ABSTRACT
Introduction:
Similar presenting manifestations in early phase and lack of awareness of aetiology of acute febrile illness (AFI) are major challenges in management of AFI.
Material and Methods:
This was a retrospective observational cross-sectional study conducted in the Department of Microbiology, NRS Medical College, from 1 July 2022 to 30 June 2023 in serologically diagnosed febrile patients attending the outpatient department or admitted. Clinical and epidemiological data and laboratory parameters were recorded in a pretested structured questionnaire study tool, and collected data were analysed on MS-Excel sheets with various charts and tables.
Results:
A total of 1711 serologically diagnosed febrile patients showed preponderance of dengue (38.3%), followed by leptospirosis (25%), scrub typhus (23.9%), malaria (12.6%), and enteric fever (1.92%). A majority of cases were male, less than 40 years of age, and from the rural population (73.2%), except in malaria (urban = 79.6%). The mean duration of fever was 9 days. Febrile cases were recorded maximum during the monsoon and postmonsoon periods (66.5%). The common manifestations are fever, headache (46.2%), pain abdomen (7.8%), nausea, and vomiting (9.4%). Thrombocytopenia with bleeding manifestation was higher in dengue (18%) cases. Mortality in dengue cases was recorded with multiorgan dysfunction syndrome (MODS). Scrub typhus cases showed seizure (8.3%) and altered sensorium (5%) due to fatal meningoencephalitis. Fatality in leptospirosis was mostly due to acute kidney injury (29.5%) and Weil’s disease (4.4%).
Conclusion:
Misdiagnosis or incorrect diagnosis and delay in initiation of appropriate treatment results in increased morbidity and mortality in AFI. Determination of epidemiological features and clinical manifestations of AFI along with timely correct diagnosis will benefit clinicians in proper treatment initiation, thereby reducing morbidity and mortality.
Keywords: Dengue, leptospirosis, malaria, scrub typhus, typhoid fever
Introduction
Acute febrile illness (AFI) is one of the major illnesses among people seeking treatment in primary care settings. It has varied aetiologies according to geographical distribution.[1] Diagnosis is often based on presenting manifestation, and patients receive empirical therapy due to similar presentation.[2] Studies from South, North, and West India showed major causes of AFI as scrub typhus, dengue, malaria, enteric fever, leptospirosis, chikungunya, Japanese encephalitis.[3,4,5] A gap in literature exists in the understanding of the clinical and epidemiological characteristics of AFI in eastern India.
Identifying pathogens of AFI, clinical manifestations, and adverse outcomes will enable triaging cases for better management at a population scale.
Aim of the Study
The aim of this study was to determine epidemiological factors and clinical presentations of five serologically diagnosed causes of AFI (dengue, malaria, scrub typhus, leptospirosis, and enteric fever) in a teaching hospital, Kolkata.
Materials and Methods
This was a hospital-based observational cross-sectional study conducted in the Department of Microbiology, Nil Ratan Sircar Medical College from 1 July 2022 to 30 June 2023 on febrile patients attending the outpatient department (OPD) or admitted in IPD with fever after approval of the Institutional Ethics Committee (IEC No - NRSMC/IEC/131/2022 Dt. 11/10/2022).
The case definition of AFI was acute onset fever and other symptoms such as headache, diarrhoea, chills, and cough without any obvious focus of infection for less than 14 days duration.[6,7]
Inclusion criteria
Serologically diagnosed dengue, malaria, scrub typhus, leptospirosis, and enteric fever cases.
Patients who provided written informed consent for this study.
Exclusion criteria
Cases of AFI other than serologically diagnosed cases of dengue, malaria, scrub typhus, leptospirosis, and enteric fever.
Patients suffering from haematological malignancies, autoimmune disorders, tuberculosis, and SARS CoV-2 and on immunosuppressants were excluded from this study.
Serological diagnostic criteria
Dengue: Dengue NS1 Ag ELISA (Oscar™) was performed if there was febrile illness within 5 days and IgM Ab ELISA (Standard E™) was performed after 5 days of illness. Dengue NS1 Ag and/or IgM Ab reactive cases and other serologies and nonreactive and blood culture negative cases were included in this study.
Scrub typhus: Scrub typhus IgM Ab ELISA (InBios™) reactive with other serologies nonreactive and blood culture-negative.
Leptospirosis: Leptospira IgM Ab ELISA (Panbio™) reactive with other serologies nonreactive and blood culture-negative
Malaria: Thick peripheral blood smear examination was done with screening of cases, and thin smear was used for species identification of malarial parasites (trophozoites of Plasmodium falciparum, Plasmodium vivax, or mixed) and RDT kit (Reckon™) (lateral flow immunochromatographic antigen-detection tests) for Pf-HRP II/Pan-pLDH antigen reactive but other serologies nonreactive and blood culture-negative.
Enteric fever: Blood culture positive for Salmonella Typhi or Salmonella Paratyphi (BacT/Alert3D™ and Vitek2 compact™) and semiquantitative slide agglutination Widal test (Pathozyme diagnostics™) show titre equals or more than of 1:160 for anti-O antibodies and more than of 1:320 for anti-H antibodies with other serologies as nonreactive. [see Table 1 for list of diagnostic kits which were used in this study].
Table 1.
Sensitivity and specificity of serological diagnostic kits
| Kit Name | Sensitivity | Specificity |
|---|---|---|
| Dengue NS1 Ag ELISA (Oscar™) | 100% | 100% |
| Dengue IgM Ab EILSA (Standard E™) | 98.2% | 95.4% |
| Scrub typhus IgM Ab ELISA (InBios™) | 84.0% | 94.82% |
| Leptospira IgM Ab ELISA (Panbio™) | 96.5% | 98.5% |
| Malaria RDT kit (Reckon™) | 100%* | 100% |
| Widal slide agglutination test (Pathozyme diagnostics™) | 91.3% | 80.9% |
*The sensitivity of Malaria (Pf/Pan) antigen test is compared to microscopic examination with more than 100 parasites per μl of blood. The sensitivity and specificity of all test kits provided here are according to the manufacturer’s kit literature.
Acute kidney injury (AKI) was defined according to KDIGO criteria[8] as follows:
Increase in serum creatinine by ≥0.3 mg/dl (≥26.5 μmol/l) within 48 hours or
Increase in serum creatinine to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days or
Urine volume <0.5 ml/kg/h for 6 hours.
According to World Health Organization (WHO), the leptospirosis fact sheet case definition of Weil’s disease was presence of jaundice, renal failure, haemorrhage, and myocarditis with arrhythmias in a patient infected with leptospirosis.[9]
Organ involvement in AFI was assessed by presence of or any new appearance of clinical manifestation and biochemical results suggestive of particular organ dysfunction during the course of the illness and was regularly followed up till their organ dysfunction improved or the patient died due to the illness. [see Table 2 for clinical manifestations and laboratory parameters which were used to assess organ dysfunction].
Table 2.
Relevant clinical manifestations and laboratory parameters suggestive of organ dysfunction
| Organ system | Clinical manifestation | Laboratory parameter/investigation |
|---|---|---|
| Haematological | Anaemia, Bleeding manifestation | Complete blood count |
| GI system | Nausea, vomiting, diarrhoea | - |
| Hepatobiliary system | Icterus, ascites | Liver function test, USG abdomen |
| Renal system | AKI | Kidney function test |
| Cardiovascular system | Myocarditis, heart failure | ECG, Echocardiography |
| Respiratory system | Shortness of breath. Pleural effusion | Chest X Ray |
| Central nervous system | Altered sensorium, Encephalitis, meningitis | CT scan, CSF study |
In our study, diagnosis of AFI was made on the basis of a combination of clinical manifestation, serological results, and relevant other laboratory parameters such as complete blood count, liver function test, and kidney function test profile.
Clinical and epidemiological data and laboratory parameters from patients, who fulfilled inclusion criteria, were recorded in a pretested structured questionnaire study tool. Data generated were collected and compiled in MS-EXCEL sheets, and relevant statistical analysis, for example, calculation of mean, standard deviation, and percentage calculation, was done and represented in the form of various charts and tables.
Results
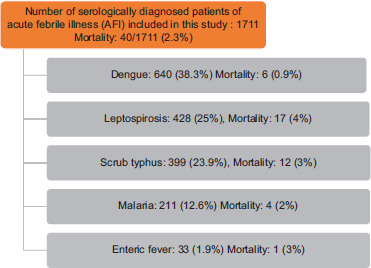
During the study period, a total of 1711 patients were serologically diagnosed with AFI. A majority of AFI cases suffered from dengue infection (38.3%), followed by leptospirosis (25%), scrub typhus (23.9%), malaria (12.6%), and enteric fever (1.9%). Higher mortality was recorded in leptospirosis (4%) with an overall mortality of AFI of 2.3% [see Table 3]. Most of the malaria cases were caused by P. vivax (58.8%), followed by P. falciparum (40.8%), and mixed infection was noted in one patient only.
Table 3.
Total number of diagnosed AFI cases, distribution of dengue, leptospirosis, scrub typhus, malaria, and enteric cases and respective mortality rates
|
A majority of patients who attended OPD or were admitted were from rural areas of North and South, 24 Parganas, Howrah, Hooghly, and Nadia, except in malaria (79.6% from urban Kolkata). A majority of the patients were less than 40 years (mean = 35.79 years, SD = 21.22) with a male preponderance except in leptospirosis [see Table 4].
Table 4.
Distribution of age, sex, and geographic location across five causes of AFI
| Dengue (n=640) | Leptospirosis (n=428) | Scrub typhus (n=399) | Malaria (n=211) | Enteric fever (n=33) | |
|---|---|---|---|---|---|
| Age mean (SD) | 25.8 (15.7) | 34.8 (22.3) | 24.5 (18.7) | 39.1 (20.5) | 41.1 (21.7) |
| Male n (%) | 365 (57.03) | 213 (49.76) | 223 (55.88) | 149 (70.61) | 19 (57.57) |
| Female n (%) | 275 (42.97) | 215 (50.24) | 176 (44.12) | 62 (29.39) | 14 (42.43) |
| Urban n (%) | 224 (35) | 111 (25.93) | 58 (14.53) | 168 (79.62) | 9 (27.27) |
| Rural n (%) | 416 (65) | 317 (74.07) | 341 (85.47) | 48 (20.38) | 24 (72.73) |
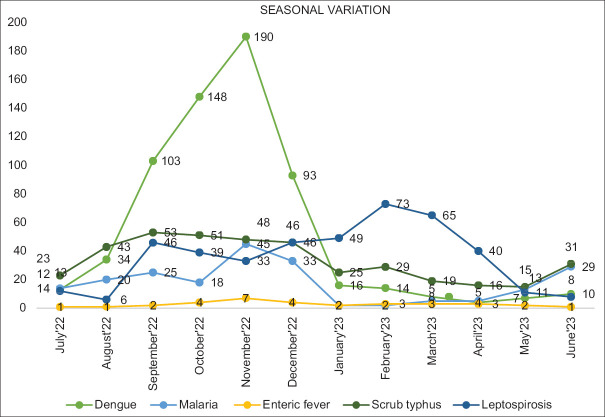
Distinctive seasonal distribution of AFI cases was observed, and a marked increase in cases of dengue was noted in post monsoon months. A post-monsoon peak was also seen in malaria, scrub typhus, and leptospirosis cases [see Figure 1].
Figure 1.
Seasonal distribution of AFI cases
The time of presentation with fever was longer in enteric fever (mean = 9 days, SD = 2.3), followed by leptospirosis (mean = 7.7, SD = 4.9). The common presentations in AFI cases were headache in dengue (73.12%), malaria (92.41%), enteric fever (39.39%), scrub typhus (18.29%), vomiting in dengue (17.34%), and scrub typhus (14.53%). Bleeding manifestation (17.96%) was markedly noted in dengue, whereas breathlessness (17.54%), seizure (8.27%), and altered sensorium (5.01%) were prominent symptoms of scrub typhus [see Table 5].
Table 5.
Clinical manifestations of AFI cases
| Dengue (n=640) | Leptospirosis (n=428) | Scrub typhus (n=399) | Malaria (n=211) | Enteric fever (n=33) | |
|---|---|---|---|---|---|
| Fever duration mean (SD) | 5.1 (5.2) | 7.7 (4.9) | 7.6 (2.7) | 5.5 (3.3) | 9 (2.3) |
| Headache n (%) | 468 (73.12) | 33 (8.48) | 73 (18.29) | 195 (92.41) | 13 (39.39) |
| Pain abdomen n (%) | 93 (14.53) | 16 (4.11) | 48 (12.03) | 12 (5.68) | 3 (9.09) |
| Nausea n (%) | 158 (24.68) | 22 (5.14) | 79 (19.79) | 17 (8.05) | 2 (6.06) |
| Vomiting n (%) | 111 (17.34) | 20 (5.14) | 58 (14.53) | 17 (8.05) | 2 (6.06) |
| Bleeding manifestation n (%) | 115 (17.96) | 13 (3.34) | 10 (2.5) | 6 (2.84) | Nil |
| Breathlessness n (%) | 17 (2.65) | 13 (3.34) | 70 (17.54) | Nil | Nil |
| Seizure n (%) | 8 (1.25) | 7 (1.79) | 33 (8.27) | Nil | Nil |
| Altered sensorium n (%) | 7 (1.09) | 17 (4.37) | 20 (5.01) | Nil | Nil |
Laboratory investigation showed the mean haemoglobin level decreased in both scrub typhus (Hb – 9.8 g/dl, SD – 1.9) and leptospirosis (Hb – 9.8g/dl SD – 1.4). The total leucocyte count was significantly higher in scrub typhus (mean TLC = 111658/cmm SD = 6289.9) as compared to other causes. In leptospirosis and scrub typhus, increased serum urea and creatinine levels were recorded, which indicated renal involvement. Liver transaminase levels were also higher in leptospirosis as compared to other causes. In dengue, platelet transfusion was required for bleeding manifestation with thrombocytopenia during 5th to 8th day of illness in most of the patients. Thrombocytopenia here is defined by World Health Organization (WHO) as a platelet count of less than or equal to 100,000/μL [see Table 6].
Table 6.
Laboratory parameters in AFI cases
| Dengue (n=640) | Leptospirosis (n=428) | Scrub typhus (n=399) | Malaria (n=211) | Enteric fever (n=33) | |
|---|---|---|---|---|---|
| Haemoglobin g/dl mean (SD) | 11 (2.35) | 9.8 (1.9) | 9.8 (1.4) | 10.3 (2.8) | 10.6 (2.5) |
| TLC cells/cmm mean (SD) | 9138 (5926.7) | 10938 (5962.4) | 11658 (6289.9) | 8406 (6102.6) | 10608 (5393.6) |
| Platelet cells/cmm mean (SD) | 143526 (80497.2) | 144321 (99472.8) | 149747 (31039.7) | 107889 (88029.9) | 200000 (11871.4) |
| Urea mg/dl mean (SD) | 49 (47.45) | 75 (82.3) | 60 (56.9) | 36.1 (24.2) | 37 (30.8) |
| Creatinine mg/dl mean (SD) | 1.5 (1.3) | 2 (1.9) | 2 (2.4) | 1.17 (1.2) | 1 (1.4) |
| Total bilirubin mg/dl mean (SD) | 2.5 (3.6) | 3 (6.2) | 2 (1.4) | 1.2 (1.8) | 1 (2.2) |
| Albumin g/dl mean (SD) | 3.2 (0.6) | 3 (0.7) | NA* | 3.2 (0.6) | 3 (0.6) |
| SGOT U/L mean (SD) | 119 (197.1) | 223 (525.4) | 146 (125) | 87 (173.6) | 68 (129.5) |
| SGPT U/L mean (SD) | 171 (262.1) | 154 (331.4) | 176 (326.8) | 67 (180.3) | 53 (68.7) |
| ALP U/L mean (SD) | 116 (61.1) | 191 (229.3) | NA* | 162 (163.4) | 169 (182.8) |
NA*: Not available
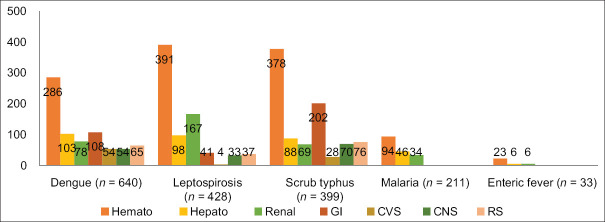
In all AFI cases, the most affected was the haematological system. Hepatic and renal involvement was marked in both leptospirosis and dengue cases, which was also evident by deranged renal and liver function test profiles. In scrub typhus, gastrointestinal (GI) system involvement was prominent (50.62%), which manifested as pain abdomen, nausea, and vomiting. Cardiovascular system (CVS) involvement was seen in severe dengue with shock (8.43%) and in scrub typhus (7.01%), which presented as breathlessness. Severe renal involvement in leptospirosis was seen in the form of AKI (29.5%) and Weil’s disease (4.4%). CNS involvement in the form of seizure (8.3%) and altered sensorium (5%) was recorded in scrub typhus cases [see Figure 2].
Figure 2.
Bar diagram showing organ involvement in AFI cases
Discussion
Our study is one of the few studies from East India which characterised spectra of AFI caused by five major pathogens in patients attending OPD or admitted in IPD in a teaching hospital.
The diagnosis of AFIs presents a challenge due to varied aetiologies and fewer diagnostic tools available, even in a teaching hospital like ours. Serological tests are valuable diagnostic tools because they are simple to perform and provide rapid results within few hours of sample receipt.
In the present study, out of 1711 serologically diagnosed AFI cases, males (56.63%) were affected more as compared to females; a majority of patients were in the age group of 25 years to 41 years and the average day of presentation with fever was 9 days. Shelke YP et al.[5] and Kumar M et al.[10] in their studies of AFI showed that males are predominantly affected, and another study done by Abhilash K et al.[3] showed that a majority of the patients were younger than 40 years of age. Shelke YP et al.[5] also showed that average day of presentation with fever was 8 days in their study. All these findings are similar with the findings of ours.
In our study, vector-borne diseases, for example, dengue, scrub typhus, leptospirosis, and malaria, had a definitive surge of cases in postmonsoon seasons, which was more prominent in dengue, due to favourable breeding places of vectors. In a similar study by Arvind N et al.[11] conducted in South India, similar upsurge of AFI cases was recorded in rainy and autumn seasons.
Our study showed dengue as the predominant cause of AFI, followed by leptospirosis, scrub typhus, malaria, and enteric fever, but previous similar studies showed scrub typhus as a major cause of AFI.[3,5]
Every year, dengue outbreak is a major public health concern in our part of the country with a significant reported morbidity and mortality.[12] In our study, severe dengue cases with bleeding manifestations and thrombocytopenia, between 5th and 8th days of illness, were treated with platelet transfusion with regular platelet level monitoring. This time period of bleeding manifestations in our study is corroborative with a critical phase of severe dengue as defined by WHO.[13]
Scrub typhus and leptospirosis are two important vector-borne causes of AFI with high mortality. Both infections are undiagnosed and underreported because similar clinical manifestations at presentation, lack of awareness, and serological tests for diagnosis of both diseases are only available in teaching hospitals.[14] Delayed diagnosis in both illnesses had poor outcome. In our study, all scrub typhus mortality (3%) was due to acute encephalitis syndrome. In a systematic review and meta-analysis by Alam A et al.,[15] the adult case fatality rate of scrub typhus encephalitis was 2.6%, which is similar with our findings (3%). In leptospirosis mortality group, common presentation was Weil’s disease, which was characterised by multiple organ system involvement as described before. In our study, AKI was a common manifestation in leptospirosis (29.5%). In a systematic review and meta-analysis on leptospirosis in India, Gupta N et al.[16] showed 35% AKI in leptospirosis due to tubulointerstitial nephritis; a similar rate was observed in our study.
Gold standard tests for diagnosing major causes of AFI are unavailable in a majority of resource-limited facilities and also time-consuming. Serological tests also have several drawbacks, e.g. cross-reactivity and misdiagnosis of coinfections as seen in scrub typhus, leptospirosis, and dengue.[14,17] Sensitivity of Widal test increases from second week of illness.[18] To overcome such challenges, syndromic testing by nested multiplex PCR, which has high sensitivity and specificity in detecting major pathogens of AFI at an early phase of illness, will aid early specific treatment initiation.[19]
WHO developed fever management protocol at the community level,[20] but geographical variations of AFI aetiologies pose challenges to implementation of that protocol, thus affecting early, effective management of AFI cases.[21] Study like ours will provide information on the distribution and prevalence of infectious aetiologies of AFIs for formulation of clinical algorithms and management protocols, which is appropriate in our geographic region.
Limitation
In our study, a limited number of pathogens were investigated due to resource constraints and limited laboratory diagnostic capacity. Gold standard tests such as IFA in scrub typhus and MAT in leptospirosis could not be done as these tests are available in selected reference laboratories. Coinfection was not included in our study because serological tests are unreliable in diagnosis of coinfection due to cross-reactivity or missed diagnosis. In enteric fever, we included only cases which are both blood culture-positive and Widal semiquantitative slide agglutination test-reactive, but both these tests have very low sensitivity in early phases of illness, so we may have missed a number of enteric fever cases in our study. Convalescent phase Widal slide agglutination test was done only in admitted patients. Serial laboratory investigation for organ dysfunction follow-up could only be performed in inpatient cases as most of the outpatient department patients did not turn up for follow-up. P-time and APTT level in all dengue cases with bleeding manifestation were not determined. Due to fund constraint, we could not perform nested multiplex PCR in patients presented with clinical manifestations of AFI; by PCR, we could have detected more cases of AFI at an early phase of illness, which will aid clinicians in informed decision making.
Conclusion
Similar manifestations in the early phase of illness, unawareness about local aetiology, and challenges in serodiagnosis are contributing factors for delay in diagnosis and initiation of appropriate treatment according to aetiology in AFI cases.
Syndromic testing by an accurate diagnostic tool, e.g. Multiplex PCR, which can detect all infections at in a single test setting in early phase of illness with high sensitivity and specificity and appropriate empirical treatment guideline, will have a greater implication in patient management protocols in AFI cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rhee C, Kharod GA, Schaad N, Furukawa NW, Vora NM, Blaney DD, et al. Global knowledge gaps in acute febrile illness etiologic investigations: A scoping review. PLoS Negl Trop Dis. 2019;13:e0007792. doi: 10.1371/journal.pntd.0007792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varghese GM. The search for effective empiric therapy for acute undifferentiated febrile illness. Clin infect Dis. 2021;73 doi: 10.1093/cid/ciaa1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abhilash KPP, Jeevan JA, Mitra S, Paul N, Murugan TP, Rangaraj A, et al. Acute undifferentiated febrile illness in patients presenting to a tertiary care Hospital in South India: Clinical spectrum and outcome. J Global Infect Dis. 2016;8:147–54. doi: 10.4103/0974-777X.192966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra V, Shukla D, Bano F, Sharma S, Nigam S, Kumar S, et al. To study the prevalence of aetiologies acute undifferentiated febrile illnesses of the patients at a tertiary care centre in Uttar Pradesh, India. J Popul Ther Clin Pharmacol. 2023;30:1719–26. [Google Scholar]
- 5.Shelke YP, Deotale VS, Maraskolhe DL. Spectrum of infections in acute febrile illness in Central India. Indian J Med Microbiol. 2017;35:480–4. doi: 10.4103/ijmm.IJMM_17_33. [DOI] [PubMed] [Google Scholar]
- 6.Division of Global Health Protection, Global Health, Centers for Disease Control and Prevention. CDC Leverages Acute Febrile Illness Surveillance System to Respond to COVID-19. Atlanta (GA): Updates from the Field; 2021. [[Last accessed on 2024 Jan 13]]. [updated 2022 Feb 15] Available from: https://www.cdc.gov/globalhealth/healthprotection/fieldupdates/2021/UFTF-AFI-Surveillance-COVID-Central-America.html . [Google Scholar]
- 7.Waggoner J, Wu HM. Health recommendations for international travel. In: Loscalzo J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison's principles of internal medicine. 21st ed. New York: McGraw-Hill Education; 2022. pp. 1000–1. [Google Scholar]
- 8.KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. Kidney Inter. 2012;2((Suppl 1)):8. [Google Scholar]
- 9.World Health Organization (WHO) Regional Office for South-East Asia (SEARO) Fact sheet: Leptospirosis. New Delhi: SEARO; 2009. [[Last accessed on 2024 Jan 13]]. Available from: https://apps.who.int/iris/handle/10665/205437 . [Google Scholar]
- 10.Kumar M, Verma R, Mishra B. Prevalence of dengue fever in Western Uttar Pradesh, India: A gender-based study. Int J Appl Basic Med Res. 2020;10:8–11. doi: 10.4103/ijabmr.IJABMR_337_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arvind N, Prabhakar K, Savitha N, Mahendra M. Clinical and microbiological profile of patients with acute febrile illness attending a tertiary care hospital in South India. J Pure Appl Microbiol. 2018;12:757–63. [Google Scholar]
- 12.National Center for Vector Borne Diseases Control (NCVBDC). Ministry of Health and Family Welfare Govt. of India. Dengue Situation in India; 2023 [Google Scholar]
- 13.Cogan JE. Dengue and Severe Dengue. World Health Organization; 2023. World Health Organization. [Google Scholar]
- 14.Rahi M, Gupte MD, Bhargava A, Varghese GM, Arora R. DHR-ICMR guidelines for diagnosis and management of rickettsial diseases in India. Indian J Med Res. 2015;141:417–22. doi: 10.4103/0971-5916.159279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam AM, Gillespie CS, Goodall J, Damodar T, Turtle L, Vasanthapuram R, et al. Neurological manifestations of scrub typhus infection: A systematic review and meta-analysis of clinical features and case fatality. PLoS Negl Trop Dis. 2022;16:e0010952. doi: 10.1371/journal.pntd.0010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta N, Wilson W, Ravindra P. Leptospirosis in India: A systematic review and meta-analysis of clinical profile, treatment and outcomes. Infez Med. 2023;31:290–305. doi: 10.53854/liim-3103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nithiyanandan S, Prashanth R, Balaji N, Magesh BR, Kumaran V. A review of acute febrile illness. Indian J Microbiol Res. 2023;9:232–40. [Google Scholar]
- 18.Maheshwari V, Kaore NM, Ramnani VK, Sarda S. A comparative evaluation of different diagnostic modalities in the diagnosis of typhoid fever using a composite reference standard: A tertiary hospital based study in Central India. J Clin Diagn Res. 2016;10:DC01–4. doi: 10.7860/JCDR/2016/20426.8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Ann Rev Biomed Eng. 2008;10:107–44. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. WHO Informal Consultation on Fever Management in Peripheral Health Care Settings: A Global Review of Evidence and Practice. viii. World Health Organization; 2013. p. 66. [Google Scholar]
- 21.FIND. Acute febrile syndrome. FIND Acute Febrile Syndr Strateg. 2012;3:1–31. [Google Scholar]