor for MetS to lessen the burden on healthcare systems. Adipocytes, which can release chemokines including CCL5, monocyte chemotactic protein, macrophage inflammatory protein, macrophage migration inhibition factor, and macrophage colony-stimulating factor, are more prevalent in people with MetS.[28,29,30] These chemokines encourage monocyte recruitment and differentiation in adipose tissue, especially visceral adipose tissue.[30] These monocytes in adipose tissue eventually develop into macrophages and release proinflammatory cytokines like IL-6, which causes the liver to create acute-phase reactants like hsCRP.[31] Previous research on cardiovascular disease (CVD) has shown that hsCRP binding might cause LDL-C within atherosclerotic plaques to undergo oxidation and enzymatic modification. HsCRP has the capacity to form plaques on its own, and its proinflammatory qualities aid in the progression of CVD.[32] This is due to the fact that atherosclerosis is essentially an inflammatory condition.[33]
Furthermore, the cytokine IL-6, which induces the liver to create hsCRP, also encourages inflammation in atherosclerosis.[34] The excessive accumulation of adipocytes in people with MetS causes the blood levels of proinflammatory molecules such hsCRP and interleukins to increase. As a result, these proinflammatory compounds contribute to the development of atherosclerosis, commencing with the first recruitment of leukocytes to the artery wall and ending with the rupture of the plaque.[35]
By altering the permeability of insulin in endothelial cells, the inflammatory properties of hsCRP may also contribute to the onset of diabetic mellitus (DM).[36] CRP-associated inflammation changes insulin delivery, which impairs insulin sensitivity in tissues with active metabolism.[37]
These research studies shed light on the possible connection between hsCRP and MetS, cardiovascular disease, and IR. In fact, our research showed a strong link between IR, CVD, and MetS. There was still a significant connection between hsCRP and MetS, even after other risk factors were taken into account. The results of our study not only point to hsCRP’s potential role as an important link between MetS, CVD, and IR, but they also imply that hsCRP may function as a separate risk factor for MetS.
Our study had a number of distinguishing advantages. First of all, it had an adequate sample size, included pertinent confounders, and used suitable statistical techniques. Second, even after taking into account a number of linked confounders in middle-aged and elderly populations, we showed that hsCRP might act as an independent risk factor for MetS. These results might help primary care doctors in their attempts to screen patients for MetS. It is crucial to recognize some of our study’s shortcomings, though.
Selection bias
Since our study is hospital-based and used a convenience sampling method, there is a potential for selection bias. Participants seeking primary healthcare services at the hospital may not be representative of the general population. It is important to acknowledge this limitation and discuss its potential impact on the study’s findings and their generalizability. The results may be more applicable to individuals seeking healthcare services at the hospital rather than the broader population.
Cross-sectional study design
The cross-sectional design used in our research limits our ability to establish causality or assess temporal relationships between hsCRP levels and MetS. We acknowledge this limitation and understand the need for a longitudinal study design with follow-up assessments to strengthen the evidence for a causal relationship. Mentioning this limitation and the potential benefits of a longitudinal study design would contribute to the discussion of the study’s findings.
By explicitly addressing these limitations, we provide a comprehensive understanding of the study’s scope and potential implications. This acknowledgment adds transparency and helps readers interpret the results appropriately, considering the inherent limitations of the study design and sample selection method.
Validation of NCEP-ATP III criteria
Regarding the validation of the modified NCEP-ATP III criteria used to define MetS in our study population, we acknowledge the importance of providing evidence for their validity. The decision to employ the modified NCEP-ATP III criteria was based on previous research studies that have successfully utilized these criteria in similar populations and settings. For instance, Krishnamoorthy Y et al.[38] conducted a study in a comparable population of older adults and found that the modified criteria demonstrated good sensitivity and specificity in identifying individuals with MetS.
Furthermore, a study by Huang Y et al.[39] examined the validity of the modified NCEP-ATP III criteria in a diverse population sample and reported high concordance with alternative validated criteria, indicating their reliability and accuracy in assessing MetS. While these studies were not conducted in our specific study population, they provide supporting evidence for the validity of the modified criteria in similar contexts. However, we acknowledge the need for future research to validate these criteria specifically within our study population.
Conclusion
The results of our study demonstrated that hsCRP acted as an independent risk factor for MetS among middle-aged and elderly individuals in the northern Kashmir region (India). These findings offer valuable insights for primary care physicians, enabling them to effectively communicate the heightened risk of MetS to individuals within this specific age range.
Institutional review board statement
The study involving human participants underwent review and approval by the Government Medical College, Baramulla IRB (GMC/B-212/B23).
Informed consent statement
Informed consent was obtained from all patients/participants, and they provided written consent before participating in the study.
Data availability statement
The corresponding author will make the raw data supporting the study’s conclusions available, without any undue reservation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to express sincere gratitude to the field staff and hospital staff for their invaluable support throughout this study. Their dedication and assistance have been instrumental in the successful execution of this research. I deeply appreciate their contributions and acknowledge their important role in this study.
References
- 1.Heianza Y, Kato K, Kodama S, Ohara N, Suzuki A, Tanaka S, et al. Risk of the development of Type 2 diabetes in relation to overall obesity, abdominal obesity and the clustering of metabolic abnormalities in Japanese individuals: Does metabolically healthy overweight really exist?The Niigata Wellness Study. Diabet Med. 2015;32:665–72. doi: 10.1111/dme.12646. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM. Metabolic syndrome: Connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol. 2006;47:1093–100. doi: 10.1016/j.jacc.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407–17. doi: 10.1111/imm.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matulewicz N, Karczewska-Kupczewska M. Insulin resistance and chronic inflammation. Postepy Hig Med Dosw (Online) 2016;70:1245–58. [PubMed] [Google Scholar]
- 5.Drabsch T, Holzapfel C, Stecher L, Petzold J, Skurk T, Hauner H. Associations between C-Reactive protein, insulin sensitivity, and resting metabolic rate in adults: A mediator analysis. Front Endocrinol (Lausanne) 2018;9:556. doi: 10.3389/fendo.2018.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. 2015;2015:508409. doi: 10.1155/2015/508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyithar MP, Bonner C, Bacon S, Kilbride SM, Schmid J, Graf R, et al. Effects of hepatocyte nuclear factor-1A and -4A on pancreatic stone protein/regenerating protein and C-reactive protein gene expression: Implications for maturity-onset diabetes of the young. J Transl Med. 2013;11:156. doi: 10.1186/1479-5876-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suhett LG, Hermsdorff HHM, Rocha NP, Silva MA, Filgueiras MS, Milagres LC, et al. Increased C-reactive protein in Brazilian children: Association with cardiometabolic risk and metabolic syndrome components (PASE Study) Cardiol Res Pract. 2019;2019:3904568. doi: 10.1155/2019/3904568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakoski SG, Cushman M, Siscovick DS, Blumenthal RS, Palmas W, Burke G, et al. The relationship between inflammation, obesity and risk for hypertension in the Multi-Ethnic Study of Atherosclerosis (MESA) J Hum Hypertens. 2011;25:73–9. doi: 10.1038/jhh.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noordam R, Oudt CH, Bos MM, Smit RAJ, van Heemst D. High-sensitivity C-reactive protein, low-grade systemic inflammation and type 2 diabetes mellitus: A two-sample Mendelian randomization study. Nutr Metab Cardiovasc Dis. 2018;28:795–802. doi: 10.1016/j.numecd.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Blaum C, Brunner FJ, Kröger F, Braetz J, Lorenz T, Goßling A, et al. Modifiable lifestyle risk factors and C-reactive protein in patients with coronary artery disease: Implications for an anti-inflammatory treatment target population. Eur J Prev Cardiol. 2021;28:152–8. doi: 10.1177/2047487319885458. [DOI] [PubMed] [Google Scholar]
- 12.Liu B, Chen G, Zhao R, Huang D, Tao L. Temporal trends in the prevalence of metabolic syndrome among middle-aged and elderly adults from 2011 to 2015 in China: The China health and retirement longitudinal study (CHARLS) BMC Public Health. 2021;21:1045. doi: 10.1186/s12889-021-11042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh TH, Lee JJ, Yu EW, Hu HY, Lin SY, Ho CY. Association between obesity and education level among the elderly in Taipei, Taiwan between 2013 and 2015: A cross-sectional study. Sci Rep. 2020;10:20285. doi: 10.1038/s41598-020-77306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, et al. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–36. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 16.Nehring SM, Goyal A, Patel BC. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2023. [[Last accessed on 2023 Jul 10]]. C Reactive Protein. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441843/ [Google Scholar]
- 17.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–7. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali N, Miah R, Hasan M, Barman Z, Mou AD, Hafsa JM, et al. Association between serum uric acid and metabolic syndrome: A cross-sectional study in Bangladeshi adults. Sci Rep. 2020;10:7841. doi: 10.1038/s41598-020-64884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CW, Chen KH, Tseng CK, Chang WC, Wu YW, Hwang JJ. The dose-response effects of uric acid on the prevalence of metabolic syndrome and electrocardiographic left ventricular hypertrophy in healthy individuals. Nutr Metab Cardiovasc Dis. 2019;29:30–8. doi: 10.1016/j.numecd.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Sigdel M, Kumar A, Gyawali P, Shrestha R, Tuladhar ET, Jha B. Association of high sensitivity C-reactive protein with the components of metabolic syndrome in diabetic and non-diabetic individuals. J Clin Diagn Res. 2014;8:CC11–3. doi: 10.7860/JCDR/2014/8085.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pravenec M, Kajiya T, Zídek V, Landa V, Mlejnek P, Simáková M, et al. Effects of human C-reactive protein on pathogenesis of features of the metabolic syndrome. Hypertension. 2011;57:731–7. doi: 10.1161/HYPERTENSIONAHA.110.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engin A. In Obesity and Lipotoxicity. Vol. 960. Springer; Cham, Switzerland: 2017. The Definition and Prevalence of Obesity and Metabolic Syndrome; pp. 1–17. [DOI] [PubMed] [Google Scholar]
- 23.Caliceti C, Calabria D, Roda A, Cicero AFG. Fructose intake, serum uric acid, and cardiometabolic disorders: A critical review. Nutrients. 2017;9:395. doi: 10.3390/nu9040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han TS, Lean ME. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis. 2016;5:2048004016633371. doi: 10.1177/2048004016633371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inzucchi SE. Clinical practice. Diagnosis of diabetes. N Engl J Med. 2012;367:542–50. doi: 10.1056/NEJMcp1103643. [DOI] [PubMed] [Google Scholar]
- 26.Wu JY, Duan XY, Li L, Dai F, Li YY, Li XJ, et al. Dyslipidemia in Shanghai, China. Prev Med. 2010;51:412–5. doi: 10.1016/j.ypmed.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Parikh RM, Mohan V. Changing definitions of metabolic syndrome. Indian J Endocrinol Metab. 2012;16:7–12. doi: 10.4103/2230-8210.91175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–49. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 30.Alexopoulos N, Katritsis D, Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. 2014;233:104–12. doi: 10.1016/j.atherosclerosis.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Thomas D, Apovian C. Macrophage functions in lean and obese adipose tissue. Metabolism. 2017;72:120–43. doi: 10.1016/j.metabol.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh U, Dasu MR, Yancey PG, Afify A, Devaraj S, Jialal I. Human C-reactive protein promotes oxidized low density lipoprotein uptake and matrix metalloproteinase-9 release in Wistar rats. J Lipid Res. 2008;49:1015–23. doi: 10.1194/jlr.M700535-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Atherosclerosis as an inflammatory disease. Curr Pharm Des. 2012;18:4266–88. doi: 10.2174/138161212802481237. [DOI] [PubMed] [Google Scholar]
- 34.Tyrrell DJ, Goldstein DR. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat Rev Cardiol. 2021;18:58–68. doi: 10.1038/s41569-020-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soeki T, Sata M. Inflammatory biomarkers and atherosclerosis. Int Heart J. 2016;57:134–9. doi: 10.1536/ihj.15-346. [DOI] [PubMed] [Google Scholar]
- 36.Jeong H, Baek SY, Kim SW, Park EJ, Lee J, Kim H, et al. C reactive protein level as a marker for dyslipidaemia, diabetes and metabolic syndrome: Results from the Korea National Health and Nutrition Examination Survey. BMJ Open. 2019;9:e029861. doi: 10.1136/bmjopen-2019-029861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H, Nie Y, Xiong H, Liu S, Li G, Huang A, et al. P2×7 Receptor expression in peripheral blood monocytes is correlated with plasma C-Reactive protein and cytokine levels in patients with type 2 diabetes mellitus: A preliminary report. Inflammation. 2015;38:2076–81. doi: 10.1007/s10753-015-0189-y. [DOI] [PubMed] [Google Scholar]
- 38.Krishnamoorthy Y, Rajaa S, Murali S, Rehman T, Sahoo J, Kar SS. Prevalence of metabolic syndrome among adult population in India: A systematic review and meta-analysis. PLoS One. 2020;15:e0240971. doi: 10.1371/journal.pone.0240971. doi:10.1371/journal.pone. 0240971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Y, Chen Z, Wang X, Zheng C, Shao L, Tian Y, et al. Comparison of the three most commonly used metabolic syndrome definitions in the Chinese population: A prospective study. Metabolites. 2022;13:12. doi: 10.3390/metabo13010012. doi:10.3390/metabo13010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author will make the raw data supporting the study’s conclusions available, without any undue reservation.


